Abstract
Alzheimer’s disease (AD) is the most prominent neurodegenerative disorder in the aging population. It is characterized by cognitive decline, gradual neurodegeneration, and the development of amyloid-β (Aβ)-plaques and neurofibrillary tangles, which constitute hyperphosphorylated tau. The early stages of neurodegeneration in AD include the loss of neurons, followed by synaptic impairment. Since the discovery of AD, substantial factual research has surfaced that outlines the disease’s causes, molecular mechanisms, and prospective therapeutics, but a successful cure for the disease has not yet been discovered. This may be attributed to the complicated pathogenesis of AD, the absence of a well-defined molecular mechanism, and the constrained diagnostic resources and treatment options. To address the aforementioned challenges, extensive disease modeling is essential to fully comprehend the underlying mechanisms of AD, making it easier to design and develop effective treatment strategies. Emerging evidence over the past few decades supports the critical role of Aβ and tau in AD pathogenesis and the participation of glial cells in different molecular and cellular pathways. This review extensively discusses the current understanding concerning Aβ- and tau-associated molecular mechanisms and glial dysfunction in AD. Moreover, the critical risk factors associated with AD including genetics, aging, environmental variables, lifestyle habits, medical conditions, viral/bacterial infections, and psychiatric factors have been summarized. The present study will entice researchers to more thoroughly comprehend and explore the current status of the molecular mechanism of AD, which may assist in AD drug development in the forthcoming era.
1. Introduction
Neurological disorders are ailments that negatively impact the brain, spinal cord, and nerves all across the body [1]. They are generally marked by a slow, continual neuronal loss that eventually disrupts the stability of homeostasis in the nervous system of humans. As a result, processes such as abstract thought, locomotion, emotion, cognition, and memory are interrupted [2]. According to accessible statistics, these detrimental consequences could affect up to 2% of the world’s population [3]. One of the main categories of non-communicable diseases responsible for people’s degraded living standards is mental health and neurodegenerative disorders [4,5]. Globally, it is approximated that 44 million individuals suffer from AD or a corresponding form of dementia, while 8.5 million people have Parkinson’s disease (PD) [6,7]. For adults beyond 65, the risk of acquiring AD doubles every five years [8]. The predicted number of individuals in 2020 suffering from dementia was 55 million; the most prevalent reason for dementia was AD, contributing to 60–80% of total dementia cases [9]. By 2030 and 2050, this proportion is anticipated to nearly double in 20 years, reaching 78 and 139 million, respectively. Approximately more than 50 million individuals suffer from epilepsy on the planet. Every year, over six million people die from stroke, and the frequency of migraine has increased to more than 10% globally [10].
Neuronal communication is conducted through the synaptic junction formed by the intimate apposition of two cells. Signal transduction incorporates neurotransmitter production, which regulates the postsynaptic neuron activity, leading to their stimulation or inhibition [11]. The plasticity of synapses is highly crucial for memory and learning and involves both presynaptic and postsynaptic neurons. The key to neural survival and functionality is the synaptic integrity itself, which indulges the contribution of calcium signaling and intracellular protein coordination [12]. Failure in the optimal maintenance of adequate synaptic connections altered the neural stress levels due to excitotoxicity or abundant excitatory stimulation and neuronal apoptosis in extreme conditions, which are plausible conditions for the onset of neurological diseases [13]. Malfunctioning synaptic transmission and modulated post-synaptic receptor compositions are a few cellular modifications linked with abnormal neural functions [14,15].
The etiology of these disorders may be influenced by a number of risk variables including oxidative stress [16], genetic variations [17], endocrine disorders, age-related [18], inflammation [19], depression [20], hypertension [21], infection [22], diabetes [23], food supplements, exposure to chemicals, metabolic conditions, and vitamin deficiencies [24]. Furthermore, any structural, electrical, and biochemical alterations in the brain, spinal cord, and nerves lead to the occurrence of a specific number of symptoms comprising poor coordination, paralysis, seizure, muscle weakness, sensational loss, pain, confusion, and fluctuating levels of consciousness [25,26]. Neurological disorders are either associated with the central nervous system (CNS) or peripheral nervous system (PNS). The most frequently occurring neurological disorders involve stroke, Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), diabetic neuropathy, and PD [27].
NDs are increasingly acquiring a lot of medical emphasis due to the slow but steady increase in incidence. The leading causes of this progressive increase in age-related problems among all age groups are digital diabetes lifestyles, inactivity, hypertension, depression, hearing loss, midlife obesity, and smoking [28,29]. Preventive measures involving lifestyle modifications, physio or other forms of therapy, neurorehabilitation, pain management techniques, medications, surgeries undertaken by neurosurgeons, and a specified diet are examples of intervention strategies for neurological disorders [27,30]. The management of all of these disorders is challenging; drug distribution to the brain is constrained since the blood–brain barrier (BBB) prevents bulky molecules from passing through [31]. Therefore, it is crucial to devise a delivery mechanism that enables molecules to enter the damaged brain region without compromising the BBB system’s regular function [32].
2. Alzheimer’s Disease
The neurodegenerative disorder called Alzheimer’s disease mainly proceeds from brain cell degeneration, and ultimately leads to demise following dementia [33]. AD could be classified as familial or sporadic based on its occurrence. Familial AD comprises inherited genetic reasons associated with families, while sporadic AD emerges due to other unknown reasons excluding genetic ones [34]. AD has a protracted course and is featured by a gradual loss of memory and a general reduction in cognitive abilities. These devastating effects of neuronal degeneration reduce a patient’s competence to deal with everyday living tasks, frequently rendering their reliance on others for caregiving. Hundreds of millions of neurons are available in a healthy brain, which are specific cells that utilize electrochemical signals for dispatching information [35]. They carry impulses across distinct areas of the brain as well as from the brain to the body’s musculature and organs. This neural communication is disrupted by AD, which causes apoptosis and a decline in functionality [36].
A person with AD experiences distinct molecular and cellular modifications in the brain, which can be recognized using a microscope to look at the brain’s tissue after the death of an organism. Research is continually being undertaken to identify the abnormalities that may lead to AD and the actual reason for the consequence. Two distinct types of aggregates are the primary neuropathological indicators of AD [37]. The first is an extracellular amyloid (Aβ) peptide deposit (plaques). The second is hyperphosphorylated Tau protein fibrillary aggregation (tangles). It is typically proposed that neuroinflammation is another pathogenic characteristic of AD [38]. In exceptional instances, AD may be brought on by genetic flaws, but the preponderance of instances is sporadic and possesses no recognized etiology. AD has not been persistently connected to any environmental pollutants. Two proteins, tau and Aβ, appear to be irregularly processed and degraded, which tend to be directly associated with pathogenesis.
Normal aging allows the brain to shrink somewhat, yet oddly enough, a considerable proportion of neurons are not lost. Unfortunately, in the case of AD, many neurons stop functioning, break connectivity with the surrounding neurons, and ultimately die, causing significant harm. AD affects metabolism, repair mechanisms, and signaling, which are vital to neurons and their circuits. AD frequently begins by the destruction of neurons and their connections in the two brain sections linked to memory (i.e., the hippocampus and entorhinal cortex) [39]. Preceded by this, it affects regions of the cerebral cortex responsible for speech, thought, and societal interaction. Numerous other areas of the brain are subsequently destroyed. As time passes, an individual with AD progressively loses the ability to function independently and the ailment is inevitably lethal [40].
The symptoms of AD change with the phases of the disease. AD is categorized into three stages: preclinical or pre-symptomatic, mild, and dementia-stage, depending on the extent of cognitive deterioration. The primary and most-often appearing symptom is the transitory loss of memory with significantly prolonged memory loss, which can be evoked in most sufferers despite it not being present [41]. Short-term memory problems lead to difficulties with multiplexing and conceptual processing, which are followed by disorganization, problems with problem-solving, a lack of enthusiasm, executive performance, and reasoning. Decision-making dysfunction in the early phases could be mild to severe. Linguistic malfunction and visual-spatial performance degradation come next [42]. During the middle to late stages, it is common to experience nervous system-associated psychiatric indications such as lethargy, wandering, social disengagement, psychosis, and anxiety. Insomnia, olfactory malfunction, dyspraxia, and extrapyramidal motor indications including akathisia, dystonia, and Parkinson’s symptoms emerge late during the illness [43,44,45,46]. When age is considered, the disease does not seem to be gender specific.
Nonetheless, because of their longer lifespan expectancy, women comprise about two-thirds of the Alzheimer’s patient population [47]. AD proceeds at different frequencies. The average lifespan of any individual suffering from AD is approximately three to eleven years upon diagnosis, but some people survive for twenty years or longer. The duration of life may vary depending on the level of impaired functionality at the time of diagnosis. AD worsens more rapidly when vascular risk conditions such as hypertension are present [48].
3. Molecular Mechanism of Alzheimer’s Disease
3.1. Amyloid β Hypothesis
For over three decades, the amyloid hypothesis, proposed by G. Higgins, J. Hardi [49], and D. Selkoe [50], has been the dominant and most widely accepted mechanistic theory of how AD develops. According to their theory, the accumulation of oligomeric Aβ (oAβ)-peptides are responsible for the pathophysiology causing downstream events such as neuroinflammation, the formation of neurofibrillary tangles (NFT), and vascular injury, encouraging dementia and cognitive deficits [51]. Their original theory primarily focused on the frequent occurrence of AD in Down’s syndrome patients due to the generation of significant amounts of Aβ-peptides since the amyloid precursor protein (APP) gene is positioned on the three 21 chromosomes [52].
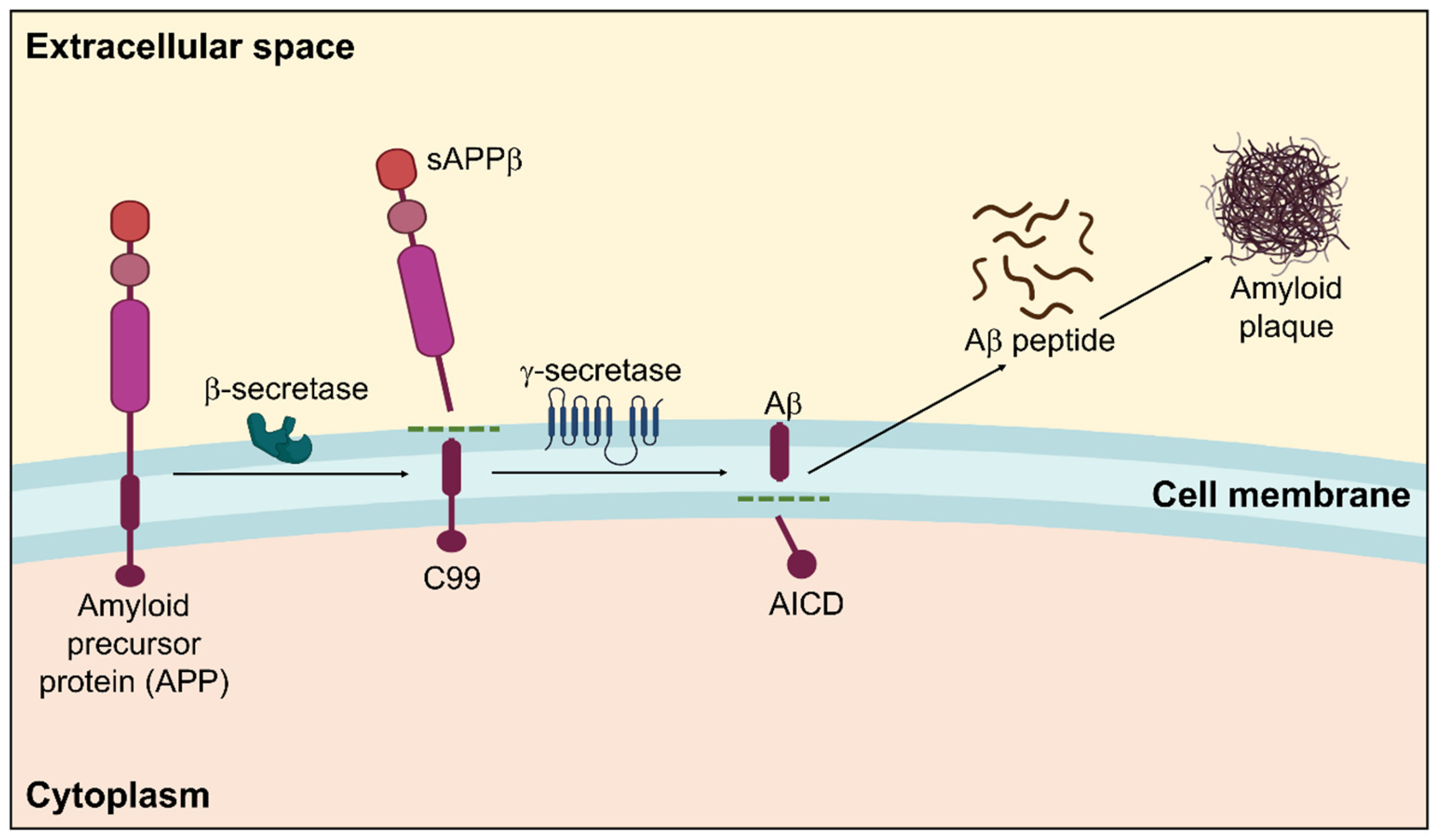
Amyloidosis is a clinicopathological phenomenon where amyloid builds up in the body’s tissues and cells, generating amyloid plaques for various intricate reasons that eventually cause organ malfunction. It may run in the family or be acquired [53]. A systematic representation of amyloidosis in AD is displayed in Figure 1.
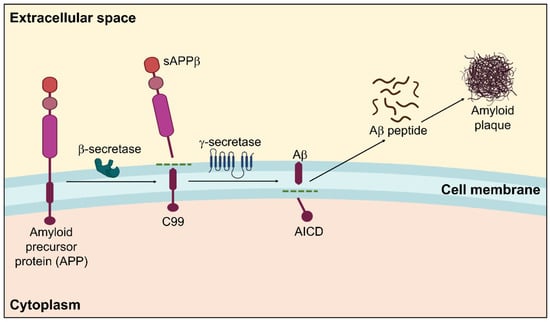
Figure 1.
Amyloidogenic pathway in the pathogenesis of Alzheimer’s disease showing the formation of amyloid plaques. Within the membrane, β-secretase cleaves APP in the first instance, followed by γ-secretase. The extracellular amyloid-β that is released by the proteolytic breakdown of APP via the amyloidogenic pathway is susceptible to self-aggregation, resulting in the development of cytotoxic oligomers and insoluble Aβ fibrils/plaques.
Amyloidosis is categorized into two types based on where amyloid fibers are deposited: localized amyloidosis, which affects a particular tissue in a specific place, and systemic amyloidosis, which affects the entire body [54,55]. These amyloid proteins make up amyloid plaques [56]. The primary element that significantly contributes to the pathophysiology of AD and is often regarded as the principal reason for AD development is the amyloid β peptide [53,57].
APP is produced by blood arteries, blood cells, neurons, and astrocytes in confined numbers and is a more significant precursor molecule than Aβ [58]. Multiple physiological functions for APP have been postulated thus far. APP is crucial for brain growth, memory, and neuroplasticity [59]. In addition to being able to safeguard neurons, it also controls intercellular relations, managing neuronal development and neuroplasticity [60].
Extracellular domains of the APP control cellular adhesion to support neural circuits. APP homodimers allow Aβ to activate calcium channels, which further modulate neural signaling and neurotransmitter discharge [61,62]. More precisely, K+-Cl− cotransporter 2 (KCC2) is stabilized on cellular membranes due to direct protein–protein interactions between APP and KCC2, which can modulate hippocampal γ-aminobutyric acid inhibition (GABAergic inhibition). APP reduction causes KCC2 to degrade more quickly through ubiquitination and tyrosine phosphorylation, which impairs γ-aminobutyric acid type A (GABAA) receptor-regulated inhibition and GABA reversal potential depolarization [58]. Soluble amyloid precursor protein (sAPP) cleavage molecules including sAPPα and sAPPβ are responsible for several facets of APP functionality, wherein the role of sAPPα has been thoroughly described. sAPPα has been demonstrated to be preventative against Aβ-induced toxicity and serves a significant role in neuroplasticity/survival [60].
Moreover, the central nervous system’s early embryonic processes and neuronal stem cell growth can be mediated by sAPPα [63]. In response to specific neuroprotective agents, it has been proposed that sAPPα could suppress cyclin-dependent kinase 5 (CDK5) activation induced by excitotoxicity and take part in diverse excitoprotective mechanisms [64]. Notably, in APP-deficient mice, sAPPα expression alone is sufficient to reverse defects, indicating that sAPPα might facilitate most APP functioning. It has been revealed that APP mutations accelerate the production of Aβ, which results in senile plaques and peripheral neuron degenerative alterations [65].
Depending on their cleavage products, APP processing can be classified as either amyloidogenic or non-amyloidogenic. In APP processing, the major proteolytic enzymes are α-, β-, and γ- secretase. The principal β-secretase in the brain is beta-site APP-cleaving enzyme 1 (BACE1) and γ-secretase. The full-length APP is broken down by α-secretase, which releases the sAPPα ectodomain beyond the cellular membrane while leaving a C-terminal APP fraction of 83 amino acids inside the plasma membrane. This process is known as the non-amyloidogenic pathway [65]. The consecutive APP proteolytic cleavage via β- and γ-secretase complex constitutes the amyloidogenic pathway. When APP is broken down by γ-secretase, amyloid peptides with varying chain lengths such as Aβ-37/38/39/40/42/43 can be produced [66,67]. The two main Aβ species in the brain are Aβ42 and Aβ40. Aβ42 has a greater potency for aggregating due to the hydrophobicity of its two terminal residues, albeit soluble Aβ40 is significantly more abundant than Aβ42. Hence, Aβ42 is primarily responsible for constituting the majority of amyloid plaques that are neurotoxic [68]. Correspondingly, Aβ42 is considered a principal performer in commencing plaque building in the pathophysiology of AD [69]. Moreover, it has been established that AD may be distinguished from other dementias by employing the Aβ42/38 ratio and levels of Aβ38/42 in the cerebral spinal fluid (CSF) [70,71,72]. By boosting Aβ synthesis and decreasing the Aβ40/Aβ42 ratio, dysregulated APP function likely aids in the etiology of AD [73]. Aβ protein is a 40–42 amino acid short peptide of 4.2 kDa [65]. Misfolded proteins with a stable conformation are called amyloid proteins. Additionally, an abnormal build-up of Aβ causes neurotoxicity [53]. Monomeric Aβ segments are soluble molecules that coalesce to produce insoluble oligomers, which then develop into neurologic plaques. The transfer of Aβ by the receptor for density lipoprotein receptor-related protein-1 (LRP1) and the receptor for advanced glycation end products (RAGE) is among the strategies our body uses to remove Aβ from the brain [74,75,76]. Clinical research has demonstrated that an imbalance between Aβ production and elimination causes abnormal metabolism, which in turn, causes extracellular protein build-up and misfolding, resulting in the establishment of amyloid plaques [77,78].
Compared to other cell types, nerve cells generate more Aβ, which is crucial for intercellular signaling and other typical physiological processes of the CNS [79]. Individuals with traumatic brain injury and PD accumulate Aβ, indicating a link between amyloid and neurodegenerative disorders [80]. Chronic stress causes the body to respond by ramping up the production of neural proteins, which results in the build-up of by-products such as phosphate. The phosphorylation of APP can be facilitated by high phosphate concentrations in the protein production area. Additionally, β-secretase engages in the subsequent phosphorylation of APP processing, which causes Aβ deposition. However, there are several circumstances where the bodily function that regulates the concentration of Aβ can become uncontrolled. For instance, natural Aβ can stimulate the production of extra APP, which is then phosphorylated and processed to become amyloid, increasing the concentration of Aβ. In peripheral neurons, Aβ elevated concentrations might also stimulate the synthesis of APP and amyloidosis. In the brain, a portion of Aβ misfolds and accumulates, generating hydrophobic exogenous oligomers that acquire the shape of plaques and fibers that harm synapses and neurons [81]. Substantial evidence such as the existence of APP mutations in familial AD patients points toward Aβ as a principal factor in disease development.
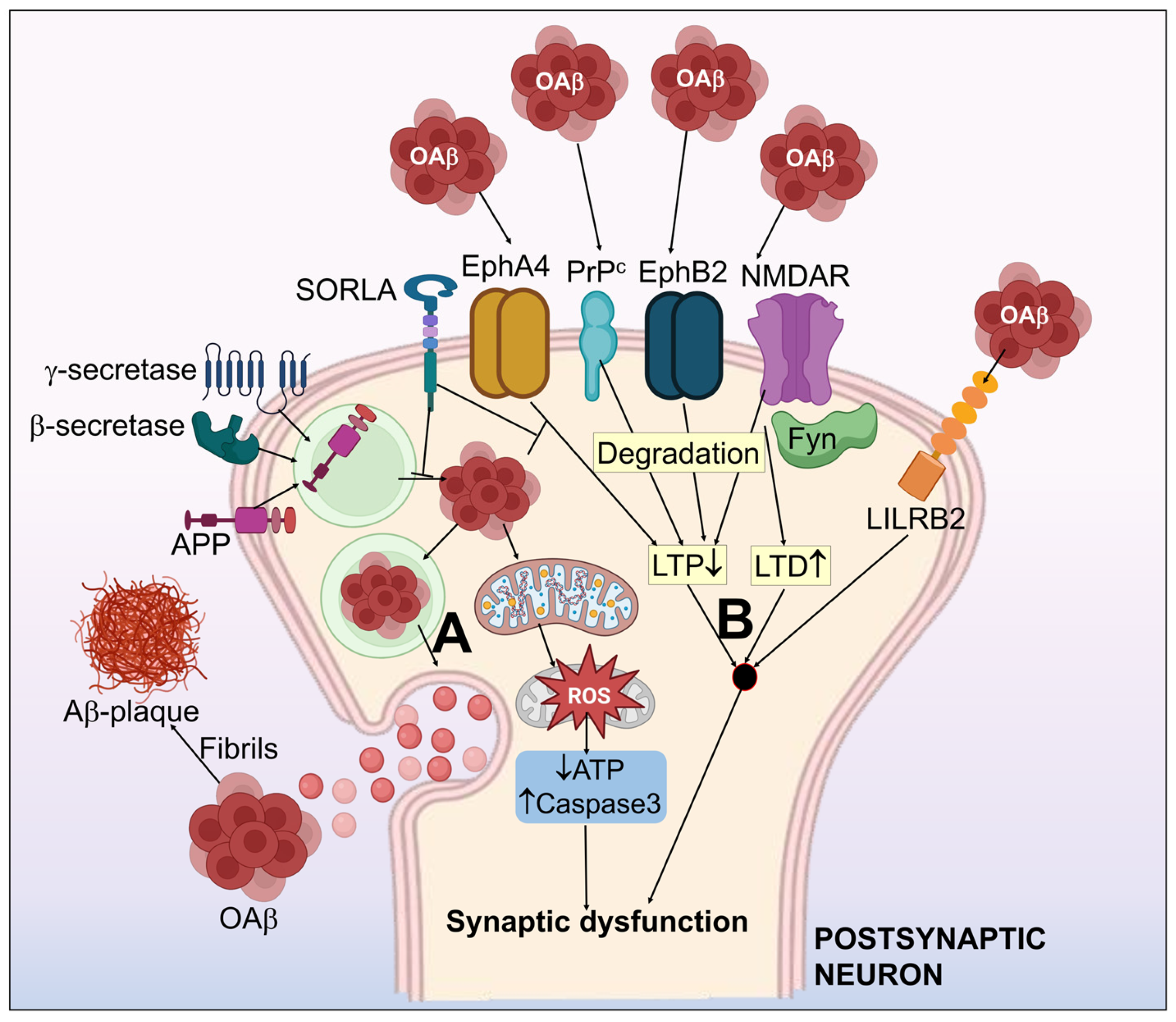
In the initial course of AD, patients have a synaptic malfunction; as the disease advances, synapses are lost. Synaptic loss is a primary pathogenic characteristic of AD and a reliable predictor of cognitive deterioration [82]. The degree of senile plaque development in the sick brain, however, does not always correlate with the severity of dementia that people with AD suffer. One viable argument is that soluble Aβ may indirectly contribute to AD pathophysiology by encouraging the development of neurofibrillary tangles [47]. The model for Aβ hypothesis in AD is displayed in Figure 2.
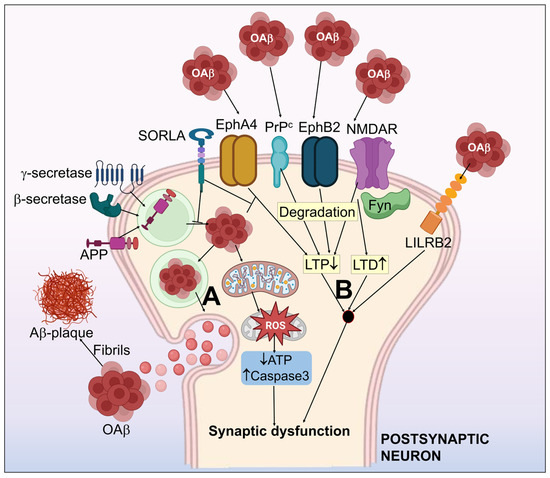
Figure 2.
Systematic illustration of the amyloid-β hypothesis in Alzheimer’s disease. (A) APP processing to form Aβ, which simultaneously assembles as aggregates of the Aβ oligomer (oAβ) and form amyloid plaques. (B) Aβ-associated synaptic dysfunction by the impairment of LTP and LTD. Aβ receptors including NMDAR, PrPc, EphA4, EphB2 & LiLRB2 have been shown to induce synaptotoxicity by interaction with Aβ. EphA4-associated synaptic and cognitive malfunction may be inhibited by SORLA. Fyn kinase functions as an essential control mechanism for NMDAR related oAβ neurotoxicity. oAβ halts the normal mitochondrial function, which results in activated capsase-3, upregulated ROS, and decrease in ATP. This further worsens the synaptic dysfunction.
3.1.1. oAβ Associated-Receptors
Even though the mechanisms behind oAβ-mediated synaptic malfunction have not yet been thoroughly defined, research findings have revealed a number of receptors that can induce Aβ synaptic toxicity. The receptors that are engaged with a reasonably high affinity toward Aβ comprise the leukocyte immunoglobulin-like receptor B2 (Lilrb2) [83], N-methyl-D-aspartate receptor (NMDAR) [84], cellular prion protein (PrPc) [85], ephrin type-B receptor 2 (EphB2) [86], and ephrin type-A receptor 4 (EphA4) [87].
LilrB2
The immune inhibitory receptor, LilrB2 is essential for maintaining the immune system’s homeostasis and repressing the immune system [88]. Current findings have correlated AD with LilrB2 and hypothesized that human LilrB2 is a potent oAβ receptor. In a mouse model of AD, paired immunoglobulin-like receptor (PirB) loss can also reverse cognition dysfunction. Cofilin and PirB engage mechanistically, and it appears that minimal amounts of the cofilin inactive phosphorylated form are present in the brains of individuals suffering from AD. The recruitment of cofilin-signaling modules due to the interaction between oAβ and PirB would cause actin depolymerization, culminating in synaptic malfunction and cognitive deficiencies [89]. A/LilrB2 inhibitors that may be bioactive such as ALI6 have been found to inhibit Aβ/LilrB2 interactions in vitro and reduce Aβ-induced neurotoxicity in the primary neurons [90].
NMDAR
In the neurological system, NMDARs, glutamate-initiated ion-gated cationic channels, are essential for exuberant synaptic transmission, development, and excitotoxicity [91,92]. Given that a subunit of NMDAR could coimmunoprecipitate with oAβ, they may interact directly [93]. Early phases of disease progress are probably when NMDAR activation by Aβ build-up takes place [94]. Aβ causes primary neurons to immediately influx Ca2+ by activating NMDARs that contain GluN2B, just like how NMDA stimulation occurs [95]. Furthermore, synthetic oAβ and Aβ derived from the AD brain could increase NMDAR-dependent long-term depression (LTD) [96,97]. These modifications could result from boosted NMDAR endocytosis and decreased NMDAR expression brought on by Aβ [98]. These findings demonstrate that the partial inhibition of NMDAR jaded stimulation with antagonists of NMDAR restores Aβ-initiated long-term potentiation (LTP) deficits and cognitive performance in distinct animal models, suggesting the importance of NMDAR in AD [99].
PrPC
PrPC is a relatively conserved protein in vertebrates at all developmental periods [100]. PrPC sized in the neuron’s pre/post-synaptic divisions, and expressed in different brain regions, specifically the hippocampus and cortex [101,102]. Numerous processes such as neuronal differentiation, neurite outgrowth, and survival could be regulated by PrPC [103]. PrPC was discovered to be a potentially high-affinity receptor for oAβ by a genome-wide association study (GWAS) [104]. Later research established that oAβ selectively binds to a 95-111aa N-terminal stretch of PrPC, particularly in oligomers of higher molecular weights [105]. In various AD mice models including APP/presenilin-1 (PS1), PrPC ablation substantially corrected synaptic LTP impairments by oAβ [106]. On the other hand, oAβ responses with PrPC had little or no impact on the formation of Aβ plaques and glial activation [107,108].
EphB2
In the maturation and adequate development of the nervous system, the Eph family that belongs to receptor tyrosine kinases perform crucial functions [109,110]. B-class ephrin ligands and Eph receptors coordinate bidirectional signaling, stimulating signals in both the receptors and ligand-bearing cells. Eph receptors govern neuroplasticity, dendritic spine formation, and neuronal-glial transmission in the brain [111]. It is intriguing that several neurological diseases including AD have recently been linked to Eph receptors and their function in synapse formation [112]. Hippocampal neurons exposed to oAβ exhibit lowered amounts of membrane EphB2, possibly due to cross-regulatory engagement between NMDAR and EphB2. EphB2′s fibronectin repeat section is where oAβ binds, leading EphB2 to be endocytosed and decomposed [113]. Surprisingly, LTP and cognitive memory deficits were corrected in an AD mice model by overexpressing EphB2 in the dentate gyrus [86]. Additionally, EphB2 overexpression can reverse the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and the NMDAR level decline is brought on by oAβ [114].
EphA4
When it comes to synaptic function, EphA and EphB serve opposite functions. During synaptic trimming, EphA4 physiological activation via an astrocytic ephrinA3 ligand at postsynaptic densities causes dendritic spine retraction via CDK5 and ephexin1. As a result, in the mouse brain, the deletion of EphA4 leads to more spines than in the wild type, which is also longer and less arranged. Interestingly, research within the last few years has correlated EphA4 to AD [115]. EphA4 messenger RNA (mRNA) expression has been observed to rise in synaptosomes in AD patients [116]. The human hippocampus also shows EphA4 accumulation in the areas around senile plaques and the AD brain exhibited higher levels of active EphA4 [117]. Since EphA4 in the hippocampus neurons are inhibited or not present, oAβ could cause the activation of EphA4 by binding to it, eliminating synaptic loss [118,119].
3.2. Tau Pathology toward Neurofibrillary Tangles
Tau is a cytosol protein mostly available in axons and is a neuronal microtubule-associated protein. The microtubule-associated protein tau (MAPT) gene possessing 16 exons is localized on chromosome 17, which encodes human tau [120,121,122]. Tau helps microtubules and related proteins assemble and remain stable [123]. By engaging on microtubules using its extensively conserved microtubule-binding repeat domains, tau also aids in regulating microtubule processes such as axon transport and neurite growth [124,125]. Microtubules oscillate between a stable phase and dynamical instability; efficient neural transmission and survival depend on optimal balancing among these two states [126]. This system relies on tau phosphorylation, which reduces tau’s capability for microtubules while maintaining their dynamic character to support the optimal neuron activity [127,128]. However, aberrant or excessive Tau phosphorylation reduces the integrity of microtubules, resulting in an elevation in neurite branching, a deduction in axonal transit, and synapse retraction, as shown in Figure 3 [126,129]. Neurodegenerative conditions like AD, ALS, and PD are featured by hyped phosphorylation of tau and the consequent micro tubular instability [130,131]. In the AD brain, hyperphosphorylated Tau could develop into oligomers, filaments of paired helical, and eventually neurofibrillary tangles [125,132,133,134]. Tau is more challenging for phosphatases to dephosphorylate once it has aggregated [135]. Oligomeric Tau could take effect as a “seed” and encourage additional Tau proteases in neighboring neurons to condense into fibrils [134,136,137]. It has been discovered that tau oligomers are the primary cause of axonal transport deficiencies in neurons, which can result in neural death [137,138]. Immunohistochemical staining was developed by researchers E. Braak and H. Braak to stage neuro-pathological Tau aggregation in the brain, and it has since been improved to make it easier for pathologists to determine the level of Tau deposition and whether AD needs to be identified in a post-mortem of the patient [139,140].
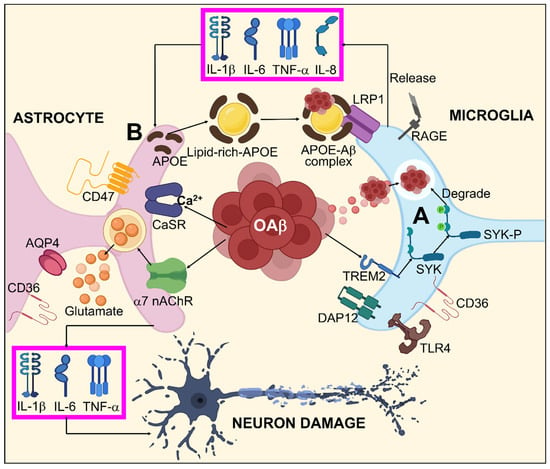
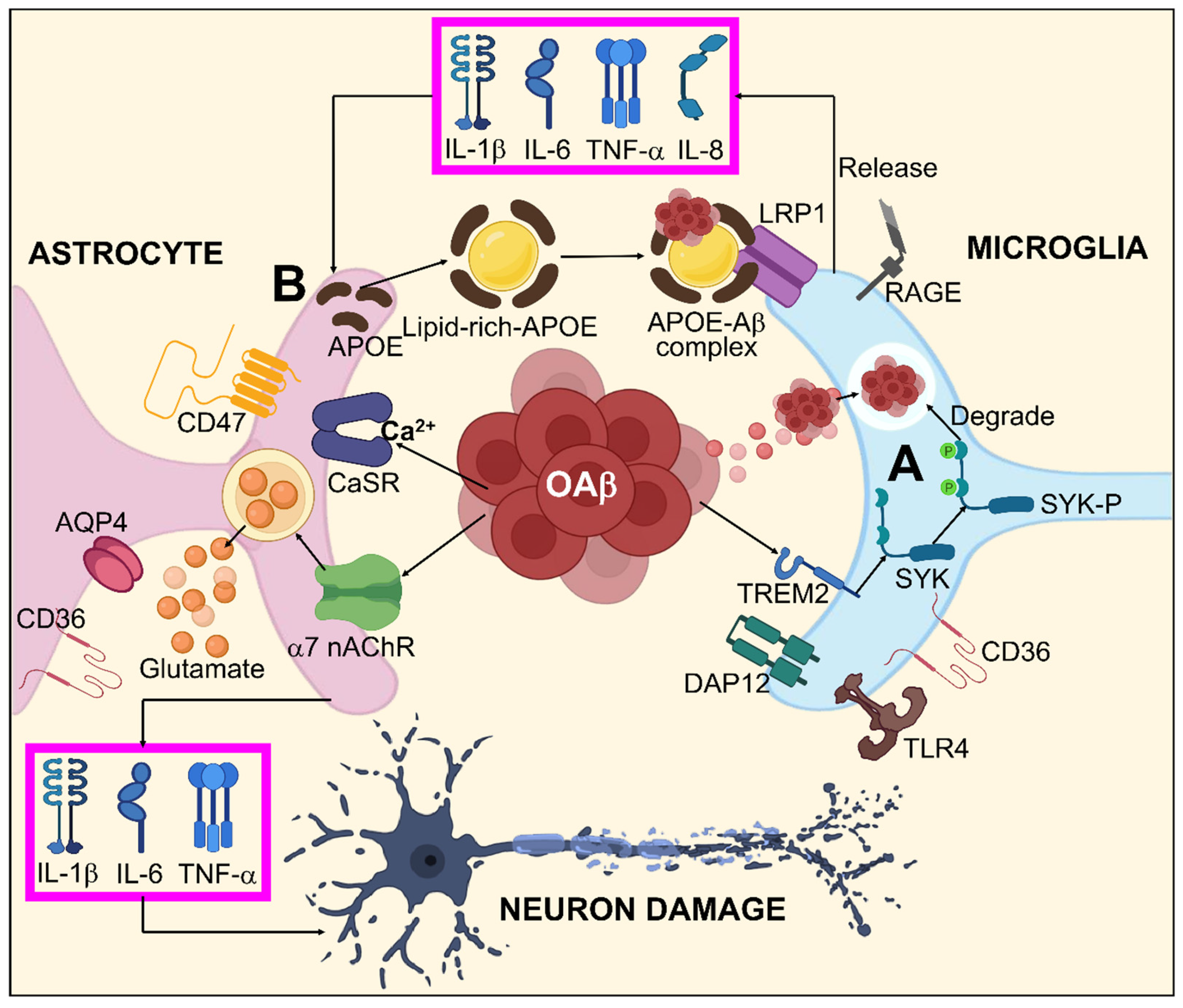
Figure 3.
Systematic illustration for Aβ-mediated glial response in AD. (A) oAβ might activate microglia by binding to different Aβ receptors including TLR4, RAGE, LRP1, CD36, and specifically to TREM2, which stimulates the SYK pathway via DAP12 inducing Aβ degeneration. (B) Aβ dependent astrocyte dysfunction by enhanced interactions between Aβ/APOPE and LRP1 results in astrocyte activation by releasing TNF-α, IL-1β, IL-6, and IL-8. Furthermore, oAβ is also capable of direct astrocyte activation by AQP4, CD36, α7-nAchR, CD47, and CaSR. This astrocyte activation leads to neuronal damage through TNF-α, IL-1β, IL-6, and excitotoxication/irregulated homeostasis of glutamate.
The presence of Aβ, neural inflammation, enzymes, and oxidative stress that modulate phosphatases and kinases can all impact the phosphorylated tau-protein conformation [141,142]. Microtubule affinity-regulating kinase (MARK), CDK5, and glycogen synthase kinase 3 (GSK3) are the three enzymes that likely have a major impact [143,144,145]. The formation of Tau into neurofibrillary tangles is in close alliance with the neurodegeneration (i.e., neural demise) and brain atrophy seen in AD [139,146,147]. The hippocampus and entorhinal cortex are the first areas of the AD brain to be impacted, accompanied by areas of the temporal lobe and neocortex. During this period, patients may experience moderate cognitive impairment (MCI) [148]. The degeneration then progresses to the frontal portions of the cortex and occipital lobe, resulting in delayed personality alterations and trouble accomplishing daily tasks [149,150]. These frontal portions shrink while the ventricles are expanded. The primary pathogenic driver of ventricular enlargement and cortical atrophy is believed to be neuronal loss [151].
3.3. Mitochondrial Dysfunction and Reactive Oxygen Species (ROS) Generation
According to multiple evidence-based studies, the pathophysiology of AD may be influenced by mitochondrial dysfunction [152]. As a result of Aβ aggregation in the mitochondria of AD brains, disrupted mitochondrial conformation, reduced adenosine triphosphate (ATP) release and respiratory function, increased mitochondria-mediated oxidative stress, and poor mitochondria dynamics occur. The brain mitochondria of the patients suffering from AD and mouse models have both been reported to possess Aβ, which is responsible for neurodegeneration [153]. Irregularities in mitochondrial structure and functioning are associated with elevated mitochondrial Aβ. For example, reduced energy consumption related to mitochondria was noted in brain areas connected to amyloid plaques. Aβ also causes anomalies in mitochondrial function; due to decreased energy generation, abnormal alterations are also observed in the mitochondrial dynamics. Additionally, proteins linked to enhanced mitochondria fission and reduced fusion of mitochondria are amplified by Aβ exposure [154]. Unfortunately, it is still uncertain how mitochondrial dysfunction contributes to AD.
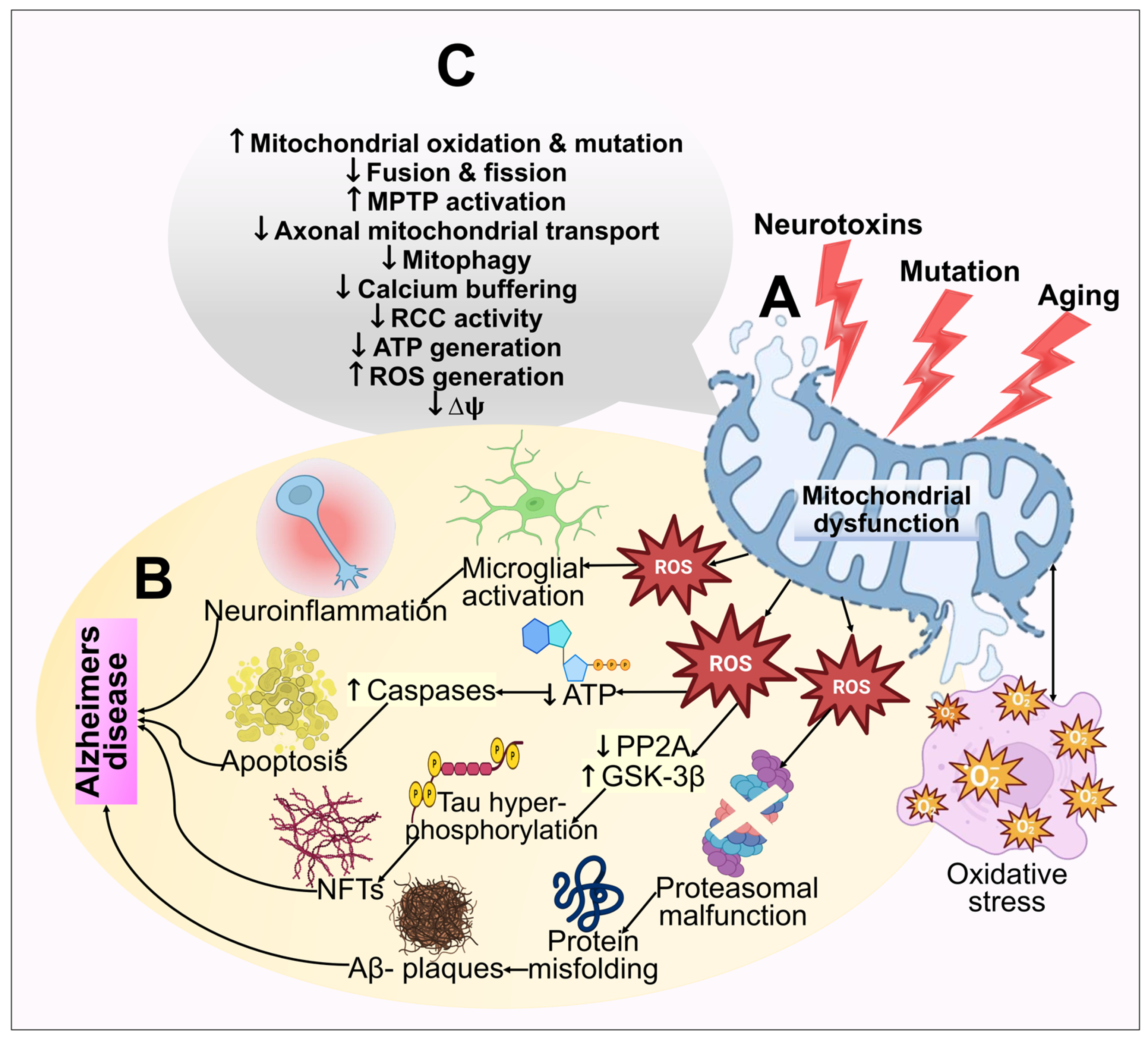
The oxygen consumption and metabolic rate of neurons are exceedingly high. As a result, to generate energy by oxidative phosphorylation, neurons depend on the numerous mitochondria in brain regions. ROS are primarily generated in mitochondria as by-products of oxidative phosphorylation, and routine homeostatic action in mitochondria frequently blocks excessive ROS formation. Furthermore, there are indications that oxidative assaults are critical in AD pathophysiology [155]. The idea that oxidative stress might be what causes AD pathogenesis triggered by Aβ is supported by the finding that oxidative stress emerges earlier in AD [156]. Aβ-peptides can elicit mitochondrial ROS generation, which releases cytochrome c and an apoptosis-inducing factor, causing malfunction of mitochondria, apoptosis, and the death of neurons [154,157]. In AD, appoptosin overexpression can cause the intrinsic caspase pathway to be activated. Prominently, decreased appoptosin expression can guard against Aβ’s neurotoxic effects [158]. Amyloid-binding cyclophilin D alcohol dehydrogenase are the few other mitochondrial proteins that have depicted a role toward mitochondrial dysfunction [159,160,161]. Mitochondrial dysfunction and oxidative stress in the pathogenesis of AD are illustrated in Figure 4.
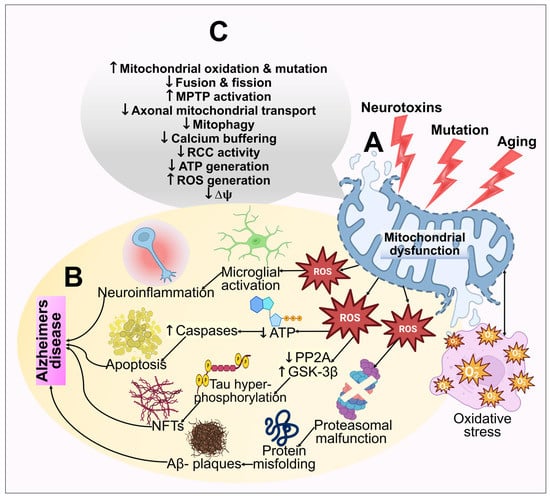
Figure 4.
An illustration of the mitochondrial dysfunction and oxidative stress in the pathogenesis of AD. (A) Multiple age-related processes, mutations, and toxic fluctuations such as metal exposure can all adversely affect mitochondria. Mitochondrial dysfunction further results in bioenergetic deficits, calcium imbalance, and free radical production. This causes oxidative stress, which exacerbates mitochondrial impairment, synaptic malfunction, cognitive decline, and memory loss. (B) The cellular redox equilibrium is disrupted by ROS generation or a compromised antioxidant arrangement, which leads to an oxidative imbalance and excessive ROS output. By adversely influencing mitochondrial energy reserves, disrupting energy metabolic processes, and impairing dynamics and mitophagy, elevated ROS reduces mitochondrial ΔΨm and ATP production. Caspase activity also rises as a result of ROS, which additionally starts the apoptotic process. However, excessive ROS generation inhibits phosphatase 2A (PP2A), which leads to glycogen synthase kinase 3 (GSK3) activation. This results in tau hyperphosphorylation and NFT buildup. (C) The functions of the mitochondria that are extensively hampered in AD have been highlighted.
3.4. Nitrosative Stress
Nitrosative stress arises when various defensive mechanisms fail to balance the formation of reactive nitrogen species (RNS), which harms intracellular constituents. The main component of RNS is nitric oxide (NO), which acts as a signaling molecule to control synaptic plasticity, neurotransmission, and brain growth. Significant cognitive impairment aligned to synapse malfunction and glial activation has been linked to nitrosative stress [162,163]. Due to S-nitrosylation, nitric oxide released as a consequence of Aβ in AD has been identified to trigger fission in mitochondria, resulting in synaptic dysfunction and neuronal death [164]. Since higher S-nitrosothiol (SNO)-CDK5 amounts were found in post-mortem samples of the AD brain and not in healthy samples, it has been determined that enhanced SNO-CDK5 activity possesses a part in the progression of AD [165]. Assessing the role of S-nitrosylation in nitrosative stress-initiated AD pathogenesis is made more accessible by the massive neuronal atrophy in the AD brain, accompanied by elevated S-nitrosylation of the peptides and a considerable proportion of altered sites of cysteine [166].
3.5. Protein Oxidation and Lipid Peroxidation
Multiple evidence-based findings imply that ROS might be crucial in the emergence of neurodegeneration in AD. ROS and RNS build up over time, which results in protein oxidation and lipid peroxidation. The brain also possesses an elevated proportion of unsaturated lipids, a high metal ion concentration, an elevated oxygen usage rate, and a poor antioxidant system. Consequently, both protein and lipid oxidation are particularly dangerous for the brain.
3.5.1. Lipid Peroxidation
The oxidative breakdown of lipid molecules is alluded to as lipid peroxidation. Removing the H-atom from lipids in the cellular membrane by free radical species sets off a series of events that lead to cellular membrane destruction. Due to its elevated oxygen uptake, a large quantity of redox metal ions, weakened antioxidant defense system, and a higher proportion of polyunsaturated fatty acids (PUFAs), the brain is thought to be particularly sensitive to lipid peroxidation [167]. By Michael’s adduction to the amino acids histidine, lysine, and cysteine, the by-product 4-hydroxy-2-nonenal (HNE) might establish covalent connections with proteins. The experimental studies conducted by Tamagno et al. [168] showed that elevated lipid peroxidation triggers the activation of BACE 1, which in turn elevates the production of Aβ. According to a study, the AD hippocampus contains higher levels of HNE-histidine Michael adducts, and the covalent alteration of the histidine side chain of Aβ leads to greater tau protein aggregation [169]. Notably, c-Jun N-terminal kinase (JNK) pathways can be activated by lipid peroxidation and Aβ (production in neurons), which results in programmed neuronal cell death. HNE is a very deadly substance that prevents muscarinic cholinergic and metabotropic glutamate receptors from coupling to phospholipase C-coupled GTP-linked proteins [170] and is an aldehydic derivative of lipid peroxidation. HNE also halts glutamate transport in cortical astrocytes and glucose transport in hippocampal neuronal cells [171].
3.5.2. Protein Oxidation
Protein carbonyls and 3-nitrotyrosine (3-NT) concentrations are elevated in response to cell protein oxidation. The interaction of the superoxide anion can produce protein carbonyls, which fragment the protein’s backbone. Moreover, the specified ROS attack on the side chains of several amino acids including lysine, proline, and arginine might lead to protein carbonyl generation. Various investigations have revealed the creation of Michael adducts amongst cysteine, lysine, and histidine and residues, which results in advance glycation end products (AGEs) [172,173]. According to Bota et al. [174], a variety of human disorders may be caused by the inadequate elimination of transformed proteins and deposits. There seems to be strong evidence that the protein carbonyl level was enhanced in the parietal lobule and hippocampus of individuals suffering from AD by 37% and 42%, respectively [175]. Currently, utilizing a redox proteomic strategy, researchers have uncovered additional altered proteins in distinct portions of the brain in AD patients including peptidyl-prolyl-cis, trans isomerase 1 (Pin1), enolase, creatine kinase BB, ubiquitin carboxyl-terminal hydrolase L-1 (UCHL-1), heat-shock 71, and dihydropyrimidinase-related protein 2. Furthermore, ATP depletion in brain cells can result in aberrant tau protein phosphorylation, possibly contributing to the start of AD [176].
3.6. DNA Damage
DNA is nucleic acid found in the mitochondria (mtDNA) and nuclear (nDNA) material of living cells. Multiple lines of research have indicated that ROS generated as an oxidative phosphorylation by-product and environmental subjection to chemicals and radiation target nuclear and mitochondrial DNA for the genotoxic attack. Furthermore, investigations have demonstrated that because mtDNA is situated near the oxidative phosphorylation cascade, it is more vulnerable to genotoxic attack than nDNA [177].
The Tau protein, in conjunction with its function in microtubule dynamics, is essential for protecting the genomic DNA of neurons from oxidative stress and ROS. The modification in the tau protein may impair nucleic acid protection mechanisms and make hippocampus neurons more vulnerable to ROS-induced oxidative stress to their nuclear RNA and genomic DNA in AD patients. It has been established that ROS can damage DNA strands and play a role in subsequent AD disease-causing processes [178]. Mullaart et al. [179] found a two-fold rise in DNA destruction in neurons of the AD brain. They theorized that this might be one of the early identifiable pathogenic events in the transition from the normal to the AD brain. 8-Hydroxyguanine (8-OHG) is the most protruding DNA marker in most biological samples including blood cells, urine, and brain tissues [180]. 8-Oxoguanine-DNA glycosylase (OGG1) is a bifunctional enzyme that has apurinic/apyrimidinic lyase and DNA glycosylase properties. Evidence from research has shown two single-nucleotide polymorphisms in OGG1 caused by the substituted amino acid A53T and A288V. These polymorphic OGG1 proteins with the A53T and A288V mutations were found in 2007 in late-stage brain tissue AD patients but not in the controls [181].
3.7. Glial Cells in AD
Another characteristic of AD is neuroinflammation, which appears as gliosis and is marked by the activation and proliferation of the two main glial cell types in the brain, astrocytes and microglia. Numerous recently discovered AD risk genes such as triggering receptors expressed on myeloid cells-2 (TREM2) are only expressed in glial cells or are greatly concentrated in them. As a result, current research has placed a lot of emphasis on the probable impact that the glia may serve in the pathogenesis of AD. Pathogenic tau and Aβ species can bring neuroinflammation and gliosis. Glial cells and inflammation can control the development of Aβ and tau in a reciprocal manner. In general, it is thought that inappropriate microglial and astrocyte activation is a harmful event during the initiation of AD, and that blocking the formation of pro-inflammatory cytokines and the malignant glial responses to pathogenic Aβ and tau may prevent AD pathogenesis.
3.7.1. Aβ Pathogenesis and Glial Cells
In AD, inappropriate Aβ collection seems to be what starts the inflammatory processes. In such a situation, oAβ might increase proliferation by activating microglia. Microglia are indigenous immune cells that govern homeostasis in the brain by managing immunological functionality, phagocytosis, and plays a reparative role [182]. Since active microglia vigorously phagocytose and destroy oAβ, they may be preventive in the beginning stages of AD. Furthermore, by the secreting brain and glial-derived neurotrophic factors, microglial activation may aid neuronal healing [183].
Moreover, active microglia can also increase oxidative stress and release pro-inflammatory cytokines such as IL-1β and IL-6 in AD and tumor necrosis factor-α (TNFα) [184,185]. Conversely, overactive microglia may mitigate synaptic function by promoting phagocytic synaptic trimming. Hence, due to putative adverse effects related to inflammation, neurotoxicity, and degeneration, chronic microglial excitation during AD development may be harmful. Neuroinflammation can also worsen Aβ build-up by interfering with phagocytic Aβ intake and elimination. The ability of tert-butyl hydroperoxide, lipopolysaccharide (LPS), IL-1β, and prostaglandin E2 to decrease the phagocytosis of microglia and increase Aβ accumulation has been demonstrated [186].
In the general brain form of glial cells, astrocytes define the boundaries dividing the nerve and non-nerve tissue throughout the meninges and vascular areas. Functional barriers created by astrocyte boundaries and scars restrict the movement of inflammatory cells into the CNS parenchyma. Astrocytes are therefore vital to limiting inflammation in the CNS [187]. Astrogliosis occurs as an outcome of Aβ accumulation and proinflammatory cytokines generated by activated microglia during the pathophysiology of AD. Active astrocytes possess a bidirectional impact on AD. On one hand, they can encourage the breakdown and removal of Aβ primarily via the production of apolipoprotein E (APOE), an essential modulator for Aβ clearance [188,189,190]. On the other hand, by releasing RNS, ROS, and pro-inflammatory cytokines that obstruct synaptic development and the growth of axons, activated astrocytes might worsen inflammation [191,192,193]. Current investigations have defined a particular subtype of A1 astrocytes that is reactive activated by TNF, IL-1, complement component 1q (C1q), and mitochondrial fragment out bursting from active microglia, suggesting that microglia might serve a substantial part in maintaining astrocytic activation [194]. A1 astrocytes are more prevalent in neurodegenerative conditions like AD where they have been demonstrated to have diminished neuroprotective functions such as supporting neurogenesis, outgrowth, and synapse formation as well as incapacitated phagocytic capabilities. A1 astrocytes can also cause neurons and oligodendrocytes to die [195]. Even though the exact mechanisms underlying the interactions between Aβ and glia are still vague, mounting evidence suggests that several glial receptors including Fc γ receptors IIb (FcγRIIb) [196], LRP1, cluster of differentiation 36 (CD36) [197,198], complement receptor 3 (CR3) [199], RAGE [200], Toll-like receptor 2/4 (TLR2/4) [201], and TREM2 perform pivotal roles in facilitating Aβ-mediated glial responses and functionalities. Aβ associated glial responses in AD are illustrated in Figure 3.
3.7.2. Tau Pathogenesis and Glia
Considering that several tau transgenic animal models and tauopathy sufferers exhibit gliosis even in the absence of Aβ pathology, pathogenic tau species can also activate microglia and astrocytes. Pro-inflammatory cytokines like IL-1β, IL-6, and TNF-α can be secreted more readily as a result of tau-mediated microglial activation [202,203,204]. Recent transcriptome studies have shown the involvement of nuclear factor kappa B (NF-κB) and nucleotide-binding domain, leucine-rich–containing family, and pyrin domain-containing-3 (NLRP3) in the process of tau-activating microglia to cause inflammation, despite this, the exact mechanism is still unclear [205,206]. Aβ and tau appear to retain similar pathways for activating microglia because Aβ also activates NF-κB signaling and the NLRP3-ASC inflammasome [207,208]. Through assimilating and destroying pathogenic tau from the AD brain, wherein the related mechanisms of the process remain obscure, microglia can drastically improve tau elimination [209]. Countless studies have revealed that the CX3C motif chemokine receptor 1 (CX3CR1) is essential for promoting tau breakdown and microglial phagocytosis. CX3CR1 interacts with tau and hyperphosphorylated tau to a limited extent [210]. Lack of CX3CR1 affects tau uptake by microglia in vitro and encourages hyperphosphorylated tau aggregation in vivo [211]. Microglia may have an impact on tau propagation through exosome generation in addition to influencing tau elimination, as exosome propagation can be stopped by depleting microglia or inhibiting exosome synthesis [212,213,214]. As a result, pathogenic tau and microglia activation may generate cyclical pathogenic episodes throughout the development of AD.
Astrocytes directly influence the pathophysiology of tau. Tau accumulation can be seen in the astrocyte nucleus in the AD brain, despite tau primarily building up in the neurons [215,216]. Tau aggregation in astrocytes affects astrocyte functioning, causes neurodegeneration, and encourages apoptosis through a series of degenerative processes including accelerating the collapse of BBB and triggering the expression of low-molecular-weight heat shock proteins [217,218,219,220]. Pathogenic tau may also impair astrocytic-mediated glutamate transport, leading to glutamate deposition in the brain and subsequent excitotoxicity [221,222]. The model for the tau hypothesis and its impact on microglia in AD is illustrated in Figure 5.
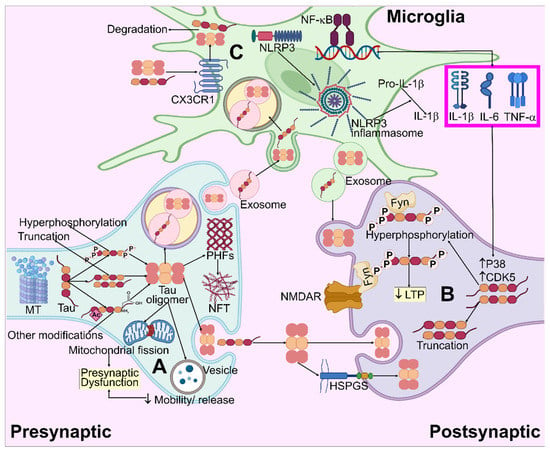
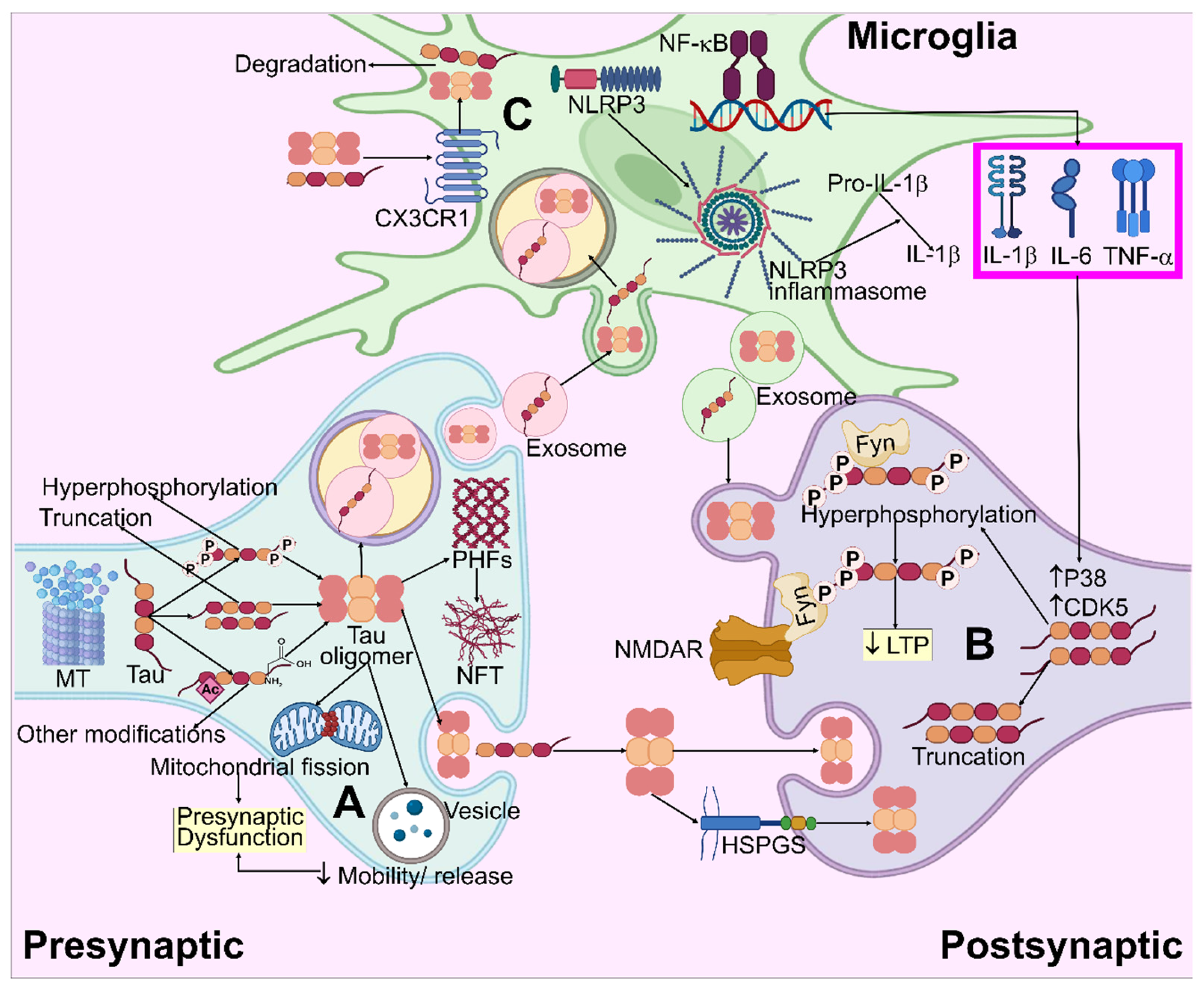
Figure 5.
Systematic illustration for tau pathogenesis in AD. (A) Tau aggregation and oligomer formation result in mitochondria fragmentation and the impairment of vesicle mobility/release, which causes presynaptic dysfunction. (B) Truncated and hyperphosphorylated tau species enter the post-synapse and modulate NMDAR/Fyn complexes, leading to LTP impairment. By means of the heparan sulfate proteoglycans (HSPGs)-mediated route, extracellular pathogenic tau species may be embodied in neurons, causing intracellular tau accumulation. (C) Extracellular tau interacts with CX3CR1, enters the microglia, and degrades. Microglial NF-κB and NLRP3 inflammasome pathways are activated by tau, which allows for the release of pro-inflammatory cytokines. These cytokines enhance the CDK5 and P38 activity, which leads to increased hyperphosphorylation.
To sum up, it seems likely that Aβ initiates tau pathogenesis in AD, where pathogenic tau and Aβ jointly cause gliosis and neuroinflammation. Inflammatory elements and reactive glial cells work synergistically to accelerate the development of Aβ and tau pathophysiology to worsen the neurodegeneration.
3.8. Proteasomal Dysfunction
By eliminating proteins that are inappropriately folded or clumped together, the ubiquitin-proteasome pathway (UPP) helps to maintain cellular integrity. When this system for removing undesirable protein complexes is disrupted, toxic and improperly folded proteins gather in brain cells, which is thought to be a pathogenic characteristic of AD. This route is crucial for the efficient elimination of aberrant protein garbage, which is essential for the viability and stability of neurons [223]. In order to continually remove defective proteins from neurons and block the aggregation of inappropriate proteins, the optimal functioning of UPP is of utmost importance [224].
Recent research has exhibited that the intracellular deposit of phosphorylated tau and Aβ protein clumps in AD patients directly impairs UPP. Brain tissue from patients with early AD had much less proteosome activity. The proteasome activity is reduced by 56% in AD patients due to the intraneural accumulation of paired helical filaments, which inhibits the proteasome. As a result, in the AD brain, the failure of UPP to remove phosphorylated tau and paired helical filament ultimately causes neuronal death [225]. Current findings have shown a strong correlation between ubiquitinated synaptic tau and hyperphosphorylation. This stable oligomerization of ubiquitinated synaptic tau results in elevated proteasome elements, proposing that a failure of the ubiquitin-proteasome system causes AD [226,227]. Although extant research has asserted a strong correlation between the build-up of hyperphosphorylated tau and malfunctioning UPP, none of these studies has explicitly stated whether the hyperphosphorylated tau is to blame for the UPP machinery’s impairment or the other way around [228]. As a result, further research employing cellular models are required to pinpoint the pathogenic event that causes aberrant neuron activity in AD patients.
3.9. Neuroinflammation
Infection, trauma, or toxic materials can cause a complicated series of inflammatory reactions in the brain system known as neuroinflammation. Microglia and astrocytes are important cells that indulge in inflammatory processes in the CNS and neuronal cells. By showing the existence of reactive microglia in the substantia nigra portion of post-mortem brain tissue from PD patients, Mc Geer et al. [229] made the initial discovery. The Aβ and tau tangles are surrounded by persistent microglial activation, which causes the loss of the homeostatic role of glial cells, developing a proinflammatory trait and exacerbating neurotoxicity. In the case of neuroinflammation, serum and brain specimens from AD patients include inflammatory mediators such TNF-α [230], IL-6, IL-β [231], and cyclooxygenase-2 (COX-2) [232].
4. Risk Factors Associated with Alzheimer’s Disease
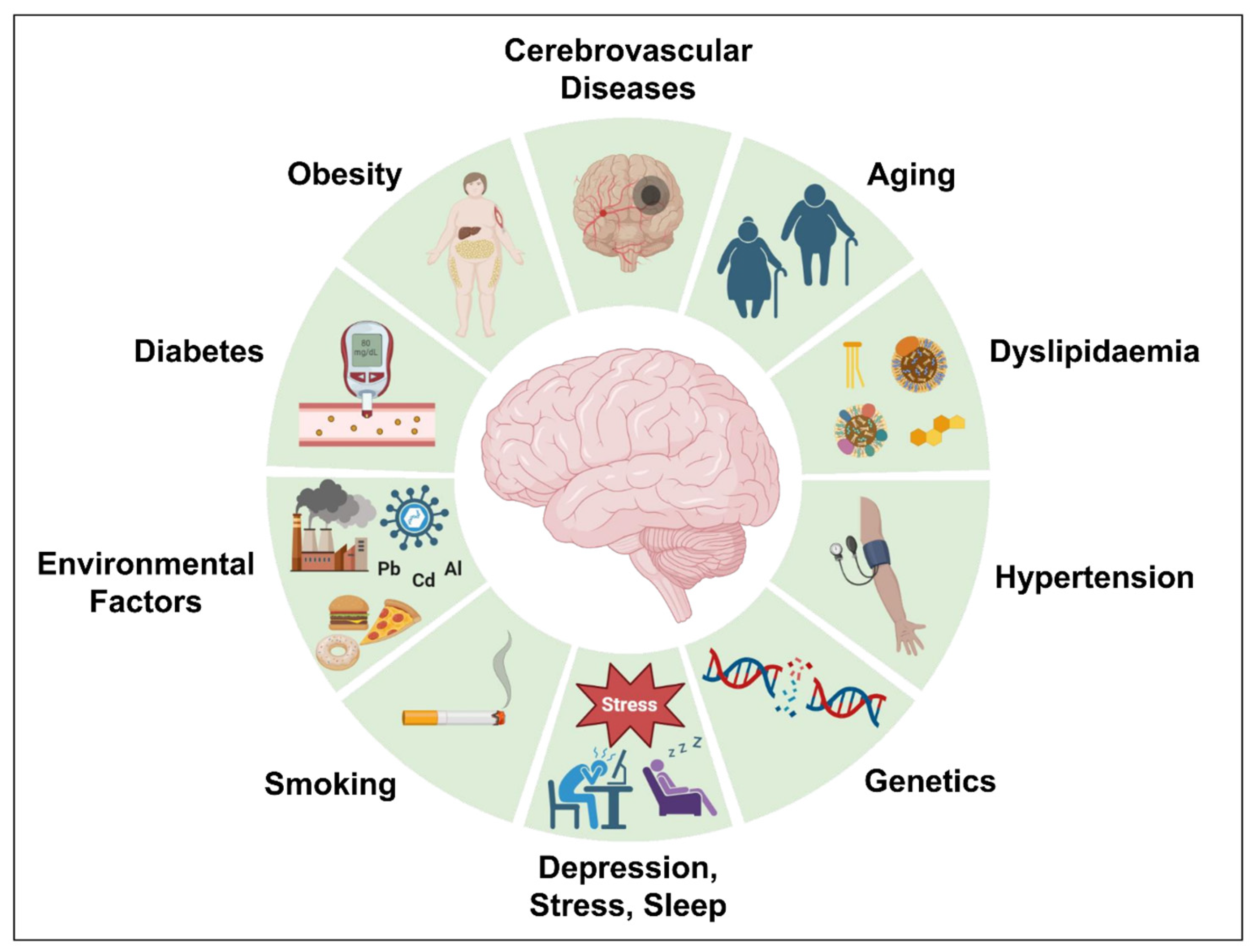
Genetics account for almost 70% of the chance of developing AD. However, acquired variables such as obesity, dyslipidemia, diabetes, hypertension, cerebrovascular disorders, and others raise the possibility of AD development. Several risk factors attributed to AD development [233] including aging, cerebrovascular diseases, obesity, diabetes, hypertension, dyslipidemia, depression, stress, abnormal sleep, and genetics have been discussed further and are illustrated in Figure 6.
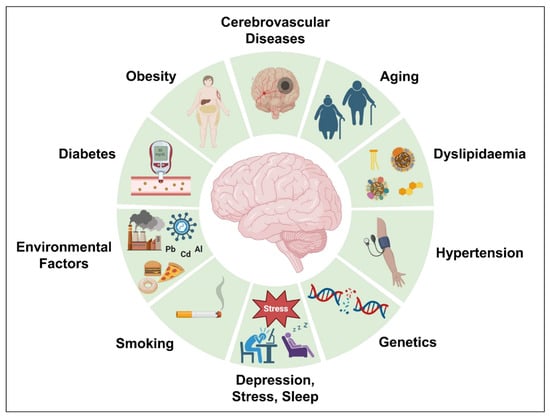
Figure 6.
Different risk factors attributed to AD development.
4.1. Aging
The principal risk factor for AD is aging. Young people rarely suffer from this disease, and the majority of instances of AD begin decades later, after the age of 65 [234]. Aging is a complicated and irreversible operation that influences many different organs and cellular systems, resulting in reduced brain size and weight, synaptic loss, and the swelling of certain ventricles along with SP deposits and NFT [235]. Many conditions such as glucose hypometabolism, dyshomeostasis of cholesterol, malfunctioning mitochondria, depression, and cognitive impairment may also become apparent as people age. These alterations make it difficult to distinguish nascent AD cases from normal aging cases [236,237].
4.2. Cerebrovascular Diseases
The risk factors for cerebral vascular diseases and AD are diverse and often co-occur. Cerebral vascular abnormalities such as hemorrhagic infarcts, vasculopathy, and mild to severe ischemic cortical infarcts might enhance the risk of dementia [238,239]. The investigational data by Liu et al. [240] stated that 6 to 47% of those with dementia also have cerebrovascular disease. These findings raise the probability that homeostatic mechanisms play a role in AD and pose the dilemma of whether dementias involving vascular processes are profoundly distinct from those caused by the build-up of Aβ42 and tau proteins or whether both pathological mechanisms have an additive impact on cognitive activity [241]. In accordance with the “double stroke” hypothesis of AD, vascular risk factors (“first stroke”) cause BBB impairment and a decrease in blood flow to the brain, which results in a reduction in the region’s blood supply (oligemia). Through both amyloidogenic and non-amyloidogenic routes, this event causes neural destruction. Primarily, the dysfunction of BBB induces oligemia and the accumulation of neurotoxic compounds, which are linked to the production of numerous focal ischemic infarcts and microinjuries brought on by hypoxia, resulting in neuronal damage. Vascular injury elevates APP expression and processing along the amyloidogenic pathway, raising the Aβ-peptide level.
Additionally, decreased Aβ peptide clearance is brought on by BBB injury. Amyloid deposition in the brain, also known as a “second stroke,” exacerbates neuronal malfunctioning and hastens the progression of neurodegeneration. The Tau protein is hyperphosphorylated due to both ATP build-up and hypoperfusion, which helps generate NFT [242].
4.3. Obesity
It is debatable whether obesity is a particular risk factor for AD. Only alcohol consumption has raised the likelihood of cognitive degradation among the several risk variables linked to cognitive deficits in a significant group of patients. Hypercholesterolemia, obesity, and depression were more likely attributed to a low AD risk [243]. The research concluded that a number of the “traditional” risk variables for AD were not linked to the initiation or progression of the disease. On the other hand, a significant association between obesity and the onset of AD has been found, raising the possibility that metabolic alterations linked to obesity harm the neurological system and cause neuronal death through necrosis or apoptosis by changing neural plasticity [244]. A greater midlife body mass index proportionately raises the likelihood of AD, and obesity has been significantly linked to the prevalence of dementia [245]. Additionally, obesity was attributed to various metabolic syndromes affecting the expression of leptin and adiponectin when combined with vascular risk factors [246].
4.4. Diabetes
Diabetes has been associated with AD, which is not strange considering that insulin plays a crucial function as a neuromodulator [247]. First, there is a correlation between AD, hyperinsulinemia, and hyperglycemia, which is brought on by insulin resistance and results in SP deposition, particularly in people who express the APOE allele ε4 [248]. Type 2 diabetes and its precursors, excessive obesity, hyperinsulinemia, and dementia, are related. The risk occurs in middle-aged people but not in older people [249]. Severe cortical thinning, increased caspase concentrations, and profound brain atrophy have been observed in transgenic APP/PS1 mice possessing hyperinsulinemia [250]. An enhanced cognitive impairment and Aβ deposition in the histocompatibility complex were observed in studies employing rats with a high-fat diet containing cholesterol [251]. Diabetes-related genes in the sortitin family of vascular protein sorting-10 domain (VpS10) genes are correlated to AD in diabetics, and their malfunction can result in Aβ accumulation and insulin/glucose malfunction [252].
4.5. Hypertension
Cumulative research by Skoog et al. [253] demonstrated that having elevated blood pressure might raise the likelihood of developing AD [254]. Hypertension contributes to the risk factors for AD by inducing cerebral edema in chronic instances, thickening of the vessel walls, and constriction of the lumen, all of which impair cerebral blood flow. The findings depict that cerebral ischemia could result in the aggregation of APP and Aβ and boost the expression of presenilin, a protein implicated in the production of Aβ. The BBB may become non-functional due to hypertension, an occurrence linked to the origination of AD [255].
4.6. Dyslipidemia
Raised cholesterol concentrations have been highlighted as prospective risk factors for AD development. Previous investigations have revealed that the cholesterol levels of AD patients are 10% higher than in healthy individuals [256]. The BBB is the main reason through which hypercholesterolemia increases AD susceptibility [257]. Various studies have illustrated that high levels of cholesterol circulation could compromise the BBB integrity. Furthermore, other than an enhanced cholinergic neural malfunction, NFT production, neuronal inflammation, cognitive decline, and cerebral micro hemorrhage occurrence consistent with AD, research on animal models shows that hypercholesterolemia is linked to hiked Aβ-peptide accumulation [258,259].
4.7. Depression
Depression in early adulthood increases the likelihood of dementia including AD developing later in life [260,261,262]. According to Zverova et al. [263], individuals having AD and indications of depression had a higher chance for cognitive deterioration in the context of cortisol levels. Wu et al. [264] stated that hippocampal shrinkage and Aβ-peptide deposits were identified in certain middle-aged individuals with significant depression, suggesting that the protein metabolism might be changed in depressed patients.
4.8. Stress
The emerging proof indicates that chronic stress may increase the likelihood of developing AD and expedite the course of the illness. Significant links exist between susceptibility to stress and elevated degrees of anxiety and dementia incidence [265]. External and environmental stress can cause psychological stress, which then promotes cellular stress, which is then made worse by oxidative destruction and inflammation [266,267,268]. Psychological stress stimulates the hypothalamic–pituitary–adrenocortical (HPA) axis, which causes the release of glucocorticoids into the blood. These substances then pass through the BBB and activate the mineral corticosteroid receptor and glucocorticoid receptor (GR) in the brain [269,270]. Chronic stress results in long-term stimulation of the HPA axis, persistent receptor depletion, and hippocampus neuronal death. According to the glucocorticoid cascading theory, the etiology of AD may be sensitive to HPA axis malfunction [271,272].
4.9. Sleep
A study by Proserpio et al. [273] established a bidirectional relation between sleep abnormalities and AD, with sleep disturbances developing before dementia sets in and often becoming worse as dementia progresses. The risk of developing dementia can also increase due to sleep problems. Studies by Shi et al. [274] showed that people with sleep difficulties had a higher risk of getting dementia. Precisely, people who experience insomnia possess a significant risk of getting AD but not vascular dementia or other types of dementia. Similarly, people with sleep-disordered breathing are more likely to experience vascular dementia, AD, and all-cause dementia.
4.10. Smoking
By using a variety of pathways, smoking may influence the chance of acquiring AD. It is well-recognized that it can increase the production of free radicals, causing higher oxidative stress and encouraging immune system pro-inflammatory activity, which in turn activates phagocytes and causes further oxidative damage. Moreover, smoking might result in cerebrovascular ailments, boosting the risk of AD [275,276].
4.11. Genetics
Over the years, genetic factors have been explored, and it has been determined that they significantly contribute to the onset of AD. Genetic factors are responsible for 70% of AD cases. It has been estimated that genetics comprise around a 70% chance of getting AD. Early AD typically occurs due to mutations in the PSEN1, PSEN2, and APP, while late-form AD is mainly linked to APOE gene polymorphism, particularly the existence of the ε4 allele [277,278]. Approximately 15% of early-onset autosomal dominant AD instances include over 30 dominant mutations in the APP gene. A total of 80% of occurrences of early AD are attributed to mutations in the PSEN1 gene, while only 5% of cases are linked to mutations in the PSEN2 gene (located at 1q31-q42) [279]. Most of the mutations in the APP gene and PSEN1 increase the Aβ42 to Aβ40 ratio, either by the increased expression of Aβ42, decreased expression of Aβ40, or both. The amyloidogenic process is encouraged by this deregulation, which promotes early Aβ accumulation in the brain tissue [237]. As established by Campion et al. [280], it is thought that additional genes complementary to APP, PSEN1, and PSEN2 are implicated in the etiology of early-onset AD.
The APOE gene, found on chromosome 19, encodes the APOE protein, which is engaged in lipid metabolism [281]. The principal risk factor for late-onset AD is the ε4 allele. When ε4 is present, the probability of developing AD rises by three times in heterozygosity and by twelve times in homozygosis. On the other hand, having the ε2 allele of APOE lowers the likelihood of acquiring AD [282,283].
4.12. Environmental Risk Factors
Air pollution, diet, metals, infections, and many other environmental risk factors could cause inflammation and oxidative stress, raising the likelihood of developing AD. Hence, it is crucial to comprehend the environmental factors and how AD relates to them [284,285]. Chronic CNS infections are considered risk factors for AD because they could result in the formation of Aβ plaques and NFT. According to Miklossy and Balin’s [286] research, chronic bacterial infections have been linked to AD. Chlamydia pneumonia infection might promote late-onset AD and raise the chance of developing it by activating astrocytes and cytotoxic microglia, interrupting calcium balance, and inducing apoptosis. The investigation by Itzhaki et al. [286] revealed the presence of herpes simplex (HSV-1) viral DNA in patients who carried the ApoE-ε4 allele, suggesting an elevated risk for AD. HSV-1 could multiply in the brain, which can activate the inflammatory process and elevate Aβ accumulation, leading to neuronal damage and AD development [287].
Copper, zinc, and iron are bio-metals that have a physiological role in biological systems. On the other hand, toxicological elements have no biological purpose (e.g., lead and aluminum) [288]. When aluminum builds up in the body, it engages with proteins and provokes misfolding, agglomeration, and phosphorylation of heavily phosphorylated proteins like the tau protein, which triggers the onset of AD. Aluminum is also bound to plasma transferrin and citrate biomolecules, which could facilitate the aluminum transport to the brain [289]. Lead can quickly penetrate the BBB, affecting neuronal development and synaptic plasticity and inflicting significant damage due to enhanced β-secretase expression and Aβ build-up. Given the self-aggregation of tau and the aggregation of Aβ plaques, the water-soluble carcinogenic element cadmium can pass through the BBB and develop neurological disorders like AD [290].
By introducing different contaminants into the atmosphere, air pollution alters the composition of the atmosphere and has recently been shown to be correlated to AD as well as cardiovascular and respiratory problems. There is a connection between oxidative stress, neuroinflammation, and neurodegeneration in people subjected to air pollution. Enhanced Aβ-42 production, aggregation, and diminished cognitive functioning are all possible consequences of air pollution [291,292].
5. Conclusions
Rapid developments in cellular biology over the recent decade have been crucial to comprehending the molecular mechanisms behind AD. The molecular pathology of AD is precisely known to be complex, featuring several theories or hypotheses where countless distinctive factors interact. Intriguing research targets studies on distinct molecular mechanisms that lead to hypoxia and oxidative stress including mitochondrial and vascular pathologic conditions. The principal neuropathological indicators of AD that have been evidently explored are Aβ- plaque formation and Tau (tangles) aggregation. The existing literature suggests that we currently understand the various mechanisms responsible for the onset and progression of AD. Thus, AD could be a set of diseases with comparable APP and Tau abnormalities brought on by several mechanisms. Further research is necessary because none of these hypotheses can fully explain all aspects of the disorder on its own. Facets like the actual root cause of AD including aberrant amyloid β generation and the mechanisms by which it affects neurons are still poorly understood. This review contains good insights concerning recent progress in Aβ- and tau-associated molecular mechanisms and glial dysfunction in AD. Furthermore, risk factors associated with AD pathogenesis have been discussed and generalized. In order to establish effective therapeutic approaches for the treatment of AD where existing pharmacological therapy cannot prevent its onset and progression, new findings that aid in understanding the molecular pathogenesis of AD and its correlations are requisite.
Author Contributions
Conceptualization, Y.R.; Methodology, A.R. and Y.R.; Writing—original draft preparation, Y.R., A.R. and A.P. (Ashutosh Pareek); Writing—review and editing, Y.R., A.R., S.M., R.K., G.S., V.J. and A.P. (Aaushi Pareek); Supervision. Y.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Banasthali Vidhyapith for providing all the necessary resources for completing this report. All of the figures used in the manuscript were designed using biorender.com.
Conflicts of Interest
The authors declare no conflict of interest concerning this article’s research, authorship, and publication.
References
- de Sá-Caputo, D.D.C.; Mario Bernardo-Filho, A.S.; Taiar, R. Introductory Chapter: Neurological Disorders-Therapy Approaches; IntechOpen: London, UK, 2021; pp. 1–11. [Google Scholar]
- Soto, C. Unfolding the Role of Protein Misfolding in Neurodegenerative Diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Orr, H. The Genetics of Neurodegenerative Diseases. J. Neurochem. 2006, 97, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.; Brazier, J.; O’Cathain, A.; Lloyd-Jones, M.; Paisley, S. Quality of Life of People with Mental Health Problems: A Synthesis of Qualitative Research. Health Qual. Life Outcomes 2012, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Putera, A.M.; Irwanto, I.; Maramis, M.M.; Prasetyo, R.V.; Soemyarso, N.A.; Noer, M.S. Effect of Mental Health Problems on the Quality of Life in Children with Lupus Nephritis. Neuropsychiatr. Dis. Treat. 2020, 16, 1583–1593. [Google Scholar] [CrossRef]
- 2022 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Gauthier, S.; Webster, C.; Servaes, S.; Morais, J.A.; Rosa-Neto, P. World Alzheimer Report 2022: Life after Diagnosis: Navigating Treatment, Care and Support; Alzheimer’s Disease International: London, UK, 2022; pp. 1–416. [Google Scholar]
- Niu, H.; Alvarez-Alvarez, I.; Guillen-Grima, F.; Aguinaga-Ontoso, I. Prevalence and Incidence of Alzheimer’s Disease in Europe: A Meta-Analysis. Neurology 2017, 32, 523–532. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef] [PubMed]
- Shin, J.H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Bykowska, O.; Gontier, C.; Sax, A.L.; Jia, D.W.; Montero, M.L.; Bird, A.D.; Houghton, C.; Pfister, J.P.; Costa, R.P. Model-Based Inference of Synaptic Transmission. Front. Synaptic Neurosci. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Glasgow, S.D.; McPhedrain, R.; Madranges, J.F.; Kennedy, T.E.; Ruthazer, E.S. Approaches and Limitations in the Investigation of Synaptic Transmission and Plasticity. Front. Synaptic Neurosci. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Cotman, C.W.; McGaugh, J.L. Synaptic Transmission. Behav. Neurosci. 1980, 151–208. [Google Scholar] [CrossRef]
- Verral, A.W. Neuronal Communication. Nat. Struct. Mol. Biol. 2019, 26, 527. [Google Scholar]
- David, M. Lovinger Neurons, Receptors, Neurotransmitters, and Alcohol. Alcohol. Res. Heal 2008, 31, 197–214. [Google Scholar]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Mahoney, E.R.; Mukherjee, S.; Lee, M.L.; Bush, W.S.; Engelman, C.D.; Lu, Q.; Fardo, D.W.; Trittschuh, E.H.; Mez, J.; et al. Genetic Variants and Functional Pathways Associated with Resilience to Alzheimer’s Disease. Brain 2020, 143, 2561–2575. [Google Scholar] [CrossRef] [PubMed]
- Sengoku, R. Aging and Alzheimer’s Disease Pathology. Neuropathology 2020, 40, 22–29. [Google Scholar] [CrossRef]
- Irwin, M.R.; Vitiello, M.V. Implications of Sleep Disturbance and Inflammation for Alzheimer’s Disease Dementia. Lancet Neurol. 2019, 18, 296–306. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease: The Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Bajwa, E.; Klegeris, A. Neuroinflammation as a Mechanism Linking Hypertension with the Increased Risk of Alzheimer’s Disease. Neural Regen. Res. 2022, 17, 2342–2346. [Google Scholar]
- Vigasova, D.; Nemergut, M.; Liskova, B.; Damborsky, J. Multi-Pathogen Infections and Alzheimer’s Disease. Microb. Cell. Fact. 2021, 20, 25. [Google Scholar] [CrossRef]
- Fiore, V.; De Rosa, A.; Falasca, P.; Marci, M.; Guastamacchia, E.; Licchelli, B.; Giagulli, V.A.; De Pergola, G.; Poggi, A.; Triggiani, V. Focus on the Correlations between Alzheimer’s Disease and Type 2 Diabetes. Endocr. Metab. Immune Disord. Drug. Targets 2019, 19, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, B.R.; Brown, R.C.; Lockwood, A.H. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environ. Health Perspect. 2005, 113, 1250–1256. [Google Scholar]
- Büeler, H. Impaired Mitochondrial Dynamics and Function in the Pathogenesis of Parkinson’s Disease. Exp. Neurol. 2009, 218, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kieper, N.; Holmström, K.M.; Ciceri, D.; Fiesel, F.C.; Wolburg, H.; Ziviani, E.; Whitworth, A.J.; Martins, L.M.; Kahle, P.J.; Krüger, R. Modulation of Mitochondrial Function and Morphology by Interaction of Omi/HtrA2 with the Mitochondrial Fusion Factor OPA1. Exp. Cell. Res. 2010, 316, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Hirtz, D.G.; Thurman, D.J.; Gwinn-Hardy, K.; Zalutsky, R. How Common Are the “Common” Neurologic Disorders? Neurology 2007, 69, 410–411. [Google Scholar] [CrossRef]
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain Health Consequences of Digital Technology Use. Dialogues Clin. Neurosci. 2020, 22, 179–187. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Thakur, K.; Albanese, E.; Giannakopoulos, P. Chapter 5 Neurological Disorders Definitions, 3rd ed.; The World Bank: Washington DC, USA, 2014; pp. 1–21. [Google Scholar]
- Erickson, M.A.; Banks, W.A. Age-Associated Changes in the Immune System and Blood-Brain Barrier Functions. Int. J. Mol. Sci. 2019, 20, 1632. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s Disease. Lancet 2016, 388, 505–517. [Google Scholar]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Sidiropoulou, K.; Pissadaki, E.K.; Poirazi, P. Inside the Brain of a Neuron. EMBO Rep. 2006, 7, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Neuroscience: The Study of the Nervous System & Its Functions. Dædalus J. Am. Acad. Arts Sci. 2015, 144, 5–9. [Google Scholar]
- Nisbet, R.M.; Polanco, J.C.; Ittner, L.M.; Götz, J. Tau Aggregation and Its Interplay with Amyloid-β. Acta Neuropathol. 2015, 129, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. Waste Clearance in the Brain and Neuroinflammation: A Novel Perspective on Biomarker and Drug Target Discovery in Alzheimer’s Disease. Cells 2022, 11, 919. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Murlimanju, B.V.; Joy, T.; Krishnamurthy, A.; Agrawal, A. Hippocampus and Its Involvement in Alzheimer’s Disease: A Review. 3 Biotech. 2022, 12, 55. [Google Scholar] [CrossRef]
- McGirr, S.; Venegas, C.; Swaminathan, A. Alzheimer’s Disease: A Brief Review. Sci. Arch. 2020, 1, 89–98. [Google Scholar]
- Hohenfeld, C.; Kuhn, H.; Müller, C.; Nellessen, N.; Ketteler, S.; Heinecke, A.; Goebel, R.; Shah, N.J.; Schulz, J.B.; Reske, M.; et al. Changes in Brain Activation Related to Visuo-Spatial Memory after Real-Time FMRI Neurofeedback Training in Healthy Elderly and Alzheimer’s Disease. Behav. Brain Res. 2020, 381, 112435. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wang, Y.M.; Zha, D.J. Brain Functional and Structural Changes in Alzheimer’s Disease with Sleep Disorders: A Systematic Review. Front. Psychiatry 2021, 12, 1880. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the Global Burden of Alzheimer’s Disease. Alzheimer’s Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef]
- Zilberzwige-Tal, S.; Gazit, E. Go with the Flow- Microfluidics Approaches for Amyloid Research. Chem. An. Asian J. 2018, 13, 3437–3447. [Google Scholar] [CrossRef]
- Maccioni, R.B.; González, A.; Andrade, V.; Cortés, N.; Tapia, J.P.; Guzmán-Martínez, L. Alzheimer’s Disease in the Perspective of Neuroimmunology. Open. Neurol. J. 2018, 12, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lutz, M.W.; Xing, Y. A Systems-Based Model of Alzheimer’s Disease. Alzheimer’s Dement. 2019, 15, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Aminoff, M.J.; Roger, P.S. Clinical Neurology, 5th ed.; McGraw Hill: New York, NY, USA, 2002; Volume 139, pp. 1–236. [Google Scholar]
- Schweighauser, M.; Arseni, D.; Bacioglu, M.; Huang, M.; Lövestam, S.; Shi, Y.; Yang, Y.; Zhang, W.; Kotecha, A.; Garringer, H.J.; et al. Age-Dependent Formation of TMEM106B Amyloid Filaments in Human Brains. Nature 2022, 605, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Duff, K.; Hardy, K.G.; Perez-Tur, J.; Hutton, M. Genetic Dissection of Alzheimer’s Disease and Related Dementias: Amyloid and Its Relationship to Tau. Nat. Neurosci. 1998, 1, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid- β Pathway in Alzheimer’s Disease. Mol. Pscychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Westermark, P.; Benson, M.D.; Buxbaum, J.N.; Cohen, A.S.; Frangione, B.; Ikeda, S.I.; Masters, C.L.; Merlini, G.; Saraiva, M.J.; Sipe, J.D. A Primer of Amyloid Nomenclature. Amyloid 2007, 14, 179–183. [Google Scholar] [CrossRef]
- Glenner, G.G.; Caine, W. Wong Alzheimer’s Disease: Initial Report of the Purification and Characterization of a Novel Cerebrovascular Amyloid Protein. Biochem. Biophys. Res. Commun. 2012, 120, 885–890. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Tewin, T. Amyloidosis in Alzheimer’s Disease: The Toxicity of Amyloid Beta (Aβ), Mechanisms of Its Accumulation and Implications of Medicinal Plants for Therapy. Evid. Based Complement. Altern. Med. 2013, 2013, 413808. [Google Scholar] [CrossRef]
- Merlini, G.; Bellotti, V. Molecular Mechanisms of Amyloidosis. N. Engl. J. Med. 2003, 349, 583–596. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Wong, P.C. Amyloid Precursor Protein Processing and Alzheimer’s Disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s Disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Nalivaeva, N.N.; Turner, A.J. The Amyloid Precursor Protein: A Biochemical Enigma in Brain Development, Function and Disease. FEBS Lett. 2013, 587, 2046–2054. [Google Scholar] [CrossRef]
- Sadleir, K.R.; Kandalepas, P.C.; Buggia-Prévot, V.; Nicholson, D.A.; Thinakaran, G.; Vassar, R. Presynaptic Dystrophic Neurites Surrounding Amyloid Plaques Are Sites of Microtubule Disruption, BACE1 Elevation, and Increased Aβ Generation in Alzheimer’s Disease. Acta Neuropathol. 2016, 132, 235–256. [Google Scholar] [CrossRef]
- Rice, H.C.; De Malmazet, D.; Schreurs, A.; Frere, S.; Van Molle, I.; Volkov, A.N.; Creemers, E.; Vertkin, I.; Nys, J.; Ranaivoson, F.M.; et al. Secreted Amyloid-b Precursor Protein Functions as a GABA B R1a Ligand to Modulate Synaptic Transmission. Science 2019, 363, eaao4827. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid Precursor Protein Trafficking, Processing, and Function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Storey, E.; Cappai, R. The Amyloid Precursor Protein of Alzheimer’s Disease and the Aβ Peptide. Neuropathol. Appl. Neurobiol. 1999, 25, 81–97. [Google Scholar] [CrossRef]
- Rogaev, E.I. Genetic Factors and a Polygenic Model of Alzheimer’s Disease. Genetika 1999, 35, 1558–1571. [Google Scholar]
- Devkota, S.; Williams, T.D.; Wolfe, M.S. Familial Alzheimer s Disease Mutations in Amyloid Protein Precursor Alter Proteolysis by γ-Secretase to Increase Amyloid β-Peptides of ≥45 Residues. J. Biol. Chem. 2021, 296, 100281. [Google Scholar] [CrossRef]
- Bibl, M.; Mollenhauer, B.; Esselmann, H.; Lewczuk, P.; Klafki, H.W.; Sparbier, K.; Smirnov, A.; Cepek, L.; Trenkwalder, C.; Rüther, E.; et al. CSF Amyloid-β-Peptides in Alzheimer’s Disease, Dementia with Lewy Bodies and Parkinson’s Disease Dementia. Brain 2006, 129, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Bibl, M.; Mollenhauer, B.; Lewczuk, P.; Esselmann, H.; Wolf, S.; Trenkwalder, C.; Otto, M.; Stiens, G.; Rüther, E.; Kornhuber, J.; et al. Validation of Amyloid-β Peptides in CSF Diagnosis of Neurodegenerative Dementias. Mol. Psychiatry 2007, 12, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Odaka, A.; Suzuki, N.; Mizusawa, H.; Nukina, N.; Ihara, Y. Visualization of Aβ42(43) and Aβ40 in Senile Plaques with End-Specific Aβ Monoclonals: Evidence That an Initially Deposited Species Is Aβ42(43). Neuron 1994, 13, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.; Gokce, O.; Luthi-Carter, R.; Lashuel, H.A. The Ratio of Monomeric to Aggregated Forms of Aβ40 and Aβ42 Is an Important Determinant of Amyloid-β Aggregation, Fibrillogenesis, and Toxicity. J. Biol. Chem. 2008, 283, 28176–28189. [Google Scholar] [CrossRef]
- Welge, V.; Fiege, O.; Lewczuk, P.; Mollenhauer, B.; Esselmann, H.; Klafki, H.W.; Wolf, S.; Trenkwalder, C.; Otto, M.; Kornhuber, J.; et al. Combined CSF Tau, p-Tau181 and Amyloid-β 38/40/42 for Diagnosing Alzheimer’s Disease. J. Neural Transm. 2009, 116, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, E.; Londos, E.; Ballard, C.; Alves, G.; Zetterberg, H.; Blennow, K.; Skogseth, R.; Minthon, L.; Aarsland, D. CSF Amyloid Β38 as a Novel Diagnostic Marker for Dementia with Lewy Bodies. J. Neurol. Neurosurg. Psychiatry 2011, 82, 160–164. [Google Scholar] [CrossRef]
- Tang, W.; Huang, Q.; Wang, Y.; Wang, Z.Y.; Yao, Y.Y. Assessment of CSF Aβ42 as an Aid to Discriminating Alzheimer’s Disease from Other Dementias and Mild Cognitive Impairment: A Meta-Analysis of 50 Studies. J. Neurol. Sci. 2014, 345, 26–36. [Google Scholar] [CrossRef]
- Jonsson, T.; Atwal, J.K.; Steinberg, S.; Snaedal, J.; Jonsson, P.V.; Bjornsson, S.; Stefansson, H.; Sulem, P.; Gudbjartsson, D.; Maloney, J.; et al. A Mutation in APP Protects against Alzheimer‘s Disease and Age-Related Cognitive Decline. Nature 2012, 488, 96–99. [Google Scholar] [CrossRef]
- Selkoe, D.J. Clearing the Brain’s Amyloid Cobwebs. Neuron 2001, 32, 177–180. [Google Scholar] [CrossRef]
- Deane, R.; Yan, S.D.; Submamaryan, R.K.; LaRue, B.; Jovanovic, S.; Hogg, E.; Welch, D.; Manness, L.; Lin, C.; Yu, J.; et al. RAGE Mediates Amyloid-β Peptide Transport across the Blood-Brain Barrier and Accumulation in Brain. Nat. Med. 2003, 9, 907–913. [Google Scholar] [CrossRef]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of Amyloid-? Peptide Across the Blood-Brain Barrier: Implication for Therapies in Alzheimer’s Disease. CNS Neurlog. Disord. 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Levin, O.S.; Vasenina, E.E. Twenty-Five Years of the Amyloid Hypothesis of Alzheimer’s Disease: Advances, Failures and New Perspectives. Zhurnal Nevrol. i Psihiatr. Im. S.S. Korsakova 2016, 116, 3–9. [Google Scholar]
- Fukumoto, H.; Tomita, T.; Matsunaga, H.; Ishibashi, Y.; Saido, T.; Iwatsubo, T. Primary Cultures of Neuronal and Non-Neuronal Rat Brain Cells Secrete Similar Proportions of Amyloid β Peptides Ending at Aβ40 and Aβ42: Neuroreport. Neuroreport 1999, 10, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Tsitsopoulos, P.P.; Marklund, N. Amyloid-ß Peptides and Tau Protein as Biomarkers in Cerebrospinal and Interstitial Fluid Following Traumatic Brain Injury: A Review of Experimental and Clinical Studies. Front. Neurol. 2013, 4, 1–17. [Google Scholar] [CrossRef]
- Maltsev, A.V.; Santockyte, R.; Bystryak, S.; Galzitskaya, O.V. Activation of Neuronal Defense Mechanisms in Response to Pathogenic Factors Triggering Induction of Amyloidosis in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 40, 19–32. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-Β-Induced Neuronal Dysfunction in Alzheimer’s Disease: From Synapses toward Neural Networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Lao, K.; Zhang, R.; Dai, Y.; Luan, J.; Guo, N.; Xu, X.; Zhang, Y.; Gou, X. Identification of Novel Aβ-LilrB2 Inhibitors as Potential Therapeutic Agents for Alzheimer’s Disease. Mol. Cell. Neurosci. 2021, 114, 103630. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Song, Y.; Li, H.; Wu, Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 43. [Google Scholar]
- Zhang, Y.; Zhao, Y.; Zhang, L.; Yu, W.; Wang, Y.; Chang, W. Cellular Prion Protein as a Receptor of Toxic Amyloid-Β42 Oligomers Is Important for Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 1–9. [Google Scholar] [CrossRef]
- Cissé, M.; Halabisky, B.; Harris, J.; Devidze, N.; Dubal, D.B.; Sun, B.; Orr, A.; Lotz, G.; Kim, D.H.; Hamto, P.; et al. Reversing EphB2 Depletion Rescues Cognitive Functions in Alzheimer Model. Nature 2011, 469, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.M.; Cerpa, W.; Muñoz, F.J.; Zanlungo, S.; Alvarez, A.R. Amyloid-β Oligomers Synaptotoxicity: The Emerging Role of EphA4/c-Abl Signaling in Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Umikawa, M.; Cui, C.; Li, J.; Chen, X.; Zhang, C.; Hyunh, H.; Kang, X.; Silvany, R.; Wan, X.; et al. Inhibitory Receptors Bind Angptls and Support Blood Stem Cells and Leukemia Development. Nature 2012, 485, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Vidal, G.S.; Djurisic, M.; William, C.M.; Michael, E.; Garcia, K.C.; Hyman, B.T.; Shatz, C.J.; Biology, D. Human LilrB2 Is a β-Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer’s Model. Science 2013, 341, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Shin, W.S.; Chan, H.; Vuong, C.K.; Dubois, B.; Li, B.; Murray, K.A.; Sawaya, M.R.; Feigon, J.; Black, D.L.; et al. Inhibiting Amyloid-ß Cytotoxicity through Its Interaction with the Cell Surface Receptor LilrB2 by Structure-Based Design. Nat. Chem. 2018, 10, 1213–1221. [Google Scholar] [CrossRef]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA Receptor Subunits: Diversity, Development and Disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Köhr, G. NMDA Receptor Function: Subunit Composition versus Spatial Distribution. Cell. Tissue Res. 2006, 326, 439–446. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-Aspartate Receptor-Dependent Mechanism That Is Blocked by the Alzheimer Drug Memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef]
- Parameshwaran, K.; Dhanasekaran, M.; Suppiramaniam, V. Amyloid Beta Peptides and Glutamatergic Synaptic Dysregulation. Exp. Neurol. 2008, 210, 7–13. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.G. Alzheimer’s Disease, β-Amyloid, Glutamate, NMDA Receptors and Memantine—Searching for the Connections. Br. J. Pharmacol. 2012, 167, 324–352. [Google Scholar] [CrossRef]
- Li, S.; Hong, S.; Shepardson, N.E.; Walsh, D.M.; Shankar, G.M.; Selkoe, D. Soluble Oligomers of Amyloid β Protein Facilitate Hippocampal Long-Term Depression by Disrupting Neuronal Glutamate Uptake. Neuron 2009, 62, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid β-Protein Dimers Isolated Directly from Alzheimer Brains Impair Synaptic Plasticity and Memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.M.; Nong, Y.; Almeida, C.G.; Paul, S.; Moran, T.; Choi, E.Y.; Nairn, A.C.; Salter, M.W.; Lombroso, P.J.; Gouras, G.K.; et al. Regulation of NMDA Receptor Trafficking by Amyloid-β. Nat. Neurosci. 2005, 8, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, P.; Feng, J.; Wu, M. Dysfunction of NMDA Receptors in Alzheimer’s Disease. Neurol. Sci. 2016, 37, 1039–1047. [Google Scholar] [CrossRef]
- Pichon, C.E.L.; Valley, M.T.; Polymenidou, M.; Chesler, A.T.; Sagdullaev, B.T.; Aguzzi, A.; Firestein, S. Olfactory Behavior and Physiology Are Disrupted in Prion Protein Knockout Mice. Nat. Neurosci. 2009, 12, 60–69. [Google Scholar] [CrossRef]
- Rial, D.; Piermartiri, T.C.; Duarte, F.S.; Tasca, C.I.; Walz, R.; Prediger, R.D. Overexpression of Cellular Prion Protein (PrPC) Prevents Cognitive Dysfunction and Apoptotic Neuronal Cell Death Induced by Amyloid-β (Aβ 1-40) Administration in Mice. Neuroscience 2012, 215, 79–89. [Google Scholar] [CrossRef]
- Zou, W.Q.; Zhou, X.; Yuan, J.; Xiao, X. Insoluble Cellular Prion Protein and Its Association with Prion and Alzheimer Diseases. Prion 2011, 5, 172–178. [Google Scholar] [CrossRef]
- Linden, R.; Martins, V.R.; Prado, M.A.M.; Cammarota, M.; Izquierdo, I.; Brentani, R.R. Physiology of the Prion Protein. Physiol. Rev. 2008, 88, 673–728. [Google Scholar] [CrossRef]
- Laurén, J.; David, A.G.; Nygaard, H.B.; Gilbert, J.W.; Strittmatter, S.M. Cellular Prion Protein Mediates Impairment of Synaptic Plasticity by Amyloid-β Oligomers. Nature 2009, 457, 1128–1132. [Google Scholar] [CrossRef]
- Kostylev, M.A.; Kaufman, A.C.; Nygaard, H.B.; Patel, P.; Haas, L.T.; Gunther, E.C.; Vortmeyer, A.; Strittmatter, S.M. Prion-Protein-Interacting Amyloid-β Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. J. Biol. Chem. 2015, 290, 17415–17438. [Google Scholar] [CrossRef]
- Gimbel, D.A.; Nygaard, H.B.; Coffey, E.E.; Gunther, E.C.; Laurén, J.; Gimbel, Z.A.; Strittmatter, S.M. Memory Impairment in Transgenic Alzheimer Mice Requires Cellular Prion Protein. J. Neurosci. 2010, 30, 6367–6374. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, A.J.; Panico, S.; Freir, D.B.; Wright, D.; Terry, C.; Risse, E.; Herron, C.E.; Malley, T.O.; Wadsworth, J.D.F.; Farrow, M.A.; et al. Amyloid-β Nanotubes Are Associated with Prion Protein-Dependent Synaptotoxicity. Nat. Commun. 2013, 4, 2416. [Google Scholar] [CrossRef] [PubMed]
- Klyubin, I.; Nicoll, A.J.; Khalili-Shirazi, A.; Farmer, M.; Canning, S.; Mably, A.; Linehan, J.; Brown, A.; Wakeling, M.; Brandner, S.; et al. Peripheral Administration of a Humanized Anti-PrP Antibody Blocks Alzheimer’s Disease Aβ Synaptotoxicity. J. Neurosci. 2014, 34, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Kullander, K.; Klein, R. Mechanisms and Functions of Eph and Ephrin Signalling. Nat. Rev. Mol. Cell. Biol. 2002, 3, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Poliakov, A.; Cotrina, M.; Wilkinson, D.G. Diverse Roles of Eph Receptors and Ephrins in the Regulation of Cell Migration and Tissue Assembly. Dev. Cell. 2004, 7, 465–480. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Pasquale, E.B. Eph Receptors in the Adult Brain. Curr. Opin. Neurobiol. 2004, 14, 288–296. [Google Scholar] [CrossRef]
- Attwood, B.K.; Patel, S.; Pawlak, R. Ephs and Ephrins: Emerging Therapeutic Targets in Neuropathology. Int. J. Biochem. Cell. Biol. 2012, 44, 578–581. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Aβ Oligomer-Induced Aberrations in Synapse Composition, Shape, and Density Provide a Molecular Basis for Loss of Connectivity in Alzheimer’s Disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kim, D.; Knox, J.A.; Johnson, E.; Mucke, L. Increasing the Receptor Tyrosine Kinase EphB2 Prevents Amyloid-β-Induced Depletion of Cell Surface Glutamate Receptors by a Mechanism That Requires the PDZ-Binding Motif of EphB2 and Neuronal Activity. J. Biol. Chem. 2016, 291, 1719–1734. [Google Scholar] [CrossRef]
- Murai, K.K.; Nguyen, L.N.; Irie, F.; Yu, Y.; Pasquale, E.B. Control of Hippocampal Dendritic Spine Morphology through Ephrin-A3/EphA4 Signaling. Nat. Neurosci. 2003, 6, 153–160. [Google Scholar] [CrossRef]
- Rosenberger, A.F.N.; Rozemuller, A.J.M.; van der Flier, W.M.; Scheltens, P.; van der Vies, S.M.; Hoozemans, J.J.M. Altered Distribution of the EphA4 Kinase in Hippocampal Brain Tissue of Patients with Alzheimer’s Disease Correlates with Pathology. Acta Neuropathol. Commun. 2014, 2, 79. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Zhao, Y.; Jiang, L.L.; Li, X.; Liu, Y.; Sun, Y.; Piña-Crespo, J.C.; Zhu, B.; Masliah, E.; Willnow, T.E.; et al. SORLA Attenuates EphA4 Signaling and Amyloid β-Induced Neurodegeneration. J. Exp. Med. 2017, 214, 3669–3685. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.K.Y.; Hung, K.W.; Huang, H.; Gu, S.; Shen, Y.; Cheng, E.Y.L.; Ip, F.C.F.; Huang, X.; Fu, W.Y.; Ip, N.Y. Blockade of EphA4 Signaling Ameliorates Hippocampal Synaptic Dysfunctions in Mouse Models of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2014, 111, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Lamberto, I.; Qin, H.; Roberta Noberini, L.P.; Bourgin, C.; Riedl, S.J.; Song, J.; Pasquale, E.B. Distinctive Binding of Three Antagonistic Peptides to the Ephrin- Binding Pocket of the EphA4 Receptor. Biochem. J. 2012, 445, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.L.; Harris, P.; Kosik, K.S.; Kurnit, D.M.; Donlon, T.A. Identification of CDNA Clones for the Human Microtubule-Associated Protein Tau and Chromosomal Localization of the Genes for Tau and Microtubule-Associated Protein 2. Mol. Brain Res. 1986, 1, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Jakes, R.; Rutherford, D.; Crowther, R.A. Multiple Isoforms of Human Microtubule-Associated Protein Tau: Sequences and Localization in Neurofibrillary Tangles of Alzheimer’s Disease. Neuron 1989, 3, 519–526. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Potier, M.C.; Ulrich, J.; Crowther, R.A. Cloning and Sequencing of the CDNA Encoding an Isoform of Microtubule-Associated Protein Tau Containing Four Tandem Repeats: Differential Expression of Tau Protein MRNAs in Human Brain. EMBO J. 1989, 8, 393–399. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 1–14. [Google Scholar] [CrossRef]
- Johnson, G.V.W.; Stoothoff, W.H. Tau Phosphorylation in Neuronal Cell Function and Dysfunction. J. Cell. Sci. 2004, 117, 5721–5729. [Google Scholar] [CrossRef]
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and Sequencing of the CDNA Encoding a Core Protein of the Paired Helical Filament of Alzheimer Disease: Identification as the Microtubule-Associated Protein Tau. Proc. Nati. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef]
- Dubey, J.; Ratnakaran, N.; Koushika, S.P. Neurodegeneration and Microtubule Dynamics: Death by a Thousand Cuts. Front. Cell. Neurosci. 2015, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Lindwall, G.; Cole, R.D. Phosphorylation Affects the Ability of Tau Protein to Promote Microtubule Assembly. J. Biol. Chem. 1984, 259, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Mandelkow, E.M.; Biernat, J.; Drewes, G.; Gustke, N.; Trinczek, B.; Mandelkow, E. Tau Domains, Phosphorylation, and Interactions with Microtubules. Neurobiol. Aging 1995, 16, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.; Frey, T.; Zeeberg, B.; Dalldorf, F.; Caplow, M. Inhibition of Microtubule Assembly by Phosphorylation of Microtubule-Associated Proteins. Biochemistry 1980, 19, 2472–2479. [Google Scholar] [CrossRef]
- Iqbal, K.; Zaidi, T.; Wen, G.Y.; Grundke-Iqbal, I.; Merz, P.A.; Shaikh, S.S.; Wisniewski, H.M.; Alafuzoff, I.; Winblad, B. Defective Brain Microtubule Assembly in Alzheimer’S Disease. Lancet 1986, 328, 421–426. [Google Scholar] [CrossRef]
- Alonso, A.C.; Grundke-Iqbal, I.; Iqbal, K. Alzheimer’s Disease Hyperphosphorylated Tau Sequesters Normal Tau into Tangles of Filaments and Disassembles Microtubules. Nat. Med. 1996, 2, 783–787. [Google Scholar] [CrossRef]
- Bancher, C.; Brunner, C.; Lassmann, H.; Budka, H.; Jellinger, K.; Wiche, G.; Seitelberger, F.; Grundke-Iqbal, I.; Iqbal, K.; Wisniewski, H.M. Accumulation of Abnormally Phosphorylated τ Precedes the Formation of Neurofibrillary Tangles in Alzheimer’s Disease. Brain Res. 1989, 477, 90–99. [Google Scholar] [CrossRef]
- Jouanne, M.; Rault, S.; Voisin-Chiret, A.S. Tau Protein Aggregation in Alzheimer’s Disease: An Attractive Target for the Development of Novel Therapeutic Agents. Eur. J. Med. Chem. 2017, 139, 153–167. [Google Scholar] [CrossRef]
- Hill, E.; Wall, M.J.; Moffat, K.G.; Karikari, T.K. Understanding the Pathophysiological Actions of Tau Oligomers: A Critical Review of Current Electrophysiological Approaches. Front. Mol. Neurosci. 2020, 13, 155. [Google Scholar] [CrossRef]
- Miao, J.; Shi, R.; Li, L.; Chen, F.; Zhou, Y.; Tung, Y.C.; Hu, W.; Gong, C.X.; Iqbal, K.; Liu, F. Pathological Tau From Alzheimer’s Brain Induces Site-Specific Hyperphosphorylation and SDS- and Reducing Agent-Resistant Aggregation of Tau in Vivo. Front. Aging Neurosci. 2019, 11, 34. [Google Scholar] [CrossRef]
- Medina, M.; Avila, J. The Role of Extracellular Tau in the Spreading of Neurofibrillary Pathology. Front. Cell. Neurosci. 2014, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, S.S.; Guerrero-Muñoz, M.J.; Castillo-Carranza, D.L. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front. Aging Neurosci. 2017, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Himmelstein, D.S.; Lancia, J.K.; Binder, L.I. Tau Oligomers and Tau Toxicity in Neurodegenerative Disease. Biochem. Soc. Trans. 2012, 40, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.M.; Mikulskis, A.; et al. Current State of Alzheimer’s Fluid Biomarkers. Acta Neuropathol. 2018, 136, 821–853. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Lelental, N.; Lachmann, I.; Holzer, M.; Flach, K.; Brandner, S.; Engelborghs, S.; Teunissen, C.E.; Zetterberg, H.; Molinuevo, J.L.; et al. Non-Phosphorylated Tau as a Potential Biomarker of Alzheimer’s Disease: Analytical and Diagnostic Characterization. J. Alzheimer’s Dis. 2017, 55, 159–170. [Google Scholar] [CrossRef]
- Hugon, J.; Mouton-Liger, F.; Cognat, E.; Dumurgier, J.; Paquet, C. Blood-Based Kinase Assessments in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 338. [Google Scholar] [CrossRef]
- Castro-Alvarez, J.F.; Alejandro Uribe-Arias, S.; Kosik, K.S.; Cardona-Gómez, G.P. Long- and Short-Term CDK5 Knockdown Prevents Spatial Memory Dysfunction and Tau Pathology of Triple Transgenic Alzheimer’s Mice. Front. Aging Neurosci. 2014, 6, 243. [Google Scholar] [CrossRef]
- Kimura, T.; Tsutsumi, K.; Taoka, M.; Saito, T.; Masuda-Suzukake, M.; Ishiguro, K.; Plattner, F.; Uchida, T.; Isobe, T.; Hasegawa, M.; et al. Isomerase Pin1 Stimulates Dephosphorylation of Tau Protein at Cyclin-Dependent Kinase (Cdk5)-Dependent Alzheimer Phosphorylation Sites. J. Biol. Chem. 2013, 288, 7968–7977. [Google Scholar] [CrossRef]
- Gómez-Isla, T.; Hollister, R.; West, H.; Mui, S.; Growdon, J.H.; Petersen, R.C.; Parisi, J.E.; Hyman, B.T. Neuronal Loss Correlates with but Exceeds Newofibriiary Tangles in Alzheimer’s Disease. Ann. Neurol. 1997, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. Imaging the Progression of Alzheimer Pathology through the Brain. Proc. Natl. Acad. Sci. USA 2002, 99, 4135–4137. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.I.; Schott, J.M.; Stevens, J.M.; Rossor, M.N.; Fox, N.C. Mapping the Evolution of Regional Atrophy in Alzheimer’s Disease: Unbiased Analysis of Fluid-Registered Serial MRI. Proc. Natl. Acad. Sci. USA 2002, 99, 4703–4707. [Google Scholar] [CrossRef] [PubMed]
- Nestor, S.M.; Rupsingh, R.; Borrie, M.; Smith, M.; Accomazzi, V.; Wells, J.L.; Fogarty, J.; Bartha, R. Ventricular Enlargement as a Possible Measure of Alzheimer’s Disease Progression Validated Using the Alzheimer’s Disease Neuroimaging Initiative Database. Brain 2008, 131, 2443–2454. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.Y.; Toga, A.W.; Thompson, P.M. Hippocampal Atrophy and Ventricular Enlargement in Normal Aging, Mild Cognitive Impairment (MCI), and Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, N.; Leon, J.; Sheng, Z.; Oka, S.; Hamasaki, H.; Iwaki, T.; Nakabeppu, Y. Molecular Pathophysiology of Impaired Glucose Metabolism, Mitochondrial Dysfunction, and Oxidative DNA Damage in Alzheimer’s Disease Brain. Mech. Ageing Dev. 2017, 161, 95–104. [Google Scholar] [CrossRef]
- Wang, X.; Su, B.; Lee, H.G.; Li, X.; Perry, G.; Smith, M.A.; Zhu, X. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer’s Disease. J. Neurosci. 2009, 29, 9090–9103. [Google Scholar] [CrossRef]
- Ebenezer, P.J.; Weidner, A.M.; Levine, H.; Markesbery, W.R.; Murphy, M.P.; Zhang, L.; Dasuri, K.; Fernandez-Kim, S.O.K.; Bruce-Keller, A.J.; Gavilán, E.; et al. Neuron Specific Toxicity of Oligomeric Amyloid-β: Role for JUN-Kinase and Oxidative Stress. J. Alzheimer’s Dis. 2010, 22, 839–848. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Bader Lange, M.L.; Sultana, R. Involvements of the Lipid Peroxidation Product, HNE, in the Pathogenesis and Progression of Alzheimer’s Disease. Biochim. Biophys. Acta 2010, 1801, 924–929. [Google Scholar] [CrossRef]
- Wanga, X.; Wanga, W.; Lia, L.; Perryb, G.; Leea, H.; Zhu, X. Oxidative Stress and Mitochondrial Dysfunction in Alzheimer’s Disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial Dysfunction Is a Trigger of Alzheimer’s Disease Pathophysiology. Biochim. Biophys. Acta 2009, 1802, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.W.; Chen, Y.; Huang, X.; Zhou, F.; Wang, W.; Xian, B.; Zhang, X.; Masliah, E.; Chen, Q.; et al. Appoptosin Is a Novel Pro-Apoptotic Protein and Mediates Cell Death in Neurodegeneration. J. Neurosci. 2012, 32, 15565–15576. [Google Scholar] [CrossRef] [PubMed]
- Lustbader, J.W.; Cirilli, M.; Lin, C.; Xu, H.W.; Takuma, K.; Wang, N.; Caspersen, C.; Chen, X.; Pollak, S.; Chaney, M.; et al. ABAD Directly Links Aβ to Mitochondrial Toxicity in Alzheimer’s Disease. Science 2004, 304, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Guo, L.; Fang, F.; Chen, D.; Sosunov, A.A.; McKhann, G.M.; Yan, Y.; Wang, C.; Zhang, H.; Molkentin, J.D.; et al. Cyclophilin D Deficiency Attenuates Mitochondrial and Neuronal Perturbation and Ameliorates Learning and Memory in Alzheimer’s Disease. Nat. Med. 2008, 14, 1097–1105. [Google Scholar] [CrossRef]
- Du, H.; Guo, L.; Zhang, W.; Rydzewska, M.; Yan, S. Cyclophilin D Deficiency Improves Mitochondrial Function and Learning/Memory in Aging Alzheimer Disease Mouse Model. Neurobiol. Aging 2011, 32, 398–406. [Google Scholar] [CrossRef]
- Okamoto, S.; Nakamura, T.; Cieplak, P.; Chan, S.F.; Liao, L.; Saleem, S.; Han, X.; Clemente, A.; Sances, S.; Brechtel, C.; et al. S-Nitrosylation—Mediated Redox Transcriptional Switch Modulates Neurogenesis and Neuronal Cell Death. Cell. Rep. 2014, 8, 217–228. [Google Scholar] [CrossRef]
- Medeiros, R.; Prediger, R.D.S.; Passos, G.F.; Pandolfo, P.; Duarte, F.S.; Franco, J.L.; Dafre, A.L.; Di Giunta, G.; Figueiredo, C.P.; Takahashi, R.N.; et al. Connecting TNF-α Signaling Pathways to INOS Expression in a Mouse Model of Alzheimer’s Disease: Relevance for the Behavioral and Synaptic Deficits Induced by Amyloid β Protein. J. Neurosci. 2007, 27, 5394–5404. [Google Scholar] [CrossRef]
- Cho, D.-H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 Mediates β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Qu, J.; Nakamura, T.; Cao, G.; Holland, E.A.; McKercher, S.R.; Lipton, S.A. S-Nitrosylation Activates Cdk5 and Contributes to Synaptic Spine Loss Induced by β-Amyloid Peptide. Proc. Natl. Acad. Sci. USA 2011, 108, 14330–14335. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, R.; Oellerich, M.; Ahmed, N.; Asif, A.R. Differential S-Nitrosylation of Proteins in Alzheimer’s Disease. Neuroscience 2014, 256, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Estimation of Peroxidative Damage. A Critical Review. Free. Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M.A.; et al. Β-site APP Cleaving Enzyme Up-regulation Induced by 4-hydroxynonenal Is Mediated by Stress-Activated Protein Kinases Pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Mitsugu, F.; Fumihisa, K.; Nobuko, S.; Motoji, S.; Yuko, S.; Shigeo, M.; Masakatsu, H.; Naoki, M.; Akihito, I. Elevated Levels of 4-Hydroxynonenal-Histidine Michael Adduct in the Hippo- Campi of Patients with Alzheimer’s Disease. Biomed. Res. 2009, 30, 227–233. [Google Scholar]
- Blanc, E.M.; Kelly, J.F.; Mark, R.J.; Waeg, G.; Mattson, M.P. Sanders-Brown 4-Hydroxynonenal an Aldehydic Product of Lipid Peroxidation Impairs Signal Transduction Associated with Muscarinic Acetylcholine and Metabotropic Glutamate Receptors: Possible Action on G Alpha/11. J. Neurochem. 1997, 69, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Lauderback, C.M.; Hackett, J.M.; Huang, F.F.; Keller, J.N.; Szweda, L.I.; Markesbery, W.R.; Butterfield, D.A. The Glial Glutamate Transporter GLT-1 Is Oxidatively Modified by 4-Hydroxy-2-Nonenal in the Alzheimer’s Disease Brain: The Role of Aβ1-42. J. Neurochem. 2001, 78, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein Carbonylation in Human Diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein Carbonyl Groups as Biomarkers of Oxidative Stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Bota, D.A.; Ngo, J.K.; Davies, K.J.A. Downregulation of the Human Lon Protease Impairs Mitochondrial Structure and Function and Causes Cell Death. Free. Radic. Biol. Med. 2005, 38, 665–677. [Google Scholar] [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M.; et al. Brain Regional Correspondence Between Alzheimer’s Disease Histopathology and Biomarkers of Protein Oxidation. J. Neurochem. 1995, 65, 2146–2156. [Google Scholar] [CrossRef]
- Castegna, A.; Aksenov, M.; Thongboonkerd, V.; Klein, J.B.; Pierce, W.M.; Booze, R.; Markesbery, W.R.; Butterfield, D.A. Proteomic Identification of Oxidatively Modified Proteins in Alzheimer’s Disease Brain. Part II: Dihydropyrimidinase-Related Protein 2, α-Enolase and Heat Shock Cognate 71. J. Neurochem. 2002, 82, 1524–1532. [Google Scholar] [CrossRef]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA Damage Is More Extensive and Persists Longer than Nuclear DNA Damage in Human Cells Following Oxidative Stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative Damage to Mitochondrial DNA Is Increased in Alzheimer’s Disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Mullaart, E.; Boerrigter, M.E.T.I.; Ravid, R.; Swaab, D.F.; Vijg, J. Increased Levels of DNA Breaks in Cerebral Cortex of Alzheimer’s Disease Patients. Neurobiol. Aging 1990, 11, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Jacoba, K.D.; Hootena, N.N.; Tadokorob, T.; Lohania, A.; Barnesa, J.; Evans, M.K. Alzheimer’s Disease Associated Polymorphisms in Human OGG1 Alter Catalytic Activity and Sensitize Cells to DNA Damage. Free Radic. Biol. Med. 2013, 63, 115–125. [Google Scholar] [CrossRef]
- Mao, G.; Pan, X.; Zhu, B.B.; Zhang, Y.; Yuan, F.; Huang, J.; Lovell, M.A.; Lee, M.P.; Markesbery, W.R.; Li, G.M.; et al. Identification and Characterization of OGG1 Mutations in Patients with Alzheimer’s Disease. Nucleic Acids Res. 2007, 35, 2759–2766. [Google Scholar] [CrossRef]
- Jin, X.; Yamashita, T. Microglia in Central Nervous System Repair after Injury. J. Biochem. 2016, 159, 491–496. [Google Scholar] [CrossRef]
- Imai, F.; Suzuki, H.; Oda, J.; Ninomiya, T.; Ono, K.; Sano, H.; Sawada, M. Neuroprotective Effect of Exogenous Microglia in Global Brain Ischemia. J. Cereb. Blood Flow. Metab. 2007, 27, 488–500. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Lindholm, K.; Konishi, Y.; Yue, X.; Hampel, H.; Zhang, D.; Shen, Y. Tumor Necrosis Factor Death Receptor Signaling Cascade Is Required for Amyloid-β Protein-Induced Neuron Death. J. Neurosci. 2004, 24, 1760–1771. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Ortiz, S.; Molina-Holgado, F.; Guaza, C. Induction of COX-2 and PGE2 Biosynthesis by IL-1β Is Mediated by PKC and Mitogen-Activated Protein Kinases in Murine Astrocytes. Br. J. Pharmacol. 2000, 131, 152–159. [Google Scholar] [CrossRef]
- Pan, X.; Zhu, Y.; Lin, N.; Zhang, J.; Ye, Q.; Huang, H.; Chen, X. Microglial Phagocytosis Induced by Fibrillar β-Amyloid Is Attenuated by Oligomeric β-Amyloid: Implications for Alzheimer’s Disease. Mol. Neurodegenrat. 2011, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M. V Astrocyte Barriers to Neurotoxic Inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Mielke, M.L.; Gómez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive Glia Not Only Associates with Plaques but Also Parallels Tangles in Alzheimer’s Disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R. The Role of Astrocytes in Amyloid β-Protein Toxicity and Clearance. Exp. Neurol. 2012, 236, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Ince, P.G.; Minett, T.; Garwood, C.J.; Shaw, P.J.; Matthews, F.E.; Brayne, C.; Simpson, J.E.; Wharton, S.B. A Reduced Astrocyte Response to β-Amyloid Plaques in the Ageing Brain Associates with Cognitive Impairment. PLoS ONE 2015, 10, e0118463. [Google Scholar] [CrossRef]
- Cregg, J.M.; DePaul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional Regeneration Beyond the Glial Scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar] [CrossRef]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.R.; Schousboe, A.; Haydon, P.G.; Jr, R.F.S.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial Cells in (Patho)Physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef]
- Scimemi, A.; Meabon, J.S.; Woltjer, R.L.; Sullivan, J.M.; Diamond, J.S.; Cook, D.G. Amyloid-Β1-42 Slows Clearance of Synaptically Released Glutamate by Mislocalizing Astrocytic GLT-1. J. Neurosci. 2013, 33, 5312–5318. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chun, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Joshi, A.U.; Minhas, P.S.; Liddelow, S.A.; Haileselassie, B.; Andreasson, I.; Ii, G.W.D.; Mochly-rosen, D. Fragmented Mitochondria Released from Microglia Trigger A1 Astrocytic Response and Propagate Inflammatory Neurodegeneration. Nat. Neurosci. 2019, 22, 1635–1648. [Google Scholar] [CrossRef]
- Fuller, J.; Stavenhagen, J.; Teeling, J.L. New Roles for Fc Receptors in Neurodegeneration-The Impact on Immunotherapy for Alzheimer’s Disease. Front. Neurosci. 2014, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Husemann, J.; Loike, J.D.; Kodama, T.; Silverstein, S.C. Scavenger Receptor Class B Type I (SR-BI) Mediates Adhesion of Neonatal Murine Microglia to Fibrillar β-Amyloid. J. Neuroimmunol. 2001, 114, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; El Khoury, J.; Medeiros, L.A.; Terada, K.; Geula, C.; Luster, A.D.; Freeman, M.W. A CD36-Initiated Signaling Cascade Mediates Inflammatory Effects of β-Amyloid. J. Biol. Chem. 2002, 277, 47373–47379. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.K.; Miller, S.D. Microglia Initiate Central Nervous System Innate and Adaptive Immune Responses through Multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef]
- Lue, L.F.; Walker, D.G.; Brachova, L.; Beach, T.G.; Rogers, J.; Schmidt, A.M.; Stern, D.M.; Yan, S.D. Involvement of Microglial Receptor for Advanced Glycation Endproducts (RAGE)in Alzheimer’s Disease: Identification of a Cellular Activation Mechanism. Exp. Neurol. 2001, 171, 29–45. [Google Scholar] [CrossRef]
- Kielian, T. Toll-like Receptors in Central Nervous System Glial Inflammation and Homeostasis. J. Neurosci. Res. 2006, 83, 711–730. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Q.; Chu, J.; Lin, L.; Li, X.G.; Chai, G.S.; Wang, Q.; Wang, J.Z.; Tian, Q. Expression of Tau40 Induces Activation of Cultured Rat Microglial Cells. PLoS ONE 2013, 8, e76057. [Google Scholar] [CrossRef]
- Ma, L.; Sun, P.; Zhang, J.C.; Zhang, Q.; Yao, S.L. Proinflammatory Effects of S100A8/A9 via TLR4 and RAGE Signaling Pathways in BV-2 Microglial Cells. Int. J. Mol. Med. 2017, 40, 31–38. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar]
- Wang, H.; Li, Y.; Ryder, J.W.; Hole, J.T.; Ebert, P.J.; Airey, D.C.; Qian, H.R.; Logsdon, B.; Fisher, A.; Ahmed, Z.; et al. Genome-Wide RNAseq Study of the Molecular Mechanisms Underlying Microglia Activation in Response to Pathological Tau Perturbation in the RTg4510 Tau Transgenic Animal Model. Mol. Neurodegener. 2018, 13, 65. [Google Scholar] [CrossRef]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brône, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau Activates NLRP3–ASC Inflammasome Exacerbating Exogenously Seeded and Non-Exogenously Seeded Tau Pathology in Vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Brian G Monks1, T.R.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 Inflammasome Is Involved in the Innate Immune Response to Amyloid-β. Nat. Immunol. 2008, 9, 857–895. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.; Gelpi, E.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, W.; Hu, X.; Hanna, M.; Caravaca, A.; Paul, S.M. Microglial Internalization and Degradation of Pathological Tau Is Enhanced by an Anti-Tau Monoclonal Antibody. Sci. Rep. 2015, 5, 11161. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Perea, J.R.; Jurado-Arjona, J.; Rábano, A.; Hernández, F.; Avila, J. Absence of CX3CR1 Impairs the Internalization of Tau by Microglia. Mol. Neurodegener. 2017, 12, 1–14. [Google Scholar] [CrossRef]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Jiang, S.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Reactive Microglia Drive Tau Pathology and Contribute to the Spreading of Pathological Tau in the Brain. Brain 2015, 138, 1738–1755. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of Microglia and Inhibition of Exosome Synthesis Halt Tau Propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Laurent, C.; Buée, L.; Blum, D. Tau and Neuroinflammation: What Impact for Alzheimer’s Disease and Tauopathies? Biomed. J. 2018, 41, 21–33. [Google Scholar] [CrossRef]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-Induced Inflammation Exacerbates Tau Pathology by a Cyclin-Dependent Kinase 5-Mediated Pathway in a Transgenic Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef]
- Ikeda, M.; Shoji, M.; Kawarai, T.; Kawarabayashi, T.; Matsubara, E.; Murakami, T.; Sasaki, A.; Tomidokoro, Y.; Ikarashi, Y.; Kuribara, H.; et al. Accumulation of Filamentous Tau in the Cerebral Cortex of Human Tau R406W Transgenic Mice. Am. J. Pathol. 2005, 166, 521–531. [Google Scholar] [CrossRef]
- Ikeda, K.; Akiyama, H.; Kondo, H.; Haga, C.; Tanno, E.; Tokuda, T.; Ikeda, S. Thorn-Shaped Astrocytes: Possibly Secondarily Induced Tau-Positive Glial Fibrillary Tangles. Acta Neuropathol. 1995, 90, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Kahlson, M.A.; Colodner, K.J. Glial Tau Pathology in Tauopathies: Functional Consequences. J. Exp. Neurosci. 2015, 9, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.S.; Lal, D.; Zhang, B.; Dabir, D.V.; Swanson, E.; Lee, V.M.Y.; Trojanowski, J.Q. Transgenic Mouse Model of Tau Pathology in Astrocytes Leading to Nervous System Degeneration. J. Neurosci. 2005, 25, 3539–3550. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, N.; García-Sierra, F.; Fu, Y.; Beckett, L.A.; Mufson, E.J.; Kuret, J.; Berry, R.W.; Binder, L.I. Tau-66: Evidence for a Novel Tau Conformation in Alzheimer’s Disease. J. Neurochem. 2001, 77, 1372–1385. [Google Scholar] [CrossRef]
- Barres, B.A. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef]
- Pita-Almenar, J.D.; Zou, S.; Colbert, C.M.; Eskin, A. Relationship between Increase in Astrocytic GLT-1 Glutamate Transport and Late-LTP. Learn. Mem. 2012, 19, 615–626. [Google Scholar] [CrossRef]
- Maragakis, N.J.; Rothstein, J.D. Glutamate Transporters in Neurologic Disease. Arch. Neurol. 2001, 58, 365–370. [Google Scholar] [CrossRef]
- Gadhave, K.; Bolshette, N.; Ahire, A.; Pardeshi, R.; Thakur, K.; Trandafir, C.; Istrate, A.; Ahmed, S.; Lahkar, M.; Muresanu, D.F.; et al. The Ubiquitin Proteasomal System: A Potential Target for the Management of Alzheimer’s Disease. J. Cell. Mol. Med. 2016, 20, 1392–1407. [Google Scholar] [CrossRef]
- Keck, S.; Nitsch, R.; Grune, T.; Ullrich, O. Proteasome Inhibition by Paired Helical Filament-tau in Brains of Patients with Alzheimer’s Disease. J. Neurochem. 2003, 85, 115–122. [Google Scholar] [CrossRef]
- Salon, M.L.; Morelli, L.; Castanño, E.M.; Soto, E.F.; Pasquini, J.M. Defective Ubiquitination of Cerebral Proteins in Alzheimer’s Disease. J. Neurosci. Res. 2000, 62, 302–310. [Google Scholar] [CrossRef]
- Tai, H.C.; Serrano-Pozo, A.; Hashimoto, T.; Frosch, M.P.; Spires-Jones, T.L.; Hyman, B.T. The Synaptic Accumulation of Hyperphosphorylated Tau Oligomers in Alzheimer Disease Is Associated with Dysfunction of the Ubiquitin-Proteasome System. Am. J. Pathol. 2012, 181, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F. Immune Function of Microglia. Glia 2001, 36, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.A.; Pickart, C.M.; Alban, A.; Landon, M.; Jamieson, C.; Ramage, R.; Mayer, R.J.; Layfield, R. Inhibition of the Ubiquitin-Proteasome System in Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2000, 97, 9902–9906. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive Microglia Are Positive for HLA-DR in the: Substantia Nigra of Parkinson’s and Alzheimer’s Disease Brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Holmes, C.; Cunningham, C.; Zotova, E.; Woolford, J.; Dean, C.; Kerr, S.; Culliford, D.; Perry, V.H. Systemic Inflammation and Disease Progression in Alzheimer Disease. Neurology 2009, 73, 768–774. [Google Scholar] [CrossRef]
- Blasko, I.; Veerhuis, R.; Stampfer-Kountchev, M.; Saurwein-Teissl, M.; Eikelenboom, P.; Grubeck-Loebenstein, B. Costimulatory Effects of Interferon-β and Interleukin-1β or Tumor Necrosis Factor α on the Synthesis of Aβ1-40 and Aβ1-42 by Human Astrocytes. Neurobiol. Dis. 2000, 7, 682–689. [Google Scholar] [CrossRef]
- Caggiano, A.O.; Kraig, R.P. Prostaglandin E Receptor Subtypes in Cultured Rat Microglia and Their Role in Reducing Lipopolysaccharide-Induced Interleukin-1β Production. J. Neurochem. 1999, 72, 565–575. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk Factors for Alzheimer Disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Guerreiro, R.; Bras, J. The Age Factor in Alzheimer’s Disease. Genome Med. 2015, 7, 1–3. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and Sex: Triad of Risk of Alzheimer’s Disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer’s Disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Miners, J.S. Cerebrovascular Disease in Ageing and Alzheimer’s Disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wong, A.; Law, A.C.K.; Mok, V.C.T. Cerebrovascular Disease, Amyloid Plaques, and Dementia. Stroke 2015, 46, 1402–1407. [Google Scholar] [CrossRef]
- Kling, M.A.; Trojanowski, J.Q.; Wolk, D.A.; Lee, V.M.-Y.; Steven, E. Arnold Vascular Disease and Dementias: Paradigm Shifts to Drive Research in New Directions. Alzheimers Dement. 2013, 9, 76–92. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Neurovascular Pathways to Neurodegeneration in Alzheimer’s Disease and Other Disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Bouras, C.; Hof, P.R.; Morrison, J.H. Neurofibrillary Tangle Densities in the Hippocampal Formation in a Non-Demented Population Define Subgroups of Patients with Differential Early Pathologic Changes. Neurosci. Lett. 1993, 153, 131–135. [Google Scholar] [CrossRef]
- Mazon, J.N.; de Mello, A.H.; Ferreira, G.K.; Rezin, G.T. The Impact of Obesity on Neurodegenerative Diseases. Life Sci. 2017, 182, 22–28. [Google Scholar] [CrossRef]
- Tolppanena, A.-M.; Ngandub, T.; Kareholtd, I.; Laatikainenb, T.; Rusanena, M.; Soininena, H.; Kivipelto, M. Midlife and Late-Life Body Mass Index and Late-Life Dementia: Results from a Prospective Population-Based Cohort. J. Alzheimer’s Dis. 2015, 38, 201–209. [Google Scholar] [CrossRef]
- Gustafson, D.R. Adiposity Hormones and Dementia. J. Neurol. Sci. 2010, 299, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Kappelle, L.J. Increased Risk of Alzheimer’s Disease in Type II Diabetes: Insulin Resistance of the Brain or Insulin-Induced Amyloid Pathology? Biochem. Soc. Trans. 2005, 33, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Sasaki, M.K.; Tanizaki, Y.; Hata, M.J.; Fujimi, K.; Matsui, Y.; Sekita, A.; Suzuki, S.O.; Kanba, S.; Kiyohara, Y.; et al. Insulin Resistance Is Associated with the Pathology of Alzheimer Disease. Neurology 2010, 75, 764–770. [Google Scholar] [CrossRef]
- José, A. Luchsinger Diabetes, Related Conditions, and Dementia. J. Neurol. Sci. 2010, 299, 35–38. [Google Scholar]
- Ramos-rodriguez, J.J.; Spires-jones, T.; Pooler, A.M.; Lechuga-sancho, A.M.; Bacskai, B.J.; Garcia-alloza, M. Progressive Neuronal Pathology and Synaptic Loss Induced by Prediabetes and Type 2 Diabetes in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.M.; Rosario, E.R.; Elteriefi, R.; Pike, C.J. Sex-Specific Effects of High Fat Diet on Indices of Metabolic Syndrome in 3xTg-AD Mice: Implications for Alzheimer’s Disease. PLoS ONE 2013, 8, e78554. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.F.; Raines, S.M.; Steele, J.W.; Ehrlich, M.E.; Lah, J.A.; Small, S.A.; Tanzi, R.E.; Attie, A.D.; Gandy, S. Diabetes-Associated SorCS1 Regulates Alzheimer’s Amyloid-β Metabolism: Evidence for Involvement of SorL1 and the Retromer Complex. J. Neurosci. 2010, 30, 13110–13115. [Google Scholar] [CrossRef] [PubMed]
- Skoog, I.; Lernfelt, B.; Landahl, S.; Palmertz, B.; Andreasson, L.A.; Nilsson, L.; Persson, G.; Odén, A.; Svanborg, A. 15-Year Longitudinal Study of Blood Pressure and Dementia. Lancet 1996, 347, 1141–1145. [Google Scholar] [CrossRef]
- Staessen, J.A.; Richart, T.; Birkenhäger, W.H. Less Atherosclerosis and Lower Blood Pressure for a Meaningful Life Perspective with More Brain. Hypertension 2007, 49, 389–400. [Google Scholar] [CrossRef]
- Skoog, I.; Gustafson, D. Update on Hypertension and Alzheimer’s Disease. Neurol. Res. 2006, 28, 605–611. [Google Scholar] [CrossRef]
- Popp, J.; Meichsner, S.; Kölsch, H.; Lewczuk, P.; Maier, W.; Kornhuber, J.; Jessen, F.; Lütjohann, D. Cerebral and Extracerebral Cholesterol Metabolism and CSF Markers of Alzheimer’s Disease. Biochem. Pharmacol. 2013, 86, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Xue-Shan, Z.; Juan, P.; Qi, W.; Zhong, R.; Li-hong, P.; Zhi-han, T.; Zhi-Sheng, J.; Gui-xue, W.; Lu-Shan, L. Imbalanced Cholesterol Metabolism in Alzheimer’s Disease. Clin. Chim. Acta 2016, 456, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Canepa, E.; Marengo, B.; Marinari, U.M.; Poli, G.; Pronzato, M.A.; Domenicotti, C. Cholesterol and Alzheimer’s Disease: A Still Poorly Understood Correlation. IUBMB Life 2012, 64, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, C.; Pirchl, M.; Humpel, C. Hypercholesterolemia in Rats Impairs the Cholinergic System and Leads to Memory Deficits. Mol. Cell. Neurosci. 2010, 45, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Amy, L.B.; Yaffe, K. Depression and Risk of Developing Dementia. Nat. Rev. Neurol. 2012, 7, 323–331. [Google Scholar]
- Ricci, S.; Fuso, A.; Ippoliti, F.; Businaro, R. Stress-Induced Cytokines and Neuronal Dysfunction in Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 28, 11–24. [Google Scholar] [CrossRef]
- Vilalta-Franch, J.; López-Pousa, S.; Llinàs-Reglà, J.; Calvó-Perxas, L.; Merino-Aguado, J.; Garre-Olmo, J. Depression Subtypes and 5-Year Risk of Dementia and Alzheimer Disease in Patients Aged 70 Years. Int. J. Geriatr. Psychiatry 2012, 28, 341–350. [Google Scholar] [CrossRef]
- Zvěřová, M.; Fišar, Z.; Jirák, R.; Kitzlerová, E.; Hroudová, J.; Raboch, J. Plasma Cortisol in Alzheimer’s Disease with or without Depressive Symptoms. Med. Sci. Monit. 2013, 19, 681–689. [Google Scholar]
- Wu, K.Y.; Lin, K.J.; Chen, C.H.; Chen, C.S.; Liu, C.Y.; Huang, S.Y.; Yen, T.C.; Hsiao, I.T. Diversity of Neurodegenerative Pathophysiology in Nondemented Patients with Major Depressive Disorder: Evidence of Cerebral Amyloidosis and Hippocampal Atrophy. Brain Behav. 2018, 8, e01016. [Google Scholar] [CrossRef]
- Wilson, R.S.; Begeny, C.T.; Boyle, P.A.; Schneider, J.A.; Bennett, D.A. Vulnerability to Stress, Anxiety, and Development of Dementia in Old. Am. J. Geriatr. Psychiatry 2011, 19, 327–334. [Google Scholar] [CrossRef]
- Donovan, N.J.; Locascio, J.J.; Marshall, G.A.; Gatchel, J.; Bernard, J.; Hanseeuw, D.M.R.; Johnson, K.A.; Reisa, A. Sperling Longitudinal Association of Amyloid-β and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am. J. Psychiatry 2018, 176, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T. Conversion of Psychological Stress into Cellular Stress Response: Roles of the Sigma-1 Receptor in the Process. Psychiatry Clin. Neurosci. 2015, 69, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Sadeh, N. Traumatic Stress, Oxidative Stress and Posttraumatic Stress Disorder: Neurodegeneration and the Accelerated-Aging Hypothesis. Mol. Psychiatry 2014, 19, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the Stress System and the Role of Glucocorticoids. Neuroimmunomodulation 2015, 22, 6–19. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Ellis, B.J.; Del Giudice, M. Beyond Allostatic Load: Rethinking the Role of Stress in Regulating Human Development. Dev. Psychopathol. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Krey, L.C.; McEwen, B.S. Prolonged Glucocorticoid Exposure Reduces Hippocampal Neuron Number: Implications for Aging. J. Neurosci. 1985, 5, 1222–1227. [Google Scholar] [CrossRef]
- Proserpio, P.; Arnaldi, D.; Nobili, F.; Nobili, L. Integrating Sleep and Alzheimer’s Disease Pathophysiology: Hints for Sleep Disorders Management. J. Alzheimer’s Dis. 2018, 63, 871–886. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.J.; Ma, M.Y.; Bao, Y.P.; Han, Y.; Wang, Y.M.; Shi, J.; Vitiello, M.V.; Lu, L. Sleep Disturbances Increase the Risk of Dementia: A Systematic Review and Meta-Analysis. Sleep. Med. Rev. 2017, 40, 4–16. [Google Scholar] [CrossRef]
- Traber, M.G.; Van Der Vliet, A.; Reznick, A.Z.; Cross, C.E. Tobacco-Related Diseases: Is There a Role for Antioxidant Micronutrient Supplementation? Clin. Chest Med. 2000, 21, 173–187. [Google Scholar] [CrossRef]
- Durazzo, T.C.; Mattsson, N.; Weiner, M.W. Smoking and Increased Alzheimer’s Disease Risk: A Review of Potential Mechanisms. Alzheimers Dement. 2014, 10, S122–S145. [Google Scholar] [CrossRef] [PubMed]
- by Dove Press, published Genes Associated with Alzheimer’s Disease: An Overview and Current Status. Mohan Giri Man. Zhang Yang Lü 2016, 11, 665–681.
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular Genetics of Early-Onset Alzheimer’s Disease Revisited. Alzheimer’s Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Gómez-Ramos, A.; Calero, O.; Soriano, E.; Avila, J.; Medina, M. Additional Mechanisms Conferring Genetic Susceptibility to Alzheimer’s Disease. Front. Cell. Neurosci. 2015, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Campion, D.; Dumanchin, C.; Hannequin, D.; Dubois, B.; Belliard, S.; Puel, M.; Thomas-Anterion, C.; Michon, A.; Martin, C.; Charbonnier, F.; et al. Early-Onset Autosomal Dominant Alzheimer Disease: Prevalence, Genetic Heterogeneity, and Mutation Spectrum. Am. J. Hum. Genet. 1999, 65, 664–670. [Google Scholar] [CrossRef]
- Corbo, R.M.; Scacchp, R. Apolipoprotein E (APOE) Allele Distribution in the World. Is APOE*4 a “thrifty” Allele? Ann. Hum. Genet. 1999, 63, 301–310. [Google Scholar] [CrossRef]
- Karch, C.M.; Goate, A.M. Alzheimer’s Disease Risk Genes and Mechanisms of Disease Pathogenesis. Biol. Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef]
- Mahley, R.W. Apolipoprotein E: From Cardiovascular Disease to Neurodegenerative Disorders. J. Mol. Med. 2016, 94, 739–746. [Google Scholar] [CrossRef]
- Granta, W.B.; Campbellb, A.; Itzhakic, R.F.; Savory, J. The Significance of Environmental Factors in the Etiology of Alzheimer’s Disease. J. Alzheimer’s Dis. 2002, 4, 179–189. [Google Scholar] [CrossRef]
- Wainaina, M.N.; Chen, Z.; Zhong, C. Environmental Factors in the Development and Progression of Late-Onset Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 253–270. [Google Scholar] [CrossRef]
- Fülöp, T.; Itzhaki, R.F.; Balin, B.J.; Miklossy, J.; Barron, A.E. Role of Microbes in the Development of Alzheimer’s Disease: State of the Art—An International Symposium Presented at the 2017 IAGG Congress in San Francisco. Front. Genet. 2018, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Zwolinska, K.; Leszek, J. The Infectious Etiology of Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s Disease. J. Alzheimer’s Dis. 2006, 10, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer Disease. Adv. Neurobiol. 2017, 18, 183–197. [Google Scholar] [PubMed]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. J. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef] [PubMed]
- Moulton, P.V.; Yang, W. Air Pollution, Oxidative Stress, and Alzheimer’s Disease. J. Environ. Public Health 2012, 2012, 472751. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Zimmer, L. Ozone Atmospheric Pollution and Alzheimer’s Disease: From Epidemiological Facts to Molecular Mechanisms. J. Alzheimer’s Dis. 2018, 62, 503–522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).