Characterization of Inflammatory Signals in BV-2 Microglia in Response to Wnt3a
Abstract
1. Introduction
2. Methods
2.1. Drug Administration
2.2. Quantitative Real-Time Polymerase Chain Reaction—RT-PCR
2.3. Protein Determinations
2.4. Immunocytochemistry
2.5. ELISA
2.6. Cell Culture
2.7. Nitroxide (NO) Assay
2.8. Cell Viability Assay
2.9. Statistics
3. Results
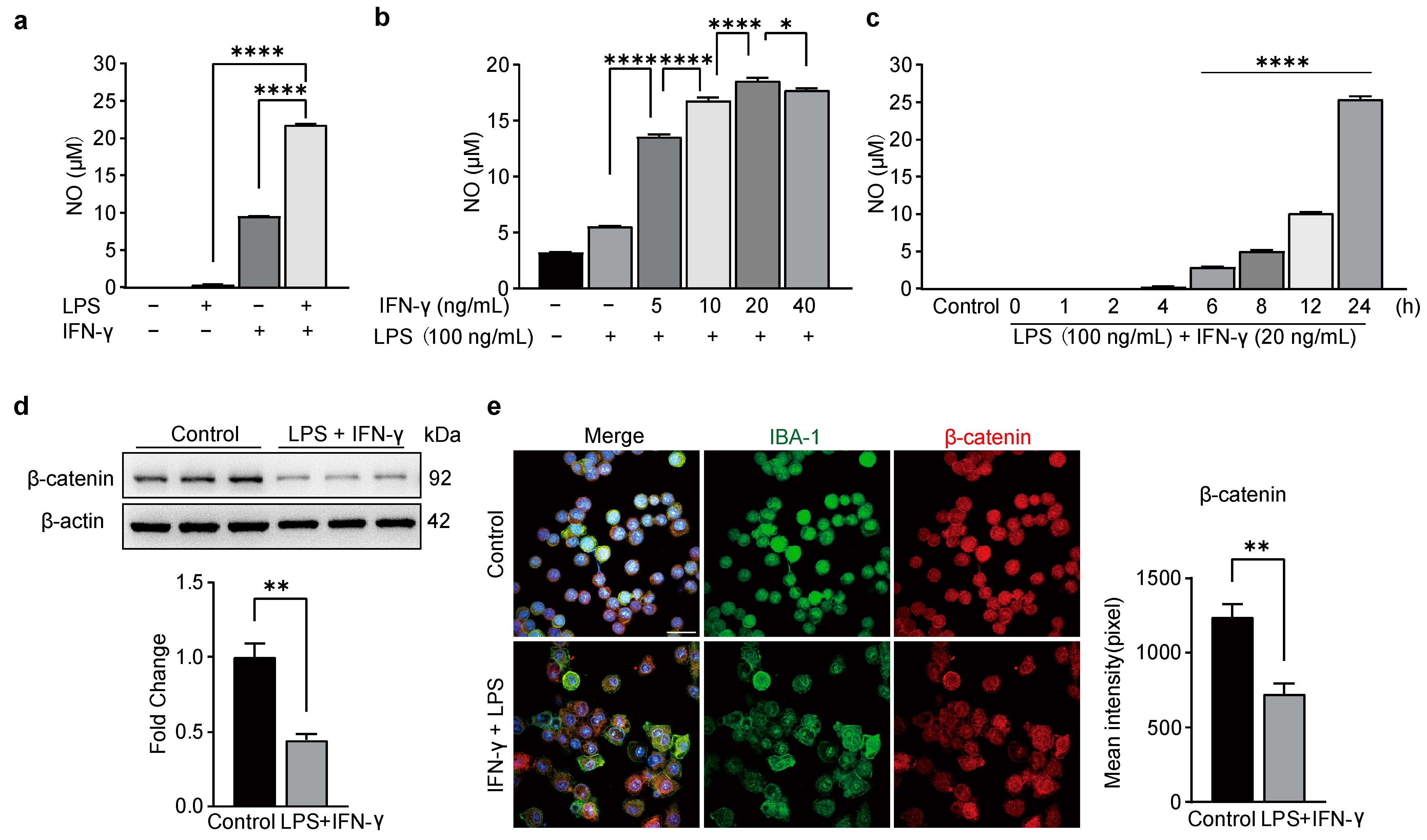
3.1. The Expression of β-Catenin Is Inhibited in LPS/IFN-γ-Treated BV2 Cells
3.2. Wnt3a Does Not Induce Cytotoxicity to BV-2 Microglial Cells
3.3. Wnt3a Activates Wnt/β-Catenin Pathway in BV-2 Cells
3.4. Overexpression of Wnt3a Inhibits Pro-Inflammation Activation in LPS/IFNγ-Treated BV-2 Cells
3.5. Overexpression of Wnt3a Inhibits the Expression of Pro-Inflammatory Mediators in LPS/IFNγ-Stimulated BV-2 Cells
3.6. Wnt3a-Overexpression Suppresses the Activation of NF-κB and ERK1/2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, D.; Jiang, Y. Sirtuin 3 attenuates neuroinflammation-induced apoptosis in BV-2 microglia. Aging 2019, 11, 9075–9089. [Google Scholar] [CrossRef]
- Chen, H.; Kankel, M.W.; Su, S.C.; Han, S.W.S.; Ofengeim, D. Exploring the genetics and non-cell autonomous mechanisms underlying ALS/FTLD. Cell. Death Differ. 2018, 25, 648–662. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Perry, V.H.; Nicoll, J.A.R.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and Inflammation in Alzheimers Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Du, G.H.; Qin, X.M.; Gao, L. Baicalein attenuates the neuroinflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-κB expressions and regulation of metabolic abnormality. Int. Immunopharmacol. 2020, 79, 106092. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.-B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Jun, Y.; Yan, Z.; Tay, S.S.W.; Ang Ling, E. Retinoic acid inhibits expression of TNF-? and iNOS in activated rat microglia. Glia 2005, 50, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, M.; Lim, S.; Sung, S.; Kim, Y. Inhibition of inducible NO synthase, cyclooxygenase-2 and interleukin-1β by torilin is mediated by mitogen-activated protein kinases in microglial BV2 cells: Inhibitory effects of torilin in BV2 cells. Br. J. Pharmacol. 2009, 156, 933–940. [Google Scholar] [CrossRef]
- Ooi, Y.Y.; Ramasamy, R.; Rahmat, Z.; Subramaiam, H.; Tan, S.W.; Abdullah, M.; Israf, D.A.; Vidyadaran, S. Bone marrow-derived mesenchymal stem cells modulate BV2 microglia responses to lipopolysaccharide. Int. Immunopharmacol. 2010, 10, 1532–1540. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.-S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Wilms, H.; Sievers, J.; Rickert, U.; Rostami-Yazdi, M.; Mrowietz, U.; Lucius, R. Dimethylfumarate inhibits microglial and astrocytic inflammation by suppressing the synthesis of nitric oxide, IL-1β, TNF-α and IL-6 in an in-vitro model of brain inflammation. J. Neuroinflamm. 2010, 7, 30. [Google Scholar] [CrossRef]
- Muley, A.; Odaka, Y.; Lewkowich, I.P.; Vemaraju, S.; Yamaguchi, T.P.; Shabwer, C.; Dickie, B.H.; Lang, R.A. Myeloid Wnt ligands are required for normal development of dermal lymphatic vasculature. PLoS ONE 2017, 12, e0181549. [Google Scholar] [CrossRef] [PubMed]
- Lobov, I.B.; Rao, S.; Carroll, T.J.; Vallance, J.E.; Ito, M.; Ondr, J.K.; Kurup, S.; Glass, D.A.; Patel, M.S.; Shu, W.; et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 2005, 437, 417–421. [Google Scholar] [CrossRef]
- Abaricia, J.O.; Shah, A.H.; Chaubal, M.; Hotchkiss, K.M.; Olivares-Navarrete, R. Wnt signaling modulates macrophage polarization and is regulated by biomaterial surface properties. Biomaterials 2020, 243, 119920. [Google Scholar] [CrossRef]
- Van Steenwinckel, J.; Schang, A.L.; Krishnan, M.L.; Degos, V.; Delahaye-Duriez, A.; Bokobza, C.; Csaba, Z.; Verdonk, F.; Montané, A.; Sigaut, S.; et al. Decreased microglial Wnt/β-catenin signalling drives microglial pro-inflammatory activation in the developing brain. Brain 2019, 142, 3806–3833. [Google Scholar] [CrossRef]
- Ille, F.; Sommer, L. Wnt signaling: Multiple functions in neural development. CMLS Cell. Mol. Life Sci. 2005, 62, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, J.; Tian, Y.; Wang, Z.; Song, Z.; Li, K.; Zhang, S.; Zhao, H. Wnt/β-Catenin Signaling Pathway Is Strongly Implicated in Cadmium-Induced Developmental Neurotoxicity and Neuroinflammation: Clues from Zebrafish Neurobehavior and In Vivo Neuroimaging. Int. J. Mol. Sci. 2022, 23, 11434. [Google Scholar] [CrossRef]
- Buchanan, F.G.; DuBois, R.N. Connecting COX-2 and Wnt in cancer. Cancer Cell 2006, 9, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 3389. [Google Scholar] [CrossRef]
- Lazarian, G.; Friedrich, C.; Quinquenel, A.; Tran, J.; Ouriemmi, S.; Dondi, S.; Martin, A.; Mihoub, I.; Chiron, D.; Bellanger, C.; et al. Stabilization of β-catenin upon B-cell receptor signaling promotes NF-kB target genes transcription in mantle cell lymphoma. Oncogene 2020, 39, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, Z.; Liang, L.; Ma, P.; Cui, D.; Chen, P.; Wu, G.; Song, X.-J. Wnt signaling contributes to withdrawal symptoms from opioid receptor activation induced by morphine exposure or chronic inflammation. Pain 2020, 161, 532–544. [Google Scholar] [CrossRef] [PubMed]
- De Caris, M.G.; Grieco, M.; Maggi, E.; Francisco, A.; Armeli, F.; Mosca, L.; Pinto, A.; D’Erme, M.; Mancini, P.; Businaro, R. Blueberry Counteracts BV-2 Microglia Morphological and Functional Switch after LPS Challenge. Nutrients 2020, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Lau, F.C.; Bielinski, D.F.; Joseph, J.A. Inhibitory effects of blueberry extract on the production of inflammatory mediators in lipopolysaccharide-activated BV2 microglia. J. Neurosci. Res. 2007, 85, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Chen, B.Y.; Sun, X.L.; Luo, Z.-J.; Rao, Z.-R.; Wang, J.-J.; Chen, L.-W. LPS-Induced proNGF Synthesis and Release in the N9 and BV2 Microglial Cells: A New Pathway Underling Microglial Toxicity in Neuroinflammation. PLoS ONE 2013, 8, e73768. [Google Scholar] [CrossRef] [PubMed]
- Burvenich, I.J.G.; Parakh, S.; Lee, F.T.; Guo, N.; Liu, Z.; Gan, H.K.; Rigopoulos, A.; O’Keefe, G.J.; Gong, S.J.; Goh, Y.W.; et al. Molecular imaging of T cell co-regulator factor B7-H3 with 89Zr-DS-5573a. Theranostics 2018, 8, 4199–4209. [Google Scholar] [CrossRef]
- Guo, Q.H.; Yang, H.J.; Wang, S.D. Olanzapine inhibits the proliferation and induces the differentiation of glioma stem-like cells through modulating the Wnt signaling pathway in vitro. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4455. [Google Scholar]
- Wang, F.; Li, C.; Shao, J.; Ma, J. Sevoflurane induces inflammation of microglia in hippocampus of neonatal rats by inhibiting Wnt/β-Catenin/CaMKIV pathway. J. Pharmacol. Sci. 2021, 146, 105–115. [Google Scholar] [CrossRef]
- Halaris, A.E.; Belendiuk, K.T.; Freedman, D.X. Antidepressant drugs affect dopamine uptake. Biochem. Pharmacol. 1975, 24, 1896–1897. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Martins, B.; Novo, J.P.; Fonseca, É.; Raposo, R.; Sardão, V.A.; Pereira, F.; Oriá, R.B.; Fontes-Ribeiro, C.; Malva, J. Necrotic-like BV-2 microglial cell death due to methylmercury exposure. Front. Pharmacol. 2022, 13, 1003663. [Google Scholar] [CrossRef] [PubMed]
- Yokoo, E.M.; Valente, J.G.; Grattan, L.; Schmidt, S.L.; Platt, I.; Silbergeld, E.K. Low level methylmercury exposure affects neuropsychological function in adults. Environ. Health 2003, 2, 8. [Google Scholar] [CrossRef]
- Aires, I.D.; Boia, R.; Rodrigues-Neves, A.C.; Madeira, M.H.; Marques, C.; Ambrósio, A.F.; Santiago, A.R. Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia 2019, 67, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.Z.; Ebeid, M.A.; El Faramawy, Y.; Saad, S.S.T.; El Magdoub, H.M.; Attia, A.A.; Aboul-Fotouh, S.; Abdel-Tawab, A.M. Effects of lithium on cytokine neuro-inflammatory mediators, Wnt/β-catenin signaling and microglial activation in the hippocampus of chronic mild stress-exposed rats. Toxicol. Appl. Pharmacol. 2020, 399, 115073. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin Prevents Lipopolysaccharide (LPS)-Induced Parkinson’s Disease via Regulation of the AKT/GSK3β-Nrf2/NF-κB Signaling Axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Yen, C.Y.; Chen, W.L.; Lin, C.-H.; Wu, Y.-R.; Chang, K.-H.; Lee-Chen, G.-J. Pathomechanism Characterization and Potential Therapeutics Identification for Parkinson’s Disease Targeting Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 1062. [Google Scholar] [CrossRef]
- Ma, B.; Liu, Y.; Zhang, X.; Zhang, R.; Zhang, Z.; Zhang, Z.; Liu, J.; Juan, Z.; Sun, X.; Sun, L.; et al. TSPO Ligands Protect against Neuronal Damage Mediated by LPS-Induced BV-2 Microglia Activation. Oxidative Med. Cell. Longev. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Chen, J.C.; Ho, F.M.; Chao, P.-D.L.; Chen, C.-P.; Jen, K.-C.G.; Hsu, H.-B.; Lee, S.-T.; Wu, W.T.; Lin, W.W. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IκB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur. Journal. Pharmacol. 2005, 521, 9–20. [Google Scholar] [CrossRef]
- Sheng, W.; Zong, Y.; Mohammad, A.; Ajit, D.; Cui, J.; Han, D.; Hamilton, J.L.; Simonyi, A.; Sun, A.Y.; Gu, Z.; et al. Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA₂-IIA expression in astrocytes and microglia. J. Neuroinflamm. 2011, 8, 121. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Masckauchán, T.N.H.; Shawber, C.J.; Funahashi, Y.; Li, C.M.; Kitajewski, J. Wnt/β-Catenin Signaling Induces Proliferation, Survival and Interleukin-8 in Human Endothelial Cells. Angiogenesis 2005, 8, 43–51. [Google Scholar] [CrossRef]
- Li, W.; Tong, H.; Huang, X.; Wang, W.; Wu, H.; Lin, S. High levels of β-catenin promote IFNγ-induced apoptosis in hepatocellular carcinoma cells. Oncol. Lett. 2012, 4, 1092–1096. [Google Scholar] [CrossRef]
- Blumenthal, A.; Ehlers, S.; Lauber, J.; Buer, J.; Lange, C.; Goldmann, T.; Heine, H.; Brandt, E.; Reiling, N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 2006, 108, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Oderup, C.; LaJevic, M.; Butcher, E.C. Canonical and Noncanonical Wnt Proteins Program Dendritic Cell Responses for Tolerance. J. Immunol. 2013, 190, 6126–6134. [Google Scholar] [CrossRef]
- Srinivasan, M.; Lahiri, D.K. Significance of NF-κB as a pivotal therapeutic target in the neurodegenerative pathologies of Alzheimer’s disease and multiple sclerosis. Expert. Opin. Ther. Targets 2015, 19, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chou, L.F.; Hong, C.H.; Chang, M.-Y.; Tsai, C.-Y.; Tian, Y.-C.; Yang, H.-Y.; Yang, C.-W. Crosstalk between E-Cadherin/β-Catenin and NF-κB Signaling Pathways: The Regulation of Host-Pathogen Interaction during Leptospirosis. Int. J. Mol. Sci. 2021, 22, 13132. [Google Scholar] [CrossRef]
- Schön, S.; Flierman, I.; Ofner, A.; Stahringer, A.; Holdt, L.M.; Kollings, F.T.; Herbst, A. β-catenin regulates NF-κB activity via TNFRSF19 in colorectal cancer cells: β-catenin regulates NF-κB activity via TNFRSF19. Int. J. Cancer 2014, 135, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Li, H.R.; Chen, Y.; Zhou, Z.; Shi, Y.Q.; Zhang, L.L.; Xin, L.; Zhao, D.B. ZNRF3 Regulates Collagen-Induced Arthritis Through NF-kB and Wnt Pathways. Inflammation 2020, 43, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Jeng, K.C.G.; Kuo, J.S.; Chen, H.-L.; Huang, H.-Y.; Chen, W.-F.; Lin, S.-Z. c-Jun N-terminal kinase and, to a lesser extent, p38 mitogen-activated protein kinase regulate inducible nitric oxide synthase expression in hyaluronan fragments-stimulated BV-2 microglia. J. Neuroimmunol. 2004, 146, 50–62. [Google Scholar] [CrossRef]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 3311–3319. [Google Scholar] [CrossRef]




| Gene | Forward | Reverse |
|---|---|---|
| iNOS | GGAGTGACGGCAAACATGACT | TCGATGCACAACTGGGTGAAC |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | CCAGAAACCGCTATGAAGTTCC | TCACCAGCATCAGTCCCAAG |
| β-actin | ATGGATGACGATATCGCTGC | TTCTGACCCATTCCCACCATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wu, Y.; Huang, M.-Y.; Song, X.-J. Characterization of Inflammatory Signals in BV-2 Microglia in Response to Wnt3a. Biomedicines 2023, 11, 1121. https://doi.org/10.3390/biomedicines11041121
Li C, Wu Y, Huang M-Y, Song X-J. Characterization of Inflammatory Signals in BV-2 Microglia in Response to Wnt3a. Biomedicines. 2023; 11(4):1121. https://doi.org/10.3390/biomedicines11041121
Chicago/Turabian StyleLi, Cheng, Ying Wu, Ming-Yue Huang, and Xue-Jun Song. 2023. "Characterization of Inflammatory Signals in BV-2 Microglia in Response to Wnt3a" Biomedicines 11, no. 4: 1121. https://doi.org/10.3390/biomedicines11041121
APA StyleLi, C., Wu, Y., Huang, M.-Y., & Song, X.-J. (2023). Characterization of Inflammatory Signals in BV-2 Microglia in Response to Wnt3a. Biomedicines, 11(4), 1121. https://doi.org/10.3390/biomedicines11041121






