Differential Modulation of Cerebellar Flocculus Unipolar Brush Cells during Vestibular Compensation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Unilateral Labyrinthectomy
2.3. Exclusion Criteria
2.4. Behavioral Assessment
2.5. Quantitative Real-Time PCR
2.6. Western Blotting
2.7. Immunostaining
2.8. Statistical Analysis
3. Results
3.1. UL-Induced Behavior Symptoms Gradually Recovered in 7 Days
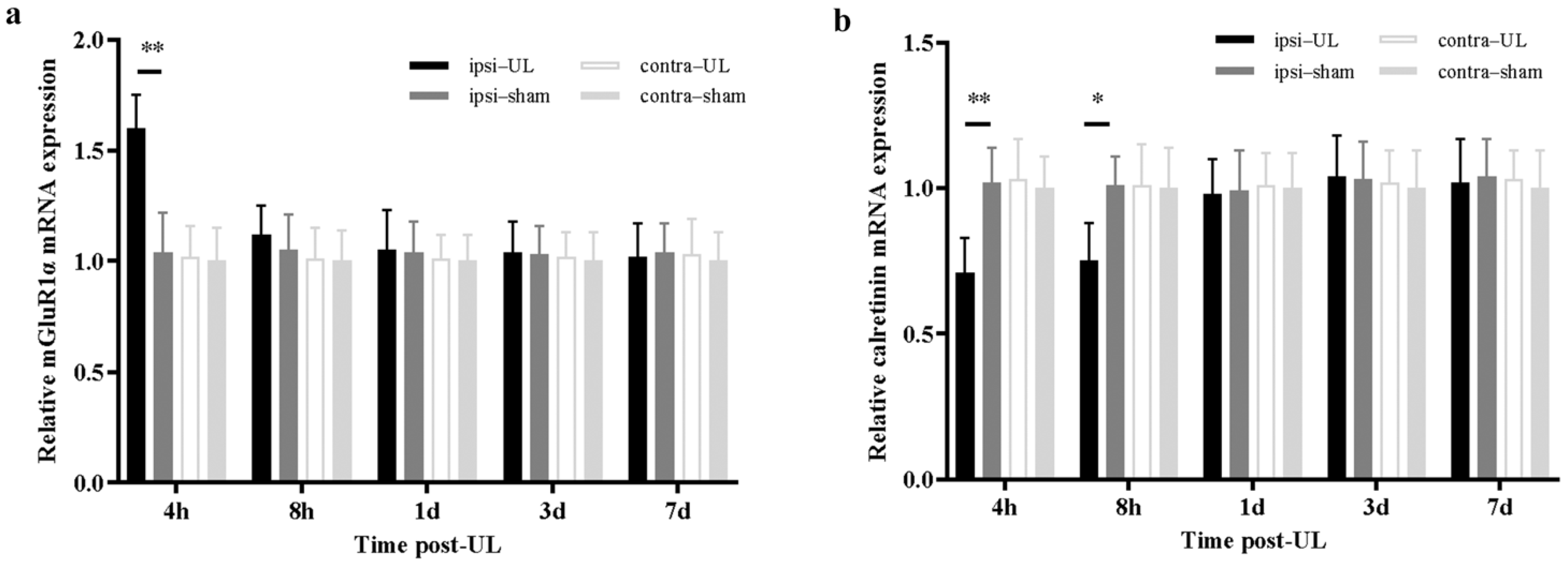
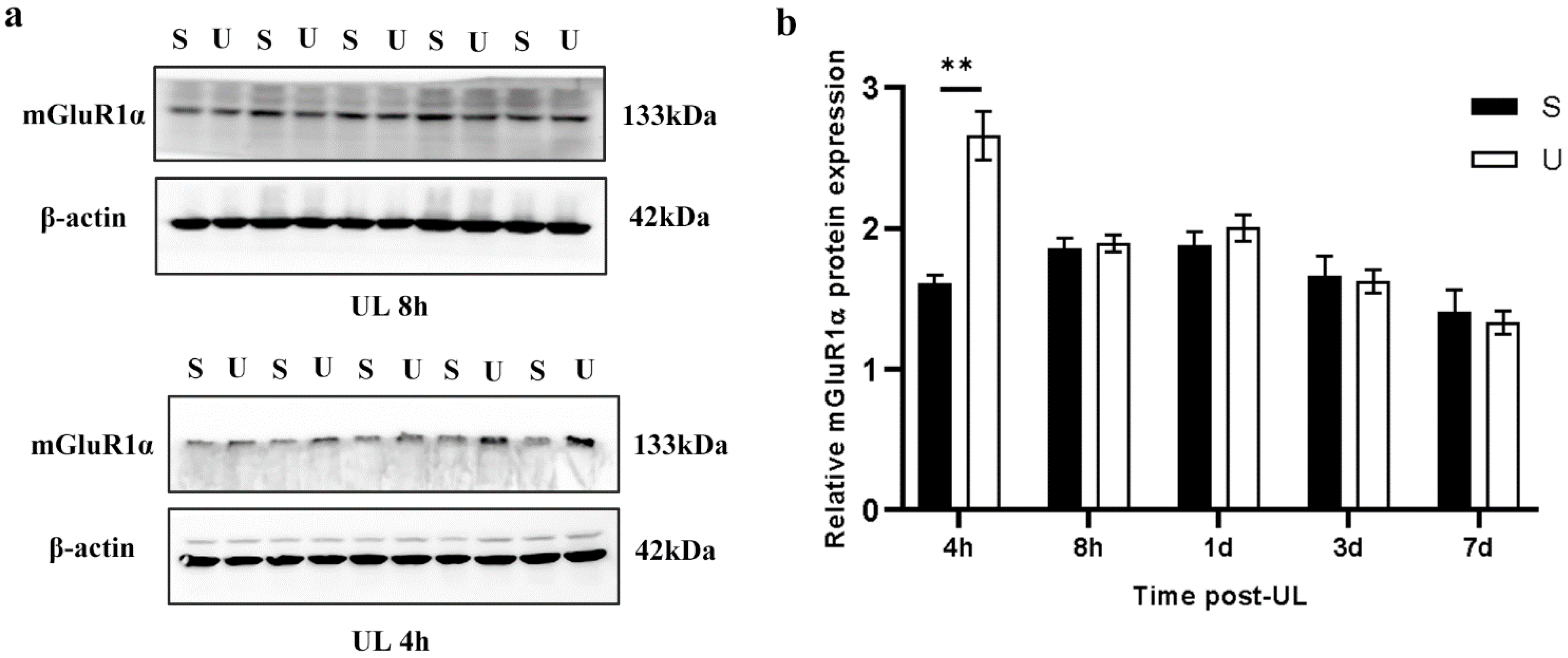
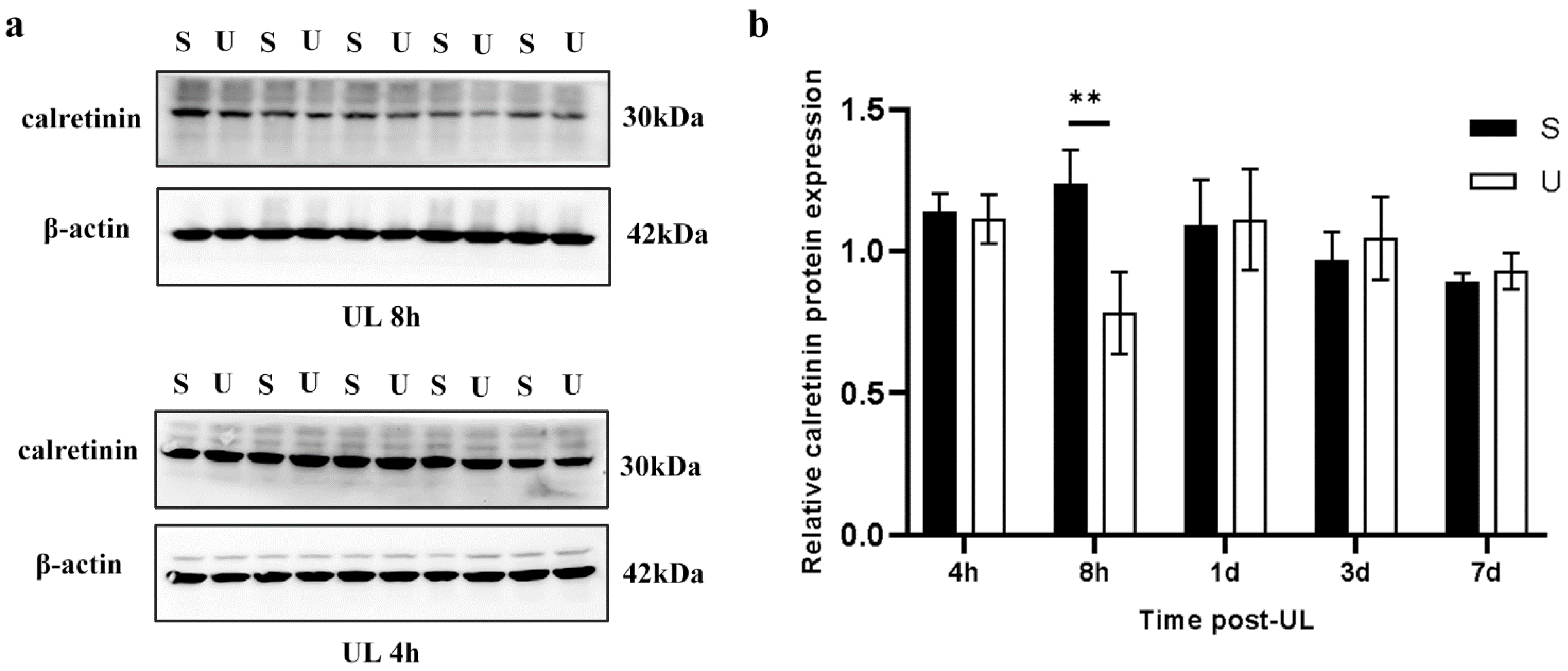
3.2. UL Increases mGluR1α While Decreasing Calretinin Levels in the Ipsilesional Flocculus
3.3. The Number of ON and OFF UBC Neurons in the Floccules Is Unaffected by UL
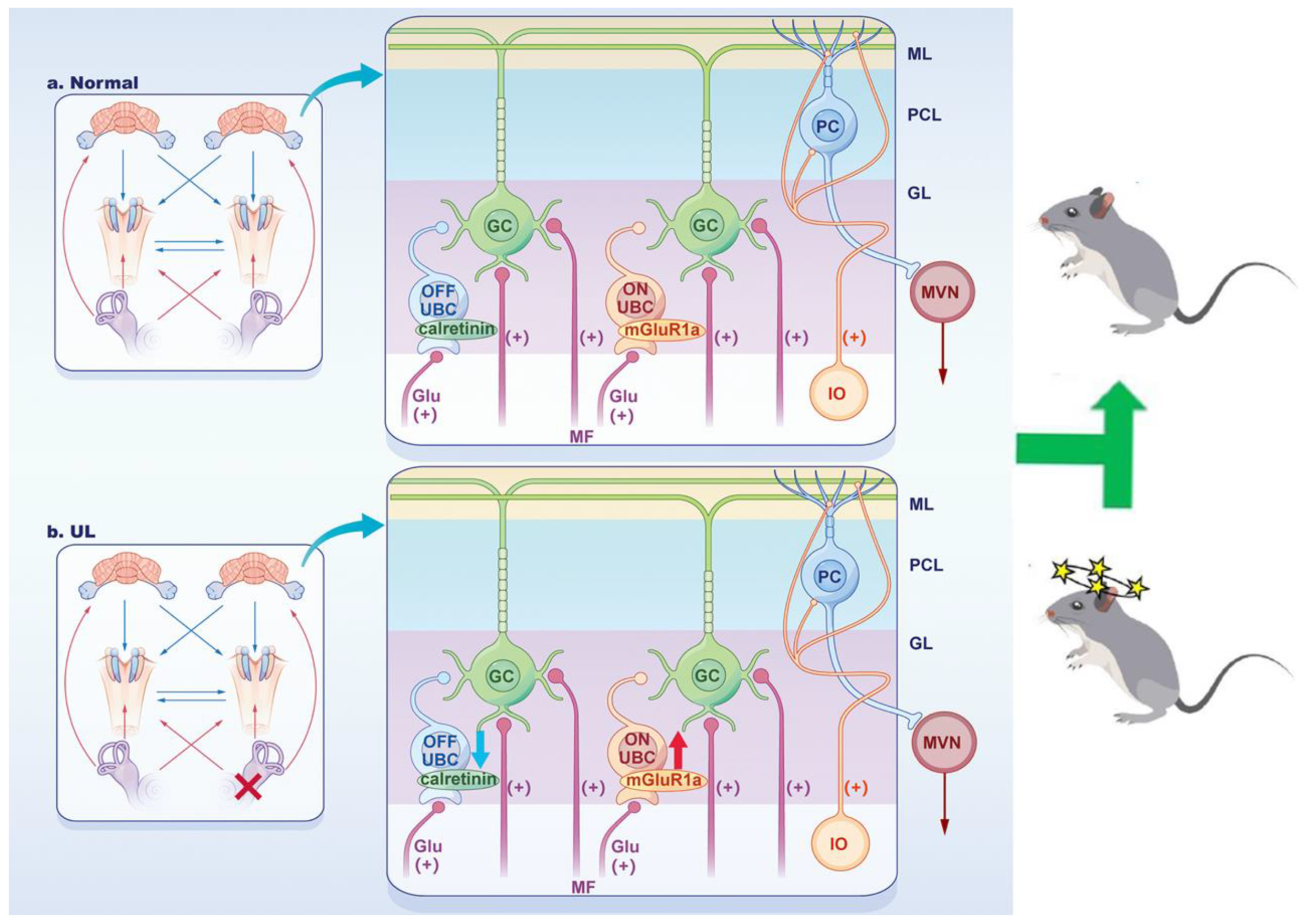
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Della-Morte, D.; Rundek, T. Dizziness and vertigo. Front. Neurol. Neurosci. 2012, 30, 22–25. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T. Epidemiology of vestibular vertigo: A neurotologic survey of the general population. Neurology 2005, 65, 898–904. [Google Scholar] [CrossRef]
- Lacour, M.; Helmchen, C.; Vidal, P.P. Vestibular compensation: The neuro-otologist’s best friend. J. Neurol. 2016, 263 (Suppl. S1), S54–S64. [Google Scholar] [CrossRef]
- Dieterich, M.; Brandt, T. The bilateral central vestibular system: Its pathways, functions, and disorders. Ann. N. Y. Acad. Sci. 2015, 1343, 10–26. [Google Scholar] [CrossRef]
- Bergquist, F.; Ludwig, M.; Dutia, M.B. Role of the commissural inhibitory system in vestibular compensation in the rat. J. Physiol. 2008, 586, 4441–4452. [Google Scholar] [CrossRef]
- Shin, M.; Moghadam, S.H.; Sekirnjak, C.; Bagnall, M.W.; Kolkman, K.E.; Jacobs, R.; Faulstich, M.; du Lac, S. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J. Neurosci. J. Soc. Neurosci. 2011, 31, 10776–10786. [Google Scholar] [CrossRef]
- Babalian, A.L.; Vidal, P.P. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. J. Neurophysiol. 2000, 84, 2514–2528. [Google Scholar] [CrossRef]
- Johnston, A.R.; Seckl, J.R.; Dutia, M.B. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J. Physiol. 2002, 545, 903–911. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, W.; Zhang, S.; Liu, B.; Leng, Y.; Zhou, R.; Kong, W. Changes in Histamine Receptors (H1, H2, and H3) Expression in Rat Medial Vestibular Nucleus and Flocculus after Unilateral Labyrinthectomy: Histamine Receptors in Vestibular Compensation. PLoS ONE 2013, 8, e66684. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, W.; Zhang, S.; Liu, B.; Liang, P.; Zhou, Y.; Zhou, T.; Zhang, K.; Leng, Y.; Kong, W. BDNF signaling in the rat cerebello-vestibular pathway during vestibular compensation: BDNF signaling in vestibular compensation. FEBS J. 2015, 282, 3579–3591. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, L.Q.; Zhang, S.L.; Liu, B.; Leng, Y.M.; Zhou, R.H.; Kong, W.J. The changes in mGluR2 and mGluR7 expression in rat medial vestibular nucleus and flocculus following unilateral labyrinthectomy. Int. J. Mol. Sci. 2013, 14, 22857. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, L.Q.; Shi, H.; Leng, Y.M.; Liu, B.; Zhang, S.L.; Kong, W.J. Expression of glycine receptors and gephyrin in rat medial vestibular nuclei and flocculi following unilateral labyrinthectomy. Int. J. Mol. Med. 2016, 38, 1481–1489. [Google Scholar] [CrossRef]
- Beraneck, M.; McKee, J.L.; Aleisa, M.; Cullen, K.E. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J. Neurophysiol. 2008, 100, 945–958. [Google Scholar] [CrossRef]
- Dutia, M.B. Mechanisms of vestibular compensation: Recent advances. Curr. Opin. Otolaryngol. Head Neck. Surg. 2010, 18, 420–424. [Google Scholar] [CrossRef]
- Farini, D.; Marazziti, D.; Geloso, M.C.; Sette, C. Transcriptome programs involved in the development and structure of the cerebellum. Cell Mol. Life Sci. 2021, 78, 6431–6451. [Google Scholar] [CrossRef]
- Dizon, M.J.; Khodakhah, K. The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex. J. Neurosci. J. Soc. Neurosci. 2011, 31, 10463–10473. [Google Scholar] [CrossRef]
- Prestori, F.; Mapelli, L.; D’Angelo, E. Diverse Neuron Properties and Complex Network Dynamics in the Cerebellar Cortical Inhibitory Circuit. Front. Mol. Neurosci. 2019, 12, 267. [Google Scholar] [CrossRef]
- Sekerková, G.; Watanabe, M.; Martina, M.; Mugnaini, E. Differential distribution of phospholipase C beta isoforms and diaglycerol kinase-beta in rodents cerebella corroborates the division of unipolar brush cells into two major subtypes. Brain Struct. Funct. 2014, 219, 719–749. [Google Scholar] [CrossRef]
- Borges-Merjane, C.; Trussell, L.O. ON and OFF unipolar brush cells transform multisensory inputs to the auditory system. Neuron 2015, 85, 1029–1042. [Google Scholar] [CrossRef]
- Balmer, T.S.; Trussell, L.O. Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. eLife 2019, 8, e44964. [Google Scholar] [CrossRef]
- National Research Council Committee for the Update of the Guide for the Care. Use of Laboratory Animals. The National Academies Collection: Reports funded by National Institutes of Health. In Guide for the Care and Use of Laboratory Animals; National Academies Press: Cambridge, MA, USA; National Academy of Sciences: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Lindner, M.; Gosewisch, A.; Eilles, E.; Branner, C.; Krämer, A.; Oos, R.; Wolf, E.; Ziegler, S.; Bartenstein, P.; Brandt, T.; et al. Ginkgo biloba Extract EGb 761 Improves Vestibular Compensation and Modulates Cerebral Vestibular Networks in the Rat. Front. Neurol. 2019, 10, 147. [Google Scholar] [CrossRef]
- Chen, Z.P.; Zhang, X.Y.; Peng, S.Y.; Yang, Z.Q.; Wang, Y.B.; Zhang, Y.X.; Chen, X.; Wang, J.J.; Zhu, J.N. Histamine H1 Receptor Contributes to Vestibular Compensation. J. Neurosci. J. Soc. Neurosci. 2019, 39, 420–433. [Google Scholar] [CrossRef]
- Jiang, W.; Rajguru, S.M. Eye Movements Evoked by Pulsed Infrared Radiation of the Rat Vestibular System. Ann. Biomed. Eng. 2018, 46, 1406–1418. [Google Scholar] [CrossRef]
- Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Harashima, C.; Jacobowitz, D.M.; Stoffel, M.; Chakrabarti, L.; Haydar, T.F.; Siarey, R.J.; Galdzicki, Z. Elevated expression of the G-protein-activated inwardly rectifying potassium channel 2 (GIRK2) in cerebellar unipolar brush cells of a Down syndrome mouse model. Cell Mol. Neurobiol. 2006, 26, 719–734. [Google Scholar] [CrossRef]
- Darlington, C.L.; Smith, P.F. Molecular mechanisms of recovery from vestibular damage in mammals: Recent advances. Prog. Neurobiol. 2000, 62, 313–325. [Google Scholar] [CrossRef]
- Kitahara, T.; Takeda, N.; Kubo, T.; Kiyama, H. Nitric oxide in the flocculus works the inhibitory neural circuits after unilateral labyrinthectomy. Brain Res. 1999, 815, 405–409. [Google Scholar] [CrossRef]
- Courjon, J.H.; Flandrin, J.M.; Jeannerod, M.; Schmid, R. The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Res. 1982, 239, 251–257. [Google Scholar] [CrossRef]
- Fukasawa, M.; Okamoto, K.; Nakamura, M.; Mikami, K.; Shimada, S.; Tanaka, Y.; Nagai, K.; Arito, M.; Kurokawa, M.S.; Masuko, K.; et al. Proteomic analysis of the rat cerebellar flocculus during vestibular compensation. J. Vestib. Res. Equilib. Orientat. 2009, 19, 83–94. [Google Scholar] [CrossRef]
- Michaelis, E.K. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol. 1998, 54, 369–415. [Google Scholar] [CrossRef]
- Diana, M.A.; Otsu, Y.; Maton, G.; Collin, T.; Chat, M.; Dieudonné, S. T-type and L-type Ca2+ conductances define and encode the bimodal firing pattern of vestibulocerebellar unipolar brush cells. J. Neurosci. J. Soc. Neurosci. 2007, 27, 3823–3838. [Google Scholar] [CrossRef]
- Kano, M.; Watanabe, T.; Uesaka, N. mGluR1 Is a Molecular “Hub” for Synapse Elimination in the Developing Cerebellum. In Cerebellum as a CNS Hub; Springer: Berlin/Heidelberg, Germany, 2021; pp. 77–89. [Google Scholar]
- Cirelli, C.; Pompeiano, M.; D’Ascanio, P.; Arrighi, P.; Pompeiano, O. c-fos Expression in the rat brain after unilateral labyrinthectomy and its relation to the uncompensated and compensated stages. Neuroscience 1996, 70, 515–546. [Google Scholar] [CrossRef]
- Holstein, G.R.; Friedrich, V.L., Jr.; Martinelli, G.P.; Ogorodnikov, D.; Yakushin, S.B.; Cohen, B. Fos expression in neurons of the rat vestibulo-autonomic pathway activated by sinusoidal galvanic vestibular stimulation. Front. Neurol. 2012, 3, 4. [Google Scholar] [CrossRef]
- Schiffmann, S.N.; Cheron, G.; Lohof, A.; d’Alcantara, P.; Meyer, M.; Parmentier, M.; Schurmans, S. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. Proc. Natl. Acad. Sci. USA 1999, 96, 5257–5262. [Google Scholar] [CrossRef]
- Nunzi, M.G.; Shigemoto, R.; Mugnaini, E. Differential expression of calretinin and metabotropic glutamate receptor mGluR1alpha defines subsets of unipolar brush cells in mouse cerebellum. J. Comp. Neurol. 2002, 451, 189–199. [Google Scholar] [CrossRef]
- Morello, F.; Partanen, J. Diversity and development of local inhibitory and excitatory neurons associated with dopaminergic nuclei. FEBS Lett. 2015, 589, 3693–3701. [Google Scholar] [CrossRef]
- Tóth, K.; Maglóczky, Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front. Neuroanat. 2014, 8, 100. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Wang, J.; Zhou, L.; Tian, E.; Chen, J.; Kong, W.; Lu, Y.; Zhang, S. Differential Modulation of Cerebellar Flocculus Unipolar Brush Cells during Vestibular Compensation. Biomedicines 2023, 11, 1298. https://doi.org/10.3390/biomedicines11051298
Liu D, Wang J, Zhou L, Tian E, Chen J, Kong W, Lu Y, Zhang S. Differential Modulation of Cerebellar Flocculus Unipolar Brush Cells during Vestibular Compensation. Biomedicines. 2023; 11(5):1298. https://doi.org/10.3390/biomedicines11051298
Chicago/Turabian StyleLiu, Dan, Jun Wang, Liuqing Zhou, E Tian, Jingyu Chen, Weijia Kong, Yisheng Lu, and Sulin Zhang. 2023. "Differential Modulation of Cerebellar Flocculus Unipolar Brush Cells during Vestibular Compensation" Biomedicines 11, no. 5: 1298. https://doi.org/10.3390/biomedicines11051298
APA StyleLiu, D., Wang, J., Zhou, L., Tian, E., Chen, J., Kong, W., Lu, Y., & Zhang, S. (2023). Differential Modulation of Cerebellar Flocculus Unipolar Brush Cells during Vestibular Compensation. Biomedicines, 11(5), 1298. https://doi.org/10.3390/biomedicines11051298







