The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Data Collection
2.3. Blood Sampling
2.4. ELISA
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
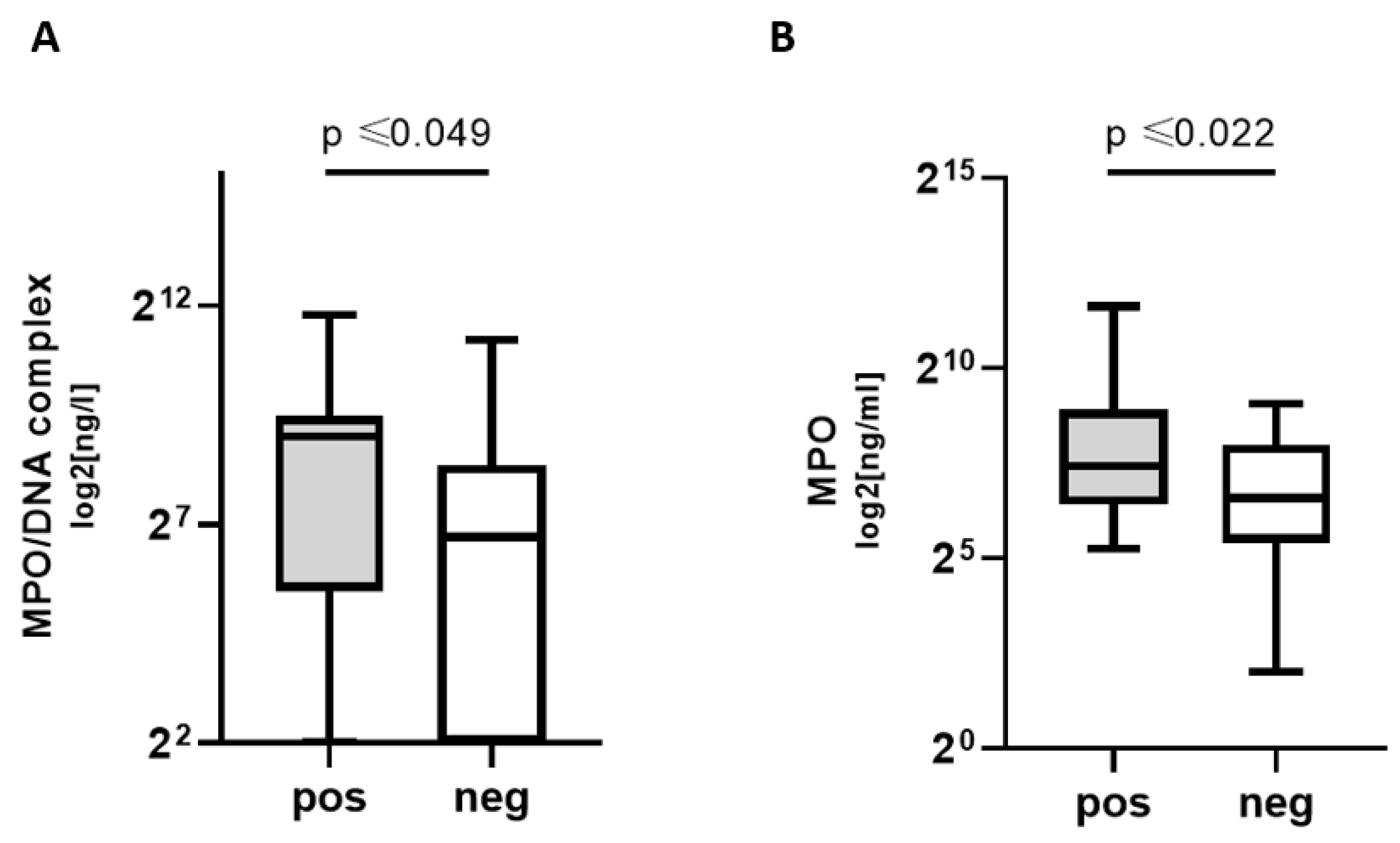
3.2. Increased NETosis, Coagulation, Platelet, and Complement Markers in COVpos
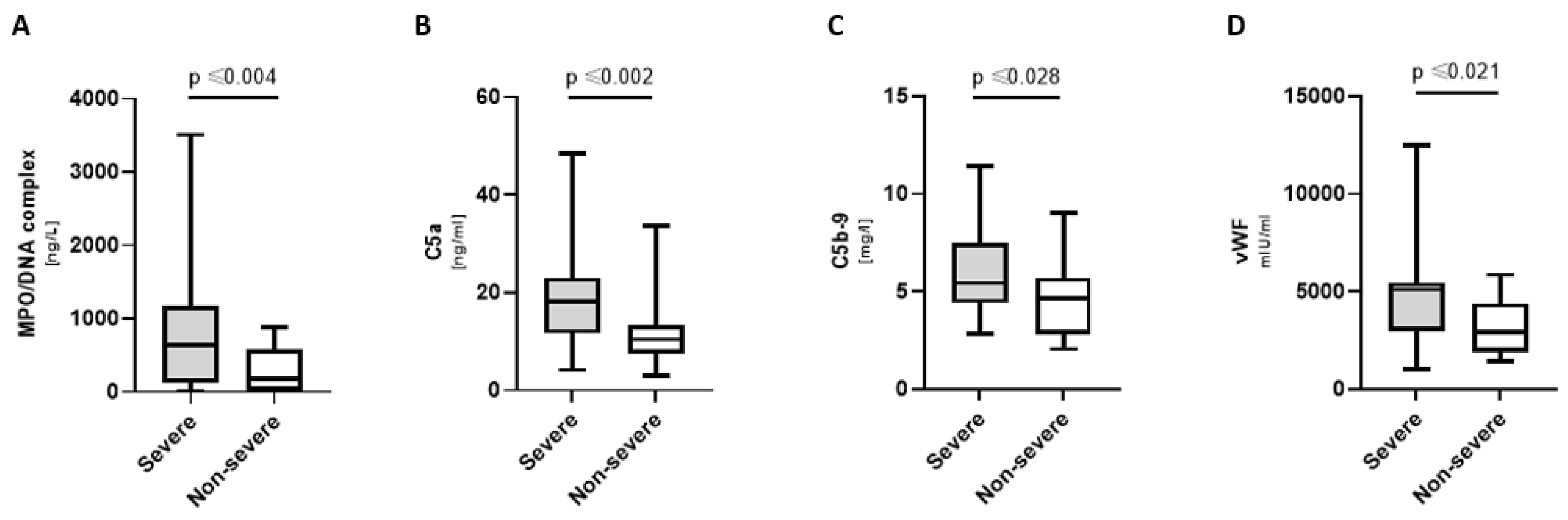
3.3. Increased NETosis, Complement, and Coagulation Markers in Severely Ill COVpos
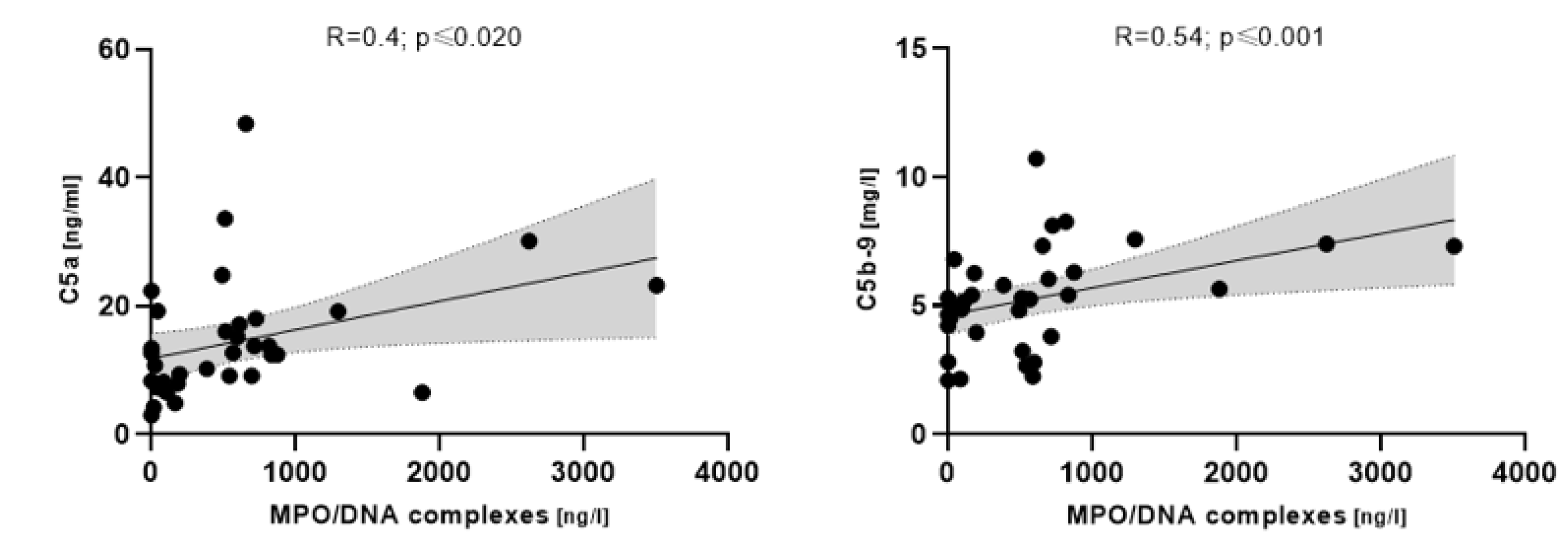
3.4. NETosis Markers Associated with Complement in COVpos
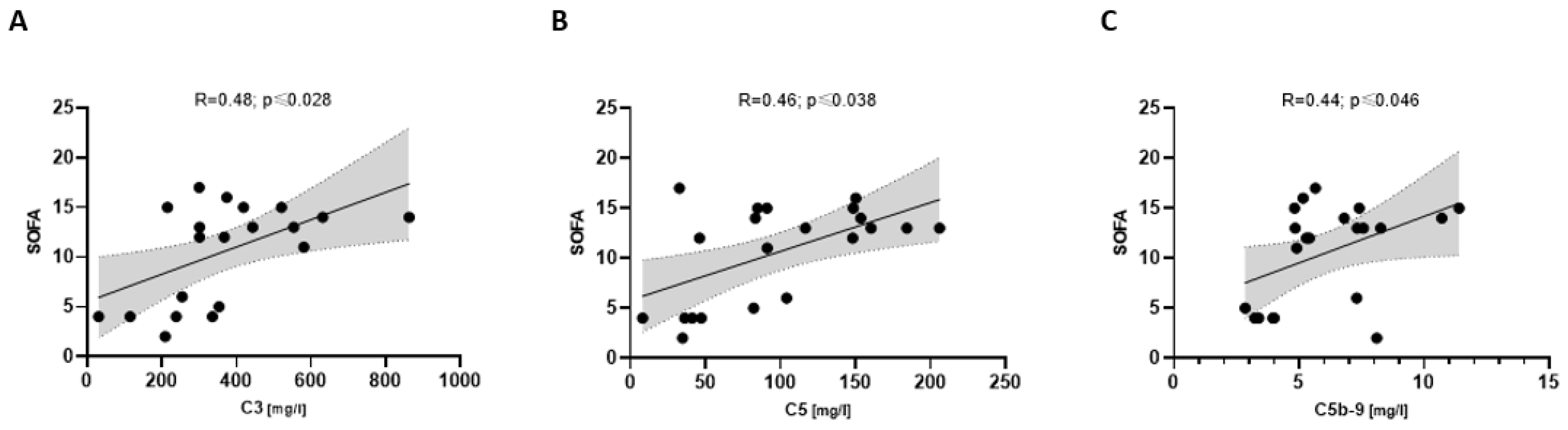
3.5. Complement Associated with Disease Severity in COVpos
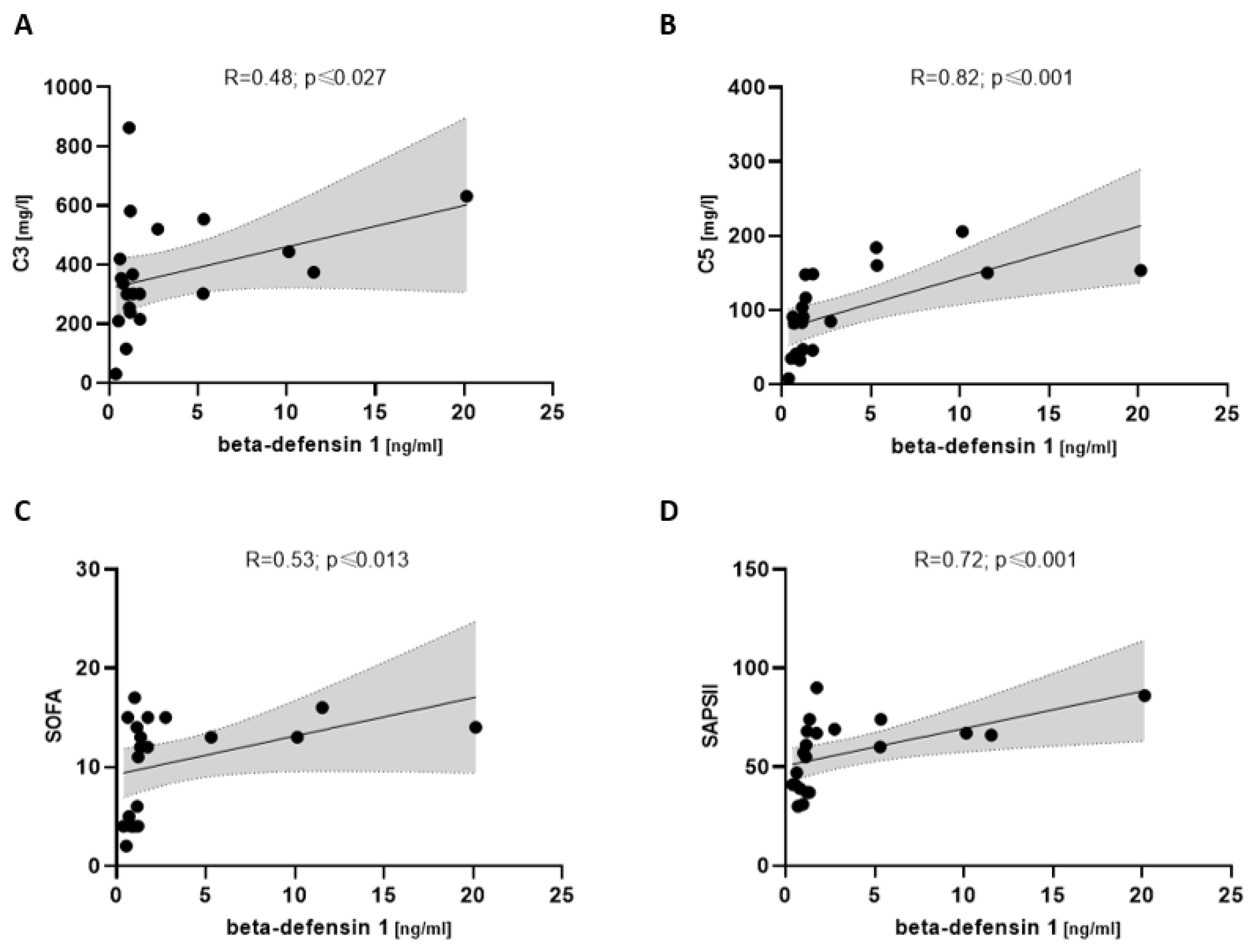
3.6. Beta-Defensin 1 Associated with Complement and Disease Severity in COVpos
4. Discussion
4.1. Central Findings
- NETosis and complement markers are higher in COVpos than in COVneg patients with acute respiratory disease.
- NETosis and complement markers were higher in severely ill COVpos patients.
- Increased complement activation markers were associated with a higher SOFA and SAPSII score.
4.2. Higher NETosis Markers Are Associated with Complement Activation Only in COVID-19
4.3. Coagulation Markers Are Increased in COVID-19 Patients
4.4. COVID-19-Related Increase in vWF
4.5. Higher Complement Markers Are Associated with Disease Severity Only in COVID-19
4.6. Beta-Defensin 1 Might Play a Role in NET-Associated Platelet Recruitment
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Paz, L.; Capodanno, D.; Montalescot, G.; Angiolillo, D.J. Coronavirus Disease 2019-Associated Thrombosis and Coagulopathy: Review of the Pathophysiological Characteristics and Implications for Antithrombotic Management. J. Am. Heart Assoc. 2021, 10, e019650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cheng, Z.; Luo, L.; Zhu, Y.; Lin, W.; Ming, Z.; Chen, W.; Hu, Y. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb. Res. 2021, 201, 23–29. [Google Scholar] [CrossRef]
- Jakobs, K.; Reinshagen, L.; Puccini, M.; Friebel, J.; Wilde, A.B.; Alsheik, A.; Rroku, A.; Landmesser, U.; Haghikia, A.; Krankel, N.; et al. Disease Severity in Moderate-to-Severe COVID-19 Is Associated with Platelet Hyperreactivity and Innate Immune Activation. Front. Immunol. 2022, 13, 844701. [Google Scholar] [CrossRef]
- Malas, M.B.; Naazie, I.N.; Elsayed, N.; Mathlouthi, A.; Marmor, R.; Clary, B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine 2020, 29, 100639. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef]
- Petito, E.; Falcinelli, E.; Paliani, U.; Cesari, E.; Vaudo, G.; Sebastiano, M.; Cerotto, V.; Guglielmini, G.; Gori, F.; Malvestiti, M.; et al. Association of Neutrophil Activation, More Than Platelet Activation, With Thrombotic Complications in Coronavirus Disease 2019. J. Infect. Dis. 2021, 223, 933–944. [Google Scholar] [CrossRef]
- De Bont, C.M.; Boelens, W.C.; Pruijn, G.J.M. NETosis, complement, and coagulation: A triangular relationship. Cell. Mol. Immunol. 2019, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U.; Bonderman, D.; Bohrmann, B.; Badimon, J.J.; Himber, J.; Riederer, M.A.; Nemerson, Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood 2000, 96, 170–175. [Google Scholar] [CrossRef]
- Giesen, P.L.; Rauch, U.; Bohrmann, B.; Kling, D.; Roque, M.; Fallon, J.T.; Badimon, J.J.; Himber, J.; Riederer, M.A.; Nemerson, Y. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. USA 1999, 96, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C.T. The regulation of natural anticoagulant pathways. Science 1987, 235, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Geddings, J.E.; Mackman, N. New players in haemostasis and thrombosis. Thromb. Haemost. 2014, 111, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Kambas, K.; Mitroulis, I.; Apostolidou, E.; Girod, A.; Chrysanthopoulou, A.; Pneumatikos, I.; Skendros, P.; Kourtzelis, I.; Koffa, M.; Kotsianidis, I.; et al. Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis. PLoS ONE 2012, 7, e45427. [Google Scholar] [CrossRef] [PubMed]
- Behzadifard, M.; Soleimani, M. NETosis and SARS-COV-2 infection related thrombosis: A narrative review. Thromb. J. 2022, 20, 13. [Google Scholar] [CrossRef]
- Von Bruhl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Kollnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; von Bruhl, M.L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef]
- Berends, E.T.; Kuipers, A.; Ravesloot, M.M.; Urbanus, R.T.; Rooijakkers, S.H. Bacteria under stress by complement and coagulation. FEMS Microbiol. Rev. 2014, 38, 1146–1171. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Zhao, M.H.; Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015, 181, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, J.; Demaria, O.; Vely, F.; Batista, L.; Chouaki Benmansour, N.; Fares, J.; Carpentier, S.; Thibult, M.L.; Morel, A.; Remark, R.; et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature 2020, 588, 146–150. [Google Scholar] [CrossRef]
- Sims, P.J.; Faioni, E.M.; Wiedmer, T.; Shattil, S.J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988, 263, 18205–18212. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.J.; Nachman, R.L. Human platelet activation by C3a and C3a des-arg. J. Exp. Med. 1983, 158, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Jamali, E.; Abbasi, M.; Tayer, A.H.; Monfared, A.A.; Tandel, P.; Tamaddon, G.; Kazerooni, E.S.; Rakhshandehroo, S.; Ranjbaran, R. The significance of surface neutrophilic MPO expression level in NETosis and NETosis-associated coagulopathies in covid-19 infected patients. Blood Cells Mol. Dis. 2022, 96, 102676. [Google Scholar] [CrossRef]
- Huckriede, J.; Anderberg, S.B.; Morales, A.; de Vries, F.; Hultstrom, M.; Bergqvist, A.; Ortiz-Perez, J.T.; Sels, J.W.; Wichapong, K.; Lipcsey, M.; et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci. Rep. 2021, 11, 15701. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1580. [Google Scholar] [CrossRef]
- Cani, E.; Dwivedi, D.J.; Liaw, K.L.; Fraser, D.D.; Yeh, C.H.; Martin, C.; Slessarev, M.; Cerroni, S.E.; Fox-Robichaud, A.A.; Weitz, J.I.; et al. Immunothrombosis Biomarkers for Distinguishing Coronavirus Disease 2019 Patients From Noncoronavirus Disease Septic Patients With Pneumonia and for Predicting ICU Mortality. Crit. Care Explor. 2021, 3, e0588. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, R.; Gentilini Cacciola, E.; Vecchio, V.; Cacciola, E. Cellular and molecular mechanisms in COVID-19 coagulopathy: Role of inflammation and endotheliopathy. J. Thromb. Thrombolysis 2022, 53, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, M.; Bouwens, B.R.C.; Wichapong, K.; Suylen, D.P.; Bouwman, F.G.; Hackeng, T.M.; Koenen, R.R. Protein arginine deiminase 4 inactivates tissue factor pathway inhibitor-alpha by enzymatic modification of functional arginine residues. J. Thromb. Haemost. 2023, 21, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C.; Sexton, T.; Smyth, S.; International COVID-19 Thrombosis Biomarkers Colloquium (ICODE) Investigators. COVID-19 and biomarkers of thrombosis: Focus on von Willebrand factor and extracellular vesicles. J. Thromb. Thrombolysis 2021, 52, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Mansouritorghabeh, H.; Parsa-Kondelaji, M. High levels of Von Willebrand factor markers in COVID-19: A systematic review and meta-analysis. Clin. Exp. Med. 2022, 22, 347–357. [Google Scholar] [CrossRef]
- Dolgushina, N.; Gorodnova, E.; Beznoshenco, O.; Romanov, A.; Menzhinskaya, I.; Krechetova, L.; Sukhikh, G. Von Willebrand Factor and ADAMTS-13 Are Associated with the Severity of COVID-19 Disease. J. Clin. Med. 2022, 11, 4006. [Google Scholar] [CrossRef]
- Fernandez-Perez, M.P.; Aguila, S.; Reguilon-Gallego, L.; de Los Reyes-Garcia, A.M.; Minano, A.; Bravo-Perez, C.; de la Morena, M.E.; Corral, J.; Garcia-Barbera, N.; Gomez-Verdu, J.M.; et al. Neutrophil extracellular traps and von Willebrand factor are allies that negatively influence COVID-19 outcomes. Clin. Transl. Med. 2021, 11, e268. [Google Scholar] [CrossRef]
- Jevtic, S.D.; Nazy, I. The COVID Complex: A Review of Platelet Activation and Immune Complexes in COVID-19. Front. Immunol. 2022, 13, 807934. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Zaid, Y.; Puhm, F.; Allaeys, I.; Naya, A.; Oudghiri, M.; Khalki, L.; Limami, Y.; Zaid, N.; Sadki, K.; Ben El Haj, R.; et al. Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020, 127, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, K.; Du, C.; Li, R.; Liu, J.; Zeng, H.; Zhu, L.; Li, A. Carboxypeptidase B blocks ex vivo activation of the anaphylatoxin-neutrophil extracellular trap axis in neutrophils from COVID-19 patients. Crit. Care 2021, 25, 51. [Google Scholar] [CrossRef] [PubMed]
- Krauel, K.; Duerschmied, D. ICODE: The international COVID-19 thrombosis biomarkers colloquium-novel soluble biomarkers: Circulating cell-free nucleic acids and other molecules. J. Thromb. Thrombolysis 2022, 53, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanian, S.; Borczuk, A.; Salvatore, S.; Fung, K.M.; Merrill, J.T.; Laurence, J.; Ahamed, J. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J. Thromb. Haemost. 2021, 19, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Meroni, P.L.; Gualtierotti, R.; Griffini, S.; Grovetti, E.; Torri, A.; Panigada, M.; Aliberti, S.; Blasi, F.; Tedesco, F.; et al. Complement activation in patients with COVID-19: A novel therapeutic target. J. Allergy Clin. Immunol. 2020, 146, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Nishimura, J.I.; Nagy, Z.; Gaal-Weisinger, J.; Panse, J.; Yoon, S.S.; Egyed, M.; Ichikawa, S.; Ito, Y.; Kim, J.S.; et al. The complement C5 inhibitor crovalimab in paroxysmal nocturnal hemoglobinuria. Blood 2020, 135, 912–920. [Google Scholar] [CrossRef]
- Law, N.; Chan, J.; Kelly, C.; Auffermann, W.F.; Dunn, D.P. Incidence of pulmonary embolism in COVID-19 infection in the ED: Ancestral, Delta, Omicron variants and vaccines. Emerg. Radiol. 2022, 29, 625–629. [Google Scholar] [CrossRef]
- Ren, S.Y.; Wang, W.B.; Gao, R.D.; Zhou, A.M. Omicron variant (B.1.1.529) of SARS-CoV-2: Mutation, infectivity, transmission, and vaccine resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Sogaard, O.S.; Tolstrup, M.; Staerke, N.B.; Lundgren, J.; Ostergaard, L.; Hvas, A.M. Inflammation and Platelet Activation After COVID-19 Vaccines—Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front. Immunol. 2021, 12, 779453. [Google Scholar] [CrossRef]
- Hetland, G.; Fagerhol, M.K.; Wiedmann, M.K.H.; Soraas, A.V.L.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Istre, M.S.; Holme, P.A.; Schultz, N.H. Elevated NETs and Calprotectin Levels after ChAdOx1 nCoV-19 Vaccination Correlate with the Severity of Side Effects. Vaccines 2022, 10, 1267. [Google Scholar] [CrossRef]
- Janiuk, K.; Jablonska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. [Google Scholar] [CrossRef]
- Fan, B.E.; Umapathi, T.; Chua, K.; Chia, Y.W.; Wong, S.W.; Tan, G.W.L.; Chandrasekar, S.; Lum, Y.H.; Vasoo, S.; Dalan, R. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J. Thromb. Thrombolysis 2021, 51, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Dalan, R.; Boehm, B.O. The implications of COVID-19 infection on the endothelium: A metabolic vascular perspective. Diabetes Metab. Res. Rev. 2021, 37, e3402. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, S.A.; Dighero-Kemp, B.; Ouedraogo, D.D.; Hensley, L.; Sakande, J. How NETosis could drive “Post-COVID-19 syndrome” among survivors. Immunol. Lett. 2020, 228, 35–37. [Google Scholar] [CrossRef] [PubMed]


| COVpos n = 47 | COVneg n = 36 | Mann–Whitney U or Chi-Squared Test | |
|---|---|---|---|
| Demographics | |||
| Age (years) 1 | 70 [55; 78] | 72.5 [58; 80.8] | 0.380 |
| Female (% per group) | 31.9 | 41.7 | 0.362 |
| Men (% per group) | 68.1 | 58.3 | 0.362 |
| BMI (kg/m^2) 1 | 26.5 [24.7; 30.4] | 25 [23.1; 28.1] | 0.140 |
| Patients with severe illness | 21 | 8 | 0.035 |
| Deceased patients | 7 | 0 | 0.010 |
| Pre-existing conditions | |||
| Coronary artery disease (% per group) | 14.9 | 30.6 | 0.088 |
| Peripheral artery disease (% per group) | 4.3 | 30.6 | 0.001 |
| Arterial hypertension (% per group) | 63.8 | 66.7 | 0.789 |
| Diabetes (% per group) | 27.7 | 22.2 | 0.575 |
| Dyslipidemia (% per group) | 29.8 | 27.8 | 0.842 |
| COPD or asthma bronchial (% per group) | 6.4 | 36.1 | 0.001 |
| Concomitant medication | |||
| Acetylsalicylic acid (% per group) | 38.3 | 30.6 | 0.466 |
| Clopidogrel (% per group) | 4.3 | 0 | 0.213 |
| Prophylactic-dose anticoagulation (% per group) | 25.5 | 58.3 | 0.003 |
| Intermediate-dose anticoagulation (% per group) | 27.7 | 8.3 | 0.028 |
| Therapeutic-dose anticoagulation (% per group) | 46.8 | 33.3 | 0.219 |
| Statin (% per group) | 25.5 | 25.0 | 0.956 |
| ACE blocker (% per group) | 25.5 | 36.1 | 0.301 |
| Angiotensin II receptor blocker (% per group) | 21.3 | 16.7 | 0.600 |
| Beta blocker (% per group) | 27.7 | 47.2 | 0.068 |
| Aldosterone antagonist (% per group) | 6.4 | 13.9 | 0.254 |
| Diuretic (% per group) | 38.3 | 47.2 | 0.417 |
| Oral glucocorticoid (% per group) | 55.3 | 19.4 | 0.001 |
| Inhalative bronchodilator (% per group) | 85.1 | 58.3 | 0.006 |
| Coagulation markers | |||
| tPA (ng/mL) 1 | 11.1 [7; 23.8] | 7.3 [4.9; 10.6] | 0.004 |
| TAT (ng/mL) 1 | 4.9 [3.7; 7.8] | 3.4 [2.5; 4.5] | 0.0001 |
| TF protein (ng/l) 1 | 343.3 [119.5; 647.7] | 133.0 [84.2; 197.1] | 0.004 |
| TF activity (pM) 1 | 356.6 [117.3; 647.7] | 117.6 [83.1; 167.3] | 0.001 |
| TFPI (ng/mL) 1 | 172.0 [130.6; 256.0] | 105.7 [67.1; 160.6] | 0.006 |
| vWF (mlU/mL) 1 | 5100.8 [2953.3; 5472.6] | 2903.9 [1897.2; 4393.7] | 0.021 |
| Beta-defensin 1 1 | 1.2 [0.9; 1.7] | 1.5 [0.8; 2.4] | 0.693 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbari, E.P.; Jakobs, K.; Puccini, M.; Reinshagen, L.; Friebel, J.; Haghikia, A.; Kränkel, N.; Landmesser, U.; Rauch-Kröhnert, U. The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies. Biomedicines 2023, 11, 1371. https://doi.org/10.3390/biomedicines11051371
Ghanbari EP, Jakobs K, Puccini M, Reinshagen L, Friebel J, Haghikia A, Kränkel N, Landmesser U, Rauch-Kröhnert U. The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies. Biomedicines. 2023; 11(5):1371. https://doi.org/10.3390/biomedicines11051371
Chicago/Turabian StyleGhanbari, Emily Parissa, Kai Jakobs, Marianna Puccini, Leander Reinshagen, Julian Friebel, Arash Haghikia, Nicolle Kränkel, Ulf Landmesser, and Ursula Rauch-Kröhnert. 2023. "The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies" Biomedicines 11, no. 5: 1371. https://doi.org/10.3390/biomedicines11051371
APA StyleGhanbari, E. P., Jakobs, K., Puccini, M., Reinshagen, L., Friebel, J., Haghikia, A., Kränkel, N., Landmesser, U., & Rauch-Kröhnert, U. (2023). The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies. Biomedicines, 11(5), 1371. https://doi.org/10.3390/biomedicines11051371






