Long-Term Benefit of Perlingual Polybacterial Vaccines in Patients with Systemic Autoimmune Diseases and Active Immunosuppression
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Subjects and Design
2.2. Immune Intervention (Immunotherapy)
2.3. Clinical Outcomes
2.4. Statistical Analysis
3. Results
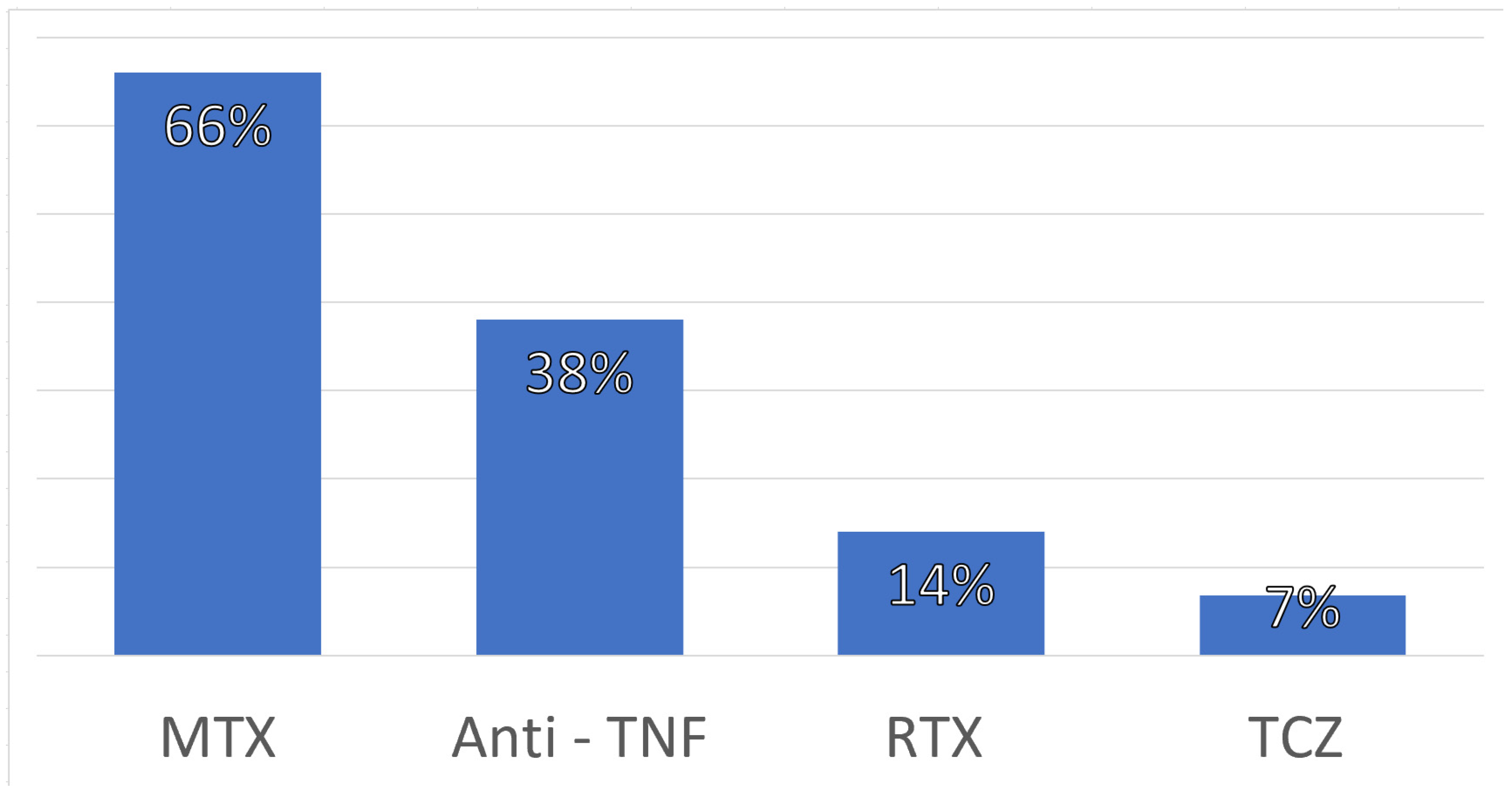
3.1. Epidemiological and Clinical Features
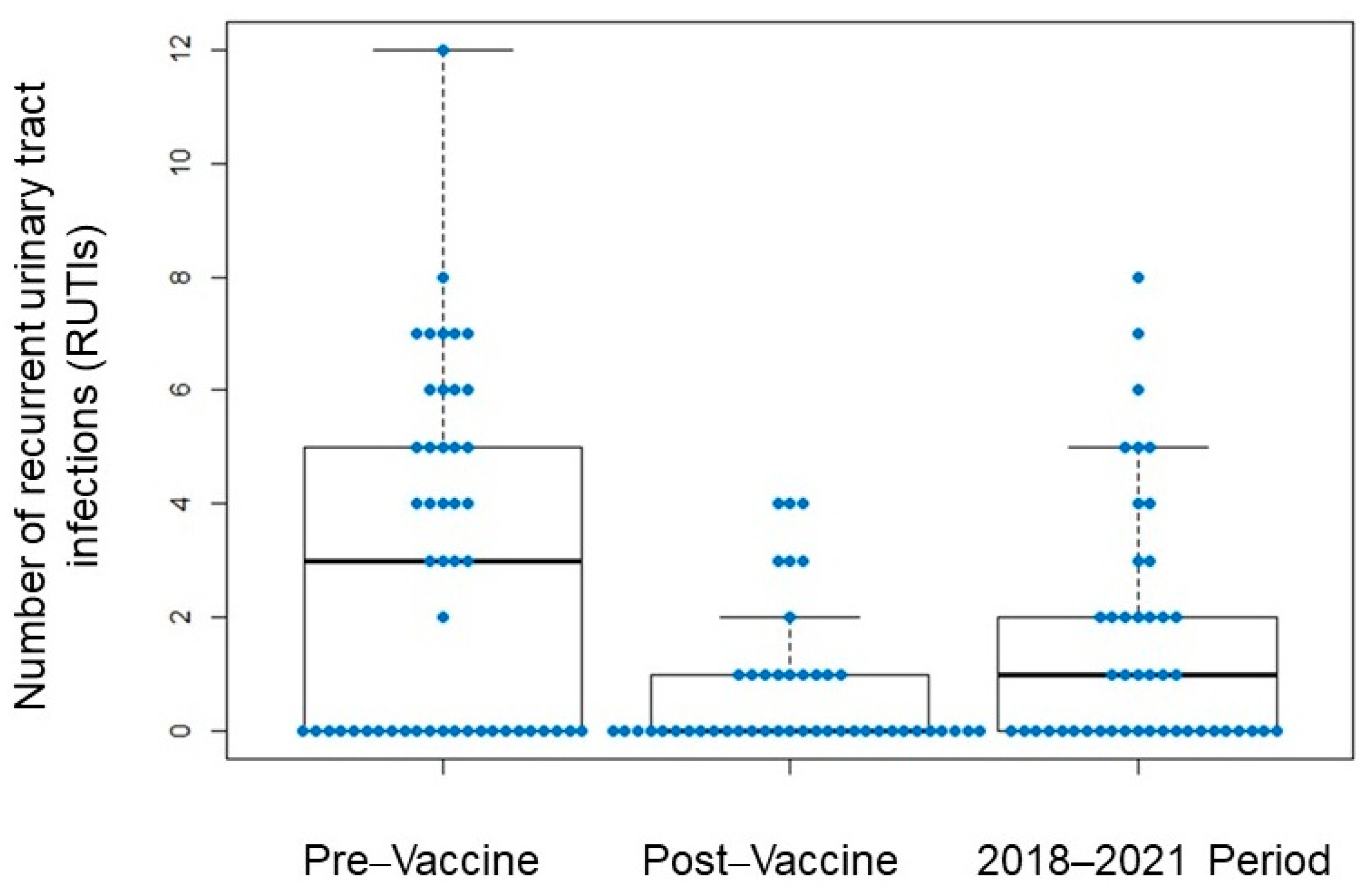
3.2. Frequency of Infections Following Suspension of Perlingual Vaccination
3.3. COVID-19 Incidence and Severity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez Lozano, C. Seguridad de las terapias biológicas: Nuevos datos de BIOBADASER. Reumatol. Clin. 2011, 6 (Suppl. 3), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, M.; Lucchino, B.; Spaziante, M.; Iannuccelli, C.; Valesini, G.; Iaiani, G. Lung Infections in Systemic Rheumatic Disease: Focus on Opportunistic Infections. Int. J. Mol. Sci. 2017, 18, 293. [Google Scholar] [CrossRef] [PubMed]
- Carmona, L.; Descalzo, M.A.; Ruiz-Montesinos, D.; Manero-Ruiz, F.J.; Perez-Pampin, E. Safety and retention rate of off-label uses of TNF antagonists in rheumatic conditions: Data from the Spanish registry BIOBADASER 2.0. Rheumatology 2010, 50, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, J.R.; Xie, F.; Chen, L.; Baddley, J.W.; Beukelman, T.; Saag, K.G.; Spettell, C.; McMahan, R.M.; Fernandes, J.; Winthrop, K.; et al. The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents. Ann. Rheum. Dis. 2011, 70, 1401–1406. [Google Scholar] [CrossRef]
- Chen, K.; Cerutti, A. Vaccination Strategies to Promote Mucosal Antibody Responses. Immunity 2010, 33, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo-Gómez, M.-F.; Foley, S.; Nickel, J.C.; García-Cenador, M.-B.; Padilla-Fernández, B.-Y.; González-Casado, I.; Martínez-Huélamo, M.; Yang, B.; Blick, C.; Ferreira, F.; et al. Sublingual MV140 for Prevention of Recurrent Urinary Tract Infections. NEJM Evid. 2022, 1, EVIDoa2100018. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Fernández-Paredes, L.; Saz-Leal, P.; Diez-Rivero, C.M.; Ochoa-Grullón, J.; Morado, C.; Macarrón, P.; Martínez, C.; Villaverde, V.; de la Peña, A.R.; et al. Sublingual Bacterial Vaccination Reduces Recurrent Infections in Patients with Autoimmune Diseases under Immunosuppressant Treatment. Front. Immunol. 2021, 12, 1707. [Google Scholar] [CrossRef]
- Tejera-Alhambra, M.; Palomares, O.; de Diego, R.P.; Diaz-Lezcano, I.; Sánchez-Ramón, S. New Biological Insights in the Immunomodulatory Effects of Mucosal Polybacterial Vaccines in Clinical Practice. Curr. Pharm. Des. 2016, 22, 6283–6293. [Google Scholar] [CrossRef]
- Sánchez-Ramón, S.; Conejero, L.; Netea, M.G.; Sancho, D.; Palomares, O.; Subiza, J.L. Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations. Front. Immunol. 2018, 9, 2936. [Google Scholar] [CrossRef]
- Sánchez Ramón, S.; Manzanares, M.; Candelas, G. Vacunas antiinfecciosas de mucosas en la profilaxis de infecciones recurrentes: Más allá de las vacunas convencionales. Reumatol. Clin. 2020, 16, 49–55. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alecsandru, D.; Valor, L.; Sánchez-Ramón, S.; Gil, J.; Carbone, J.; Navarro, J.; Rodríguez, J.J.; Rodríguez-Sainz, C.; Fernández-Cruz, E. Sublingual therapeutic immunization with a polyvalent bacterial preparation in patients with recurrent respiratory infections: Immunomodulatory effect on antigen-specific memory CD4+ T cells and impact on clinical outcome. Clin. Exp. Immunol. 2011, 164, 100–107. [Google Scholar] [CrossRef] [PubMed]
- García González, L.A.; Arrutia Díez, F. Mucosal bacterial immunotherapy with MV130 highly reduces the need of tonsillectomy in adults with recurrent tonsillitis. Hum. Vaccin. Immunother. 2019, 15, 2150–2153. [Google Scholar] [CrossRef] [Green Version]
- Guevara-Hoyer, K.; Saz-Leal, P.; Diez-Rivero, C.; Ochoa-Grullón, J.; Fernández-Arquero, M.; De Diego, R.P.; Sánchez-Ramón, S. Trained Immunity Based-Vaccines as a Prophylactic Strategy in Common Variable Immunodeficiency. A Proof of Concept Study. Biomedicines 2020, 8, 203. [Google Scholar] [CrossRef]
- Brandi, P.; Conejero, L.; Cueto, F.J.; Martínez-Cano, S.; Dunphy, G.; Gómez, M.J.; Relaño, C.; Saz-Leal, P.; Enamorado, M.; Quintas, A.; et al. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections. Cell Rep. 2022, 38, 110184. [Google Scholar] [CrossRef] [PubMed]
- del Fresno, C.; García-Arriaza, J.; Martínez-Cano, S.; Heras-Murillo, I.; Jarit-Cabanillas, A.; Amores-Iniesta, J.; Brandi, P.; Dunphy, G.; Suay-Corredera, C.; Pricolo, M.R.; et al. The Bacterial Mucosal Immunotherapy MV130 Protects Against SARS-CoV-2 Infection and Improves COVID-19 Vaccines Immunogenicity. Front. Immunol. 2021, 12, 748103. [Google Scholar] [CrossRef]
- Benito-Villalvilla, C.; Cirauqui, C.; Diez-Rivero, C.; Casanovas, M.; Subiza, J.; Palomares, O. MV140, a sublingual polyvalent bacterial preparation to treat recurrent urinary tract infections, licenses human dendritic cells for generating Th1, Th17, and IL-10 responses via Syk and MyD88. Mucosal Immunol. 2017, 10, 924–935. [Google Scholar] [CrossRef] [Green Version]
- Cirauqui, C.; Benito-Villalvilla, C.; Sánchez-Ramón, S.; Sirvent, S.; Diez-Rivero, C.M.; Conejero, L.; Brandi, P.; Hernández-Cillero, L.; Ochoa, J.L.; Pérez-Villamil, B.; et al. Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways. Eur. J. Immunol. 2017, 48, 180–193. [Google Scholar] [CrossRef] [Green Version]
- Molero-Abraham, M.; Sanchez-Trincado, J.L.; Gomez-Perosanz, M.; Torres-Gomez, A.; Subiza, J.L.; Lafuente, E.M.; Reche, P.A. Human Oral Epithelial Cells Impair Bacteria-Mediated Maturation of Dendritic Cells and Render T Cells Unresponsive to Stimulation. Front. Immunol. 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzo-Gómez, M.F.; Padilla-Fernández, B.; García-Cenador, M.B.; Virseda-Rodríguez, Á.J.; Martín-García, I.; Sánchez-Escudero, A.; Vicente-Arroyo, M.J.; Mirón-Canelo, J.A. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front. Cell Infect. Microbiol. 2015, 5, 50. [Google Scholar]
- Nieto, A.; Mazón, A.; Nieto, M.; Calderón, R.; Calaforra, S.; Selva, B.; Uixera, S.; Palao, M.J.; Brandi, P.; Conejero, L.; et al. Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 462–472. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD or N (%) | |
|---|---|
| Demographic characteristics | |
| Age (years) | 54.68 ± 14.8 |
| Sex (%females) | 92.7% |
| Rheumatic diseases | |
| Rheumatoid arthritis (RA) | 43.9% |
| Systematic lupus erythematosus (SLE) | 19.5% |
| Mixed connective tissue disease (MCTD) | 7.3% |
| Others | 29.3% |
| Recurrent infectious diseases | |
| Recurrent respiratory tract infections | 34.1% |
| Recurrent urinary tract infections | 46.3% |
| Both | 19.5% |
| Inmunological status | |
| Antibody deficiency | 19.5% |
| Hypogammaglobulinemia | 7.3% |
| Others | 12.2% |
| Patients without Infections (2018 to 2022) | Patients with Infections (2018 to 2022) | p | |
|---|---|---|---|
| No. 17 | No. 24 | ||
| RRTI (N = 30) | 13 (43.4%) | 17 (56.6%) | NS |
| RUTI (N = 25) | 13 (52.0%) | 12 (48%) | NS |
| Age (years) | 55.2 ± 16.6 (54.5) 1 | 59.2 ± 12.6 (58.0) 1 | NS |
| Sex (M/F) | 1/17 | 2/24 | NS |
| Rheumatoid arthritis | 8 | 9 | NS |
| SLE | 8 | 6 | NS |
| DMARDc * | 14 (82.3%) | 16 (66.6%) | NS |
| DMARDb ** | 1 (6%) | 1 (4%) | NS |
| Ttos combinados (DMARDc + DMARDb) | 2 (11.7%) | 7 (29%) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sancristóbal, I.; de la Fuente, E.; Álvarez-Hernández, M.P.; Guevara-Hoyer, K.; Morado, C.; Martínez-Prada, C.; Freites-Nuñez, D.; Villaverde, V.; Fernández-Arquero, M.; Fernández-Gutiérrez, B.; et al. Long-Term Benefit of Perlingual Polybacterial Vaccines in Patients with Systemic Autoimmune Diseases and Active Immunosuppression. Biomedicines 2023, 11, 1168. https://doi.org/10.3390/biomedicines11041168
Pérez-Sancristóbal I, de la Fuente E, Álvarez-Hernández MP, Guevara-Hoyer K, Morado C, Martínez-Prada C, Freites-Nuñez D, Villaverde V, Fernández-Arquero M, Fernández-Gutiérrez B, et al. Long-Term Benefit of Perlingual Polybacterial Vaccines in Patients with Systemic Autoimmune Diseases and Active Immunosuppression. Biomedicines. 2023; 11(4):1168. https://doi.org/10.3390/biomedicines11041168
Chicago/Turabian StylePérez-Sancristóbal, Inés, Eduardo de la Fuente, María Paula Álvarez-Hernández, Kissy Guevara-Hoyer, Concepción Morado, Cristina Martínez-Prada, Dalifer Freites-Nuñez, Virginia Villaverde, Miguel Fernández-Arquero, Benjamín Fernández-Gutiérrez, and et al. 2023. "Long-Term Benefit of Perlingual Polybacterial Vaccines in Patients with Systemic Autoimmune Diseases and Active Immunosuppression" Biomedicines 11, no. 4: 1168. https://doi.org/10.3390/biomedicines11041168






