Biomarkers of Atrial Fibrillation Recurrence in Patients with Paroxysmal or Persistent Atrial Fibrillation Following External Direct Current Electrical Cardioversion
Abstract
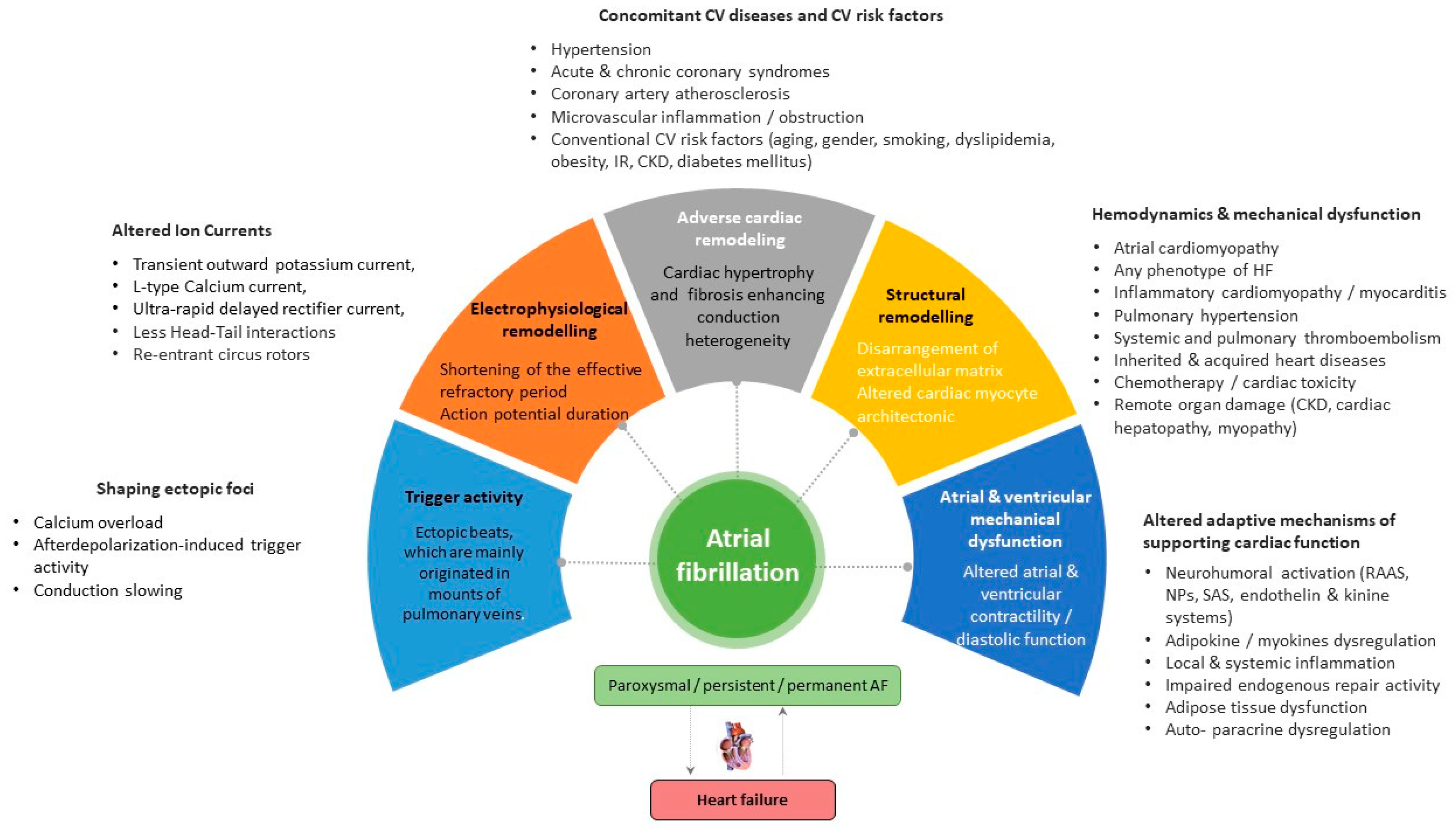
:1. Introduction
2. Promoting Factors and Electrophysiological/Anatomical Substrates of AF
2.1. Electrophysiological Remodeling
2.2. Adverse Cardiac Remodeling
3. Electrical Cardioversion of AF: Safety and Outcomes
4. Predictors for AF Recurrence Following Electrical Cardioversion
4.1. Natriuretic Peptides
4.2. Biomarkers of Fibrosis
4.2.1. Galectin-3
4.2.2. sST2
4.2.3. Other Biomarkers of Fibrosis
4.3. Biomarkers of Inflammation
4.3.1. GDF15
4.3.2. hs-CRP
4.4. Myokines and Adipocytokines
4.4.1. Apelin
4.4.2. Irisin
4.4.3. Bone-Related Proteins
4.5. Biomarkers of Oxidative Stress and Endothelial Dysfunction
4.5.1. Cell-Free Circulating DNA
4.5.2. mRNA
4.5.3. Asymmetric Dimethylarginine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Biomarkers | Population | Observation Period | Significance/Outcomes | References |
|---|---|---|---|---|
| Biomechanical stress | ||||
| BNP | 58 patients with persistent AF and preserved LVEF | 6 months | Baseline BNP level and the magnitude of its decrease after successful cardioversion predicted AF recurrence | [82] |
| NT-proBNP and BMP10 | 100 non-CVD patients with and without AF recurrence | 30-day follow-up | Low NT-proBNP levels and BMP10 levels after electric cardioversion predicted sinus rhythm restoration | [83] |
| BNP and NT-proBNP | 43 patients with persistent AF | 18 months | Pre- and post-procedural levels of BNP and NT-proBNP did not predict new episodes of AF | [84] |
| NT-proBNP | 199 patients with persistent AF | 30 days | The levels of NT-proBNP > 500 ng/L predicted recurrence of AF in 30 days after successful electrical cardioversion | [85] |
| NT-pro-BNP | 40 patients with persistent AF | 1 month | Elevated baseline NT-pro-BNP predicted AF recurrence after electric cardioversion | [86] |
| NT-pro-BNP | 171 patients with persistent AF without HF | 1 month | Pre-cardioversion and post-cardioversion NT-pro-BNP levels did not predict a relapse of AF in patients without HF | [87] |
| ANP and BNP | 71 HF patients with persistent AF | 1 month | Low ANP and high BNP levels before electric cardioversion independently predicted recurrent AF | [88] |
| ANP and BNP | 60 patients with persistent AF | 12 months | The BNP level ≥700 fmol/mL on day 7 after cardioversion predicted AF recurrence. ANP level was not predictive of AF recurrence | [89] |
| NT-proBNP | 200 patients with newly onset AF with and without HF | 1 month | NT-proBNP levels of either ≤450 pg/mL or >1800 pg/mL had positive and negative predictive values for cardioversion in rate-control and rhythm-control strategies | [90] |
| Cardiac fibrosis | ||||
| Gal-3 | 90 patients with persistent AF | 3 months | Serum Gal-3 level independently predicted early AF recurrence following successful direct-current electrical cardioversion. | [98] |
| Gal-3 | 82 patients with persistent AF | 1 month | Baseline serum levels of Gal-3 were not associated with a risk of recurrent AF | [101] |
| Gal-3 | 75 non-HF patients with paroxysmal or persistent AF | 1 year | Pre-procedural Gal-3 levels did not predict recurrent AF | [102] |
| sST2 | 80 patients with persistent AF without HF | 12 months | Serum levels of sST2 predict sinus rhythm maintenance after cardioversion of AF in patients without HF | [112] |
| FGF-23 | 79 patients with persistent AF | 12 months | FGF-23, but not Gal-3, PIIINP, and ICTP, had weak predictive ability for relapsing AF | [113] |
| PIIINP | 88 patients with maintenance of sinus rhythm and 54 patients with AF recurrence | 24 months | Baseline PIIINP levels >0.72 U/mL independently predicted AF recurrence after electric cardioversion | [114] |
| Inflammation | ||||
| GDF15 | 82 patients with persistent AF | 1 month | GDF-15 levels correlated positively with the CHA2DS2-VASc score, but not associated with a risk of recurrent AF after electric cardioversion | [101] |
| hs-CRP | 102 patients with non-valvular persistent AF | 1 year | Low levels of hs-CRP were associated with long-term maintenance of sinus rhythm after electrical cardioversion for AF | [137] |
| hs-CRP | 53 patients with persistent AF and a mean LVEF of 58.7 ± 6% | 3 weeks | No changes in hs-CRP levels and decrease in NT-proBNP levels after effective cardioversion. Pre-procedural levels of hs-CRP predicted recurrence rate of AF | [138] |
| hs-CRP | 57 patients with a mean LVEF of 58.7 ± 6% | 3 weeks | Pre-procedural levels of hs-CRP, but not NT-proBNP, predicted recurrence rate of AF | [139] |
| hs-CRP | 60 patients who received amiodarone for sinus rhythm maintenance | 3 years | Pre-procedural levels of CRP >0.43 mg/dL were an independent predictor of AF recurrence | [140] |
| hs-CRP | 106 patients with a history of symptomatic AF lasting ≥ 1 day | 36 days | Pre-procedural hs-CRP levels ≥0.06 mg/dL predicted both AF recurrence and maintenance of sinus rhythm | [141] |
| hs-CRP | 56 patients with persistent AF | 180 days | Pre-procedural hs-CRP <0.8 mg/L was significantly associated with lower AF recurrence rates and maintenance of sinus rhythm | [142] |
| hs-CRP | 216 patients with persistent AF | 12 months | The baseline and 2-day levels of hs-CRP levels contributed a risk of AF recurrence | [145] |
| Apelin and NT-proBNP | 40 patients with persistent AF and 15 controls in sinus rhythm | 1 month | Pre-procedural apelin levels were lower and NT-pro-BNP levels were higher in patients with AF compared to controls. Cardioversion led to an increase in apelin levels and a decrease in NT-proBNP levels. Apelin did not predict AF recurrence, but NT-proBNP did | [176] |
| Biomarkers of oxidative stress and mitochondrial dysfunction | ||||
| cfc-mtDNA | 59 non-AF patients undergoing cardiac surgery, 100 patients with paroxysmal AF, 116 patients with persistent AF, 20 longstanding-persistent AF individuals and 84 control individuals | - | Elevated cfc-mtDNA levels were found in patients with paroxysmal AF undergoing electrical cardioversion or pulmonary vein isolation, as well as in patients with AF relapse after AF treatment. In patients with persistent AF and longstanding persistent AF, the levels of cfc-mtDNA gradually decreased | [204] |
| miR-199a-5p and miR-22-5p | 49 HFrEF with AF and 49 HFrEF with sinus rhythm | - | Elevated levels of circulating miR-199a-5p and miR-22-5p were associated with AF in HFrEF patients | [207] |
| miR-21 | 60 persistent AF patients and 60 matched sinus rhythm volunteers | - | Circulating miR-21 positively correlates with the quantification of left atrial fibrosis and is associated with the risk of persistent AF in patients with left atrial enlargement | [208] |
| miR-1-3p | 64 consecutive patients with cryptogenic stroke, 9 patients with AF and 9 individuals with sinus rhythm | 6 and 12 months | Elevated plasma levels of miR-1-3p predicted AF | [209] |
| miR-21, miR-133a, miR-133b, miR-150, miR-328, and miR-499 | 5 acute new-onset AF patients, 16 well-controlled AF and 15 control | - | miR-21, miR-133b, and miR-499, which downregulate apoptosis and fibrosis, were found to be directly related to AF | [213] |
| Biomarkers of endothelial dysfunction | ||||
| ADMA | 64 patients with persistent AF | 1 month | High levels of ADMA were strongly associated with an increased risk of AF relapse after electrical cardioversion | [222] |
| ADMA | 98 patients with persistent AF | 6 months | Changes in ADMA did not predict rhythm outcome after electrical cardioversion | [223] |
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of Atrial Fibrillation: European Perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R. Vorhofflimmern als Komorbidität bei Herzinsuffizienz. Internist 2018, 59, 415–419. [Google Scholar] [CrossRef]
- Alonso, A.; Almuwaqqat, Z.; Chamberlain, A. Mortality in Atrial Fibrillation: Is It Changing? Trends Cardiovasc. Med. 2021, 31, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Galezewska, M.; Markowska, M.; Braszak, A.; Bryl, W.; Bogdanski, P. Atrial Fibrillation and Obesity: Should Doctors Focus on This Comorbidity? Minerva Med. 2019, 110, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.L.; Glover, B.M. The Impact of Lifestyle Intervention on Atrial Fibrillation. Curr. Opin. Cardiol. 2018, 33, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.; Pasch, L.B.; Newton, P.J.; Bajorek, B.V.; Ferguson, C. Addressing Multimorbidity and Polypharmacy in Individuals with Atrial Fibrillation. Curr. Cardiol. Rep. 2018, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Linz, D.; Schotten, U. Dynamics of Atrial Fibrillation Mechanisms and Comorbidities. Annu. Rev. Physiol. 2021, 83, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Schoonderwoerd, B.A.; Van Gelder, I.C.; Van Veldhuisen, D.J.; Van den Berg, M.P.; Crijns, H.J.G.M. Electrical and Structural Remodeling: Role in the Genesis and Maintenance of Atrial Fibrillation. Prog. Cardiovasc. Dis. 2005, 48, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Cha, T.-J.; Ehrlich, J.R.; Zhang, L.; Shi, Y.-F.; Tardif, J.-C.; Leung, T.K.; Nattel, S. Dissociation between Ionic Remodeling and Ability to Sustain Atrial Fibrillation during Recovery from Experimental Congestive Heart Failure. Circulation 2004, 109, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Vanbeselaere, V.; Truyers, C.; Elli, S.; Buntinx, F.; De Witte, H.; Degryse, J.; Henrard, S.; Vaes, B. Association between Atrial Fibrillation, Anticoagulation, Risk of Cerebrovascular Events and Multimorbidity in General Practice: A Registry-Based Study. BMC Cardiovasc. Disord. 2016, 16, 61. [Google Scholar] [CrossRef]
- Bernard, M.L. Atrial Fibrillation and Multimorbidity. Mayo Clin. Proc. 2019, 94, 2381–2382. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Lip, G.Y.H. The Patient Pathway Review for Atrial Fibrillation. Crit. Pathw. Cardiol. 2022, 21, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Furniss, S.S.; Sneyd, J.R. Safe Sedation in Modern Cardiological Practice. Heart 2015, 101, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Fried, A.M.; Strout, T.D.; Perron, A.D. Electrical Cardioversion for Atrial Fibrillation in the Emergency Department: A Large Single-Center Experience. Am. J. Emerg. Med. 2021, 42, 115–120. [Google Scholar] [CrossRef]
- Brandes, A.; Crijns, H.J.G.M.; Rienstra, M.; Kirchhof, P.; Grove, E.L.; Pedersen, K.B.; Van Gelder, I.C. Cardioversion of Atrial Fibrillation and Atrial Flutter Revisited: Current Evidence and Practical Guidance for a Common Procedure. EP Eur. 2020, 22, 1149–1161. [Google Scholar] [CrossRef]
- Voskoboinik, A.; Kalman, E.; Plunkett, G.; Knott, J.; Moskovitch, J.; Sanders, P.; Kistler, P.M.; Kalman, J.M. A Comparison of Early versus Delayed Elective Electrical Cardioversion for Recurrent Episodes of Persistent Atrial Fibrillation: A Multi-Center Study. Int. J. Cardiol. 2019, 284, 33–37. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef]
- Cheung, C.C.; Nattel, S.; Macle, L.; Andrade, J.G. Management of Atrial Fibrillation in 2021: An Updated Comparison of the Current CCS/CHRS, ESC, and AHA/ACC/HRS Guidelines. Can. J. Cardiol. 2021, 37, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Toufan, M.; Kazemi, B.; Molazadeh, N. The Significance of the Left Atrial Volume Index in Prediction of Atrial Fibrillation Recurrence after Electrical Cardioversion. J. Cardiovasc. Thorac. Res. 2017, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A Review of Factors Associated with Maintenance of Sinus Rhythm after Elective Electrical Cardioversion for Atrial Fibrillation. Clin. Cardiol. 2018, 41, 862–870. [Google Scholar] [CrossRef]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm 2018, 15, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with atrial fibrillation and coronary artery disease—Double trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Nairn, D.; Chen, J.; Mueller-Edenborn, B.; Pilia, N.; Mayer, L.; Eichenlaub, M.; Moreno-Weidmann, Z.; Allgeier, J.; Trenk, D.; et al. Structural and electrophysiological determinants of atrial cardiomyopathy identify remodeling discrepancies between paroxysmal and persistent atrial fibrillation. Front. Cardiovasc. Med. 2023, 9, 1101152. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; López-Mínguez, J.R.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and anatomical substrates in the genesis and perpetuation of atrial fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.F.; Cardona, K.; Martinez, L.; Saiz, J.; Trenor, B. Electrophysiological and structural remodeling in heart failure modulate arrhythmogenesis. 2D simulation study. PLoS ONE 2014, 9, e103273. [Google Scholar] [CrossRef]
- Wang, Y.; Hill, J.A. Electrophysiological remodeling in heart failure. J. Mol. Cell. Cardiol. 2010, 48, 619–632. [Google Scholar] [CrossRef]
- Janse, M.J. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc. Res. 2004, 61, 208–217. [Google Scholar] [CrossRef]
- Coronel, R.; Wilders, R.; Verkerk, A.O.; Wiegerinck, R.F.; Benoist, D.; Bernus, O. Electrophysiological changes in heart failure and their implications for arrhythmogenesis. Biochim. Biophys. Acta 2013, 1832, 2432–2441. [Google Scholar] [CrossRef]
- Aistrup, G.L.; Balke, C.W.; Wasserstrom, J.A. Arrhythmia triggers in heart failure: The smoking gun of [Ca2+]i dysregulation. Heart Rhythm 2011, 8, 1804–1808. [Google Scholar] [CrossRef]
- Akar, F.G.; Rosenbaum, D.S. Transmural electrophysiological heterogeneities underlying arrhythmogenesis in heart failure. Circ. Res. 2003, 93, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, X.; Dong, F.; Wang, Y.; Hui, J.; Lin, Z.; Yang, J.; Xu, Y. Temporal alterations and cellular mechanisms of transmural repolarization during progression of mouse cardiac hypertrophy and failure. Acta Physiol. 2013, 208, 95–110. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Li, Y.; Ren, X.; Xing, Y.; Shang, H. Effects of Wenxin Keli on Cardiac Hypertrophy and Arrhythmia via Regulation of the Calcium/Calmodulin Dependent Kinase II Signaling Pathway. Biomed. Res. Int. 2017, 2017, 1569235. [Google Scholar] [CrossRef]
- Kuba, K.; Sato, T.; Imai, Y.; Yamaguchi, T. Apelin and Elabela/Toddler; double ligands for APJ/Apelin receptor in heart development, physiology, and pathology. Peptides 2019, 111, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.; da Costa, B.R.; Collet, T.H.; Feller, M.; Floriani, C.; Bauer, D.C.; Cappola, A.R.; Heckbert, S.R.; Ceresini, G.; Gussekloo, J.; et al. Thyroid Function within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation 2017, 136, 2100–2116. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Nie, L.; Long, J.; Zhao, J.; Liu, X.; Wang, L.; Liu, D.; Wang, S.; Liu, S.; Yang, J. Hydrogen sulfide alleviates hypothyroidism-induced myocardial fibrosis in rats through stimulating autophagy and inhibiting TGF-β1/Smad2 pathway. Korean J. Physiol. Pharmacol. 2023, 27, 1–8. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chang, G.J.; Lai, Y.J.; Chen, W.J.; Chang, S.H.; Hung, L.M.; Kuo, C.T.; Yeh, Y.H. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc. Diabetol. 2019, 18, 125. [Google Scholar] [CrossRef]
- Glukhov, A.V.; Fedorov, V.V.; Kalish, P.W.; Ravikumar, V.K.; Lou, Q.; Janks, D.; Schuessler, R.B.; Moazami, N.; Efimov, I.R. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation 2012, 125, 1835–1847. [Google Scholar] [CrossRef]
- Wiegerinck, R.F.; van Veen, T.A.; Belterman, C.N.; Schumacher, C.A.; Noorman, M.; de Bakker, J.M.; Coronel, R. Transmural dispersion of refractoriness and conduction velocity is associated with heterogeneously reduced connexin-43 in a rabbit model of heart failure. Heart Rhythm 2008, 5, 1178–1185. [Google Scholar] [CrossRef]
- Akar, F.G.; Nass, R.D.; Hahn, S.; Cingolani, E.; Shah, M.; Hesketh, G.G.; DiSilvestre, D.; Tunin, R.S.; Kass, D.A.; Tomaselli, G.F. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1223–H1230. [Google Scholar] [CrossRef]
- Yan, J.; Killingsworth, C.; Walcott, G.; Zhu, Y.; Litovsky, S.; Huang, J.; Ai, X.; Pogwizd, S.M. Molecular remodeling of Cx43, but not structural remodeling, promotes arrhythmias in an arrhythmogenic canine model of nonischemic heart failure. J. Mol. Cell. Cardiol. 2021, 158, 72–81. [Google Scholar] [CrossRef]
- Poelzing, S.; Rosenbaum, D.S. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1762–H1770. [Google Scholar] [CrossRef] [PubMed]
- Vitale, E.; Rosso, R.; Lo Iacono, M.; Cristallini, C.; Giachino, C.; Rastaldo, R. Apelin-13 Increases Functional Connexin-43 through Autophagy Inhibition via AKT/mTOR Pathway in the Non-Myocytic Cell Population of the Heart. Int. J. Mol. Sci. 2022, 23, 13073. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, X.; Zhang, L.; Li, X.; Liu, Q. Apelin-13 regulates angiotensin ii-induced Cx43 downregulation and autophagy via the AMPK/mTOR signaling pathway in HL-1 cells. Physiol. Res. 2020, 69, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Song, J.J.; Yang, X.C.; Zhong, G.Z.; Zhong, J.C. MiRNA-122-5p inhibitor abolishes angiotensin II-mediated loss of autophagy and promotion of apoptosis in rat cardiofibroblasts by modulation of the apelin-AMPK-mTOR signaling. In Vitro Cell. Dev. Biol. Anim. 2022, 58, 136–148. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Dong, Z.; Liu, X.; Liu, Y.; Li, X.; Xu, Y.; Guo, Y.; Wang, N.; Zhang, M.; et al. MicroRNA-122-5p Aggravates Angiotensin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling. J. Cardiovasc. Transl. Res. 2022, 15, 535–547. [Google Scholar] [CrossRef]
- Beyer, C.; Tokarska, L.; Stühlinger, M.; Feuchtner, G.; Hintringer, F.; Honold, S.; Fiedler, L.; Schönbauer, M.S.; Schönbauer, R.; Plank, F. Structural Cardiac Remodeling in Atrial Fibrillation. JACC Cardiovasc. Imaging 2021, 14, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.R., 2nd; Rathakrishnan, B.; Campbell, K.; Thomas, K.L.; Piccini, J.P.; Bahnson, T.; Stiber, J.A.; Daubert, J.P. Sinus Node Dysfunction and Atrial Fibrillation: A Reversible Phenomenon? Pacing Clin. Electrophysiol. 2017, 40, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Mainardi, L.; Rodriguez Matas, J.F. Cellular heterogeneity and repolarisation across the atria: An in silico study. Med. Biol. Eng. Comput. 2022, 60, 3153–3168. [Google Scholar] [CrossRef]
- Da Costa, A.; Mourot, S.; Roméyer-Bouchard, C.; Thévenin, J.; Samuel, B.; Kihel, A.; Isaaz, K. Anatomic and electrophysiological differences between chronic and paroxysmal forms of common atrial flutter and comparison with controls. Pacing Clin. Electrophysiol. 2004, 27, 1202–1211. [Google Scholar] [CrossRef]
- Soulat-Dufour, L.; Lang, S.; Addetia, K.; Ederhy, S.; Adavane-Scheuble, S.; Chauvet-Droit, M.; Jean, M.L.; Nhan, P.; Ben Said, R.; Kamami, I.; et al. Restoring Sinus Rhythm Reverses Cardiac Remodeling and Reduces Valvular Regurgitation in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, V.; Henriquez, C.S. Modelling cardiac fibroblasts: Interactions with myocytes and their impact on impulse propagation. Europace 2007, 9 (Suppl. S6), vi29–vi37. [Google Scholar] [CrossRef]
- Duffy, H.S. Fibroblasts, myofibroblasts, and fibrosis: Fact, fiction, and the future. J. Cardiovasc. Pharmacol. 2011, 57, 373–375. [Google Scholar] [CrossRef]
- Rohr, S. Cardiac fibroblasts in cell culture systems: Myofibroblasts all along? J. Cardiovasc. Pharmacol. 2011, 57, 389–399. [Google Scholar] [CrossRef]
- Dhein, S.; Salameh, A. Remodeling of Cardiac Gap Junctional Cell-Cell Coupling. Cells 2021, 10, 2422. [Google Scholar] [CrossRef]
- Ravens, U.; Peyronnet, R. Electrical Remodelling in Cardiac Disease. Cells 2023, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Wolfes, J.; Ellermann, C.; Frommeyer, G.; Eckardt, L. Evidence-based treatment of atrial fibrillation around the globe: Comparison of the latest ESC, AHA/ACC/HRS, and CCS guidelines on the management of atrial fibrillation. Rev. Cardiovasc. Med. 2022, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.H.; Vinson, D.R.; Drummond, K.; Strout, T.D.; Thode, H.C.; McInturff, J.J. Electrical cardioversion of emergency department patients with atrial fibrillation. Ann. Emerg. Med. 2004, 44, 20–30. [Google Scholar] [CrossRef]
- Michael, J.A.; Stiell, I.G.; Agarwal, S.; Mandavia, D.P. Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann. Emerg. Med. 1999, 33, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Shahar, A.; Novikov, I.; Agmon, U.; Ziv, A.; Hod, H. Treatment of stable atrial fibrillation in the emergency department: A population-based comparison of electrical direct-current versus pharmacological cardioversion or conservative management. Cardiology 2009, 112, 270–278. [Google Scholar] [CrossRef]
- Von Besser, K.; Mills, A.M. Is discharge to home after emergency department cardioversion safe for the treatment of recent-onset atrial fibrillation? Ann. Emerg. Med. 2011, 58, 517–520. [Google Scholar] [CrossRef]
- Xavier Scheuermeyer, F.; Grafstein, E.; Stenstrom, R.; Innes, G.; Poureslami, I.; Sighary, M. Thirty-day outcomes of emergency department patients undergoing electrical cardioversion for atrial fibrillation or flutter. Acad. Emerg. Med. 2010, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; De Ferrari, G.M.; Pandozi, C.; Alboni, P.; Capucci, A.; Disertori, M.; Gaita, F.; Lombardi, F.; Maggioni, A.P.; Mugelli, A.; et al. Atrial fibrillation requiring urgent medical care. Approach and outcome in the various departments of admission. Data from the atrial Fibrillation/flutter Italian REgistry (FIRE). Ital. Heart J. 2004, 5, 205–213. [Google Scholar] [PubMed]
- Cohn, B.G.; Keim, S.M.; Yealy, D.M. Is emergency department cardioversion of recent-onset atrial fibrillation safe and effective? J. Emerg. Med. 2013, 45, 117–127. [Google Scholar] [CrossRef]
- Cristoni, L.; Tampieri, A.; Mucci, F.; Iannone, P.; Venturi, A.; Cavazza, M.; Lenzi, T. Cardioversion of acute atrial fibrillation in the short observation unit: Comparison of a protocol focused on electrical cardioversion with simple antiarrhythmic treatment. Emerg. Med. J. 2011, 28, 932–937. [Google Scholar] [CrossRef]
- Houghton, A.R.; Sharman, A.; Pohl, J.E. Determinants of successful direct current cardioversion for atrial fibrillation and flutter: The importance of rapid referral. Br. J. Gen. Pract. 2000, 50, 710–711. [Google Scholar]
- Boriani, G.; Diemberger, I.; Biffi, M.; Domenichini, G.; Martignani, C.; Valzania, C.; Branzi, A. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: Predictors of long-term outcome. Int. J. Clin. Pract. 2007, 61, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.T.; Lyngborg, K.; Pedersen, F.; Corell, P. Predictive factors of maintenance of sinus rhythm after direct current (DC) cardioversion of atrial fibrillation/atrial flutter. Ugeskr. Laeger 2005, 167, 3408–3412. [Google Scholar] [PubMed]
- Mittal, S.; Ayati, S.; Stein, K.M.; Schwartzman, D.; Cavlovich, D.; Tchou, P.J.; Markowitz, S.M.; Slotwiner, D.J.; Scheiner, M.A.; Lerman, B.B. Transthoracic cardioversion of atrial fibrillation: Comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation 2000, 101, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Inácio, J.F.; da Rosa Mdos, S.; Shah, J.; Rosário, J.; Vissoci, J.R.; Manica, A.L.; Rodrigues, C.G. Monophasic and biphasic shock for transthoracic conversion of atrial fibrillation: Systematic review and network meta-analysis. Resuscitation 2016, 100, 66–75. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Cui, K.; Huang, W.; Fan, J.; Lei, H. Midregional pro-atrial natriuretic peptide is a superior biomarker to N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure patients with preserved ejection fraction. Medicine 2018, 97, e12277. [Google Scholar] [CrossRef]
- Okutucu, S.; Gorenek, B. Current Recommendations on Atrial Fibrillation: A Comparison of the Recent European and Canadian Guidelines. Cardiology 2022, 147, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Sullivan, L.M.; Pencina, M.J.; Meigs, J.B.; Tofler, G.H.; Selhub, J.; Jacques, P.F.; Wolf, P.A.; et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010, 121, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cao, H.; Su, L.; Ling, Z.; Liu, Z.; Lan, X.; Xu, Y.; Chen, W.; Yin, Y. NT-proBNP, but not ANP and C-reactive protein, is predictive of paroxysmal atrial fibrillation in patients undergoing pulmonary vein isolation. J. Interv. Card. Electrophysiol. 2012, 33, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Beck-da-Silva, L.; de Bold, A.; Fraser, M.; Williams, K.; Haddad, H. Brain natriuretic peptide predicts successful cardioversion in patients with atrial fibrillation and maintenance of sinus rhythm. Can. J. Cardiol. 2004, 20, 1245–1248. [Google Scholar]
- Xu, X.; Tang, Y. Relationship between Brain Natriuretic Peptide and Recurrence of Atrial Fibrillation after Successful Electrical Cardioversion: An Updated Meta-Analysis. Braz. J. Cardiovasc. Surg. 2017, 32, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Ari, H.; Binici, S.; Ari, S.; Akkaya, M.; Koca, V.; Bozat, T.; Gürdoğan, M. The predictive value of plasma brain natriuretic peptide for the recurrence of atrial fibrillation six months after external cardioversion. Turk. Kardiyol. Dern. Ars. 2008, 36, 456–460. [Google Scholar]
- Meyre, P.B.; Aeschbacher, S.; Blum, S.; Voellmin, G.; Kastner, P.M.; Hennings, E.; Kaufmann, B.A.; Kühne, M.; Osswald, S.; Conen, D. Biomarkers associated with rhythm status after cardioversion in patients with atrial fibrillation. Sci. Rep. 2022, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wozakowska-Kapłon, B.; Bartkowiak, R.; Grabowska, U.; Janiszewska, G. B-type natriuretic peptide level after sinus rhythm restoration in patients with persistent atrial fibrillation—clinical significance. Kardiol. Pol. 2010, 68, 781–786. [Google Scholar]
- Andersson, J.; Rosenqvist, M.; Tornvall, P.; Boman, K. NT-proBNP predicts maintenance of sinus rhythm after electrical cardioversion. Thromb. Res. 2015, 135, 289–291. [Google Scholar] [CrossRef]
- Kallergis, E.M.; Manios, E.G.; Kanoupakis, E.M.; Mavrakis, H.E.; Goudis, C.A.; Maliaraki, N.E.; Saloustros, I.G.; Milathianaki, M.E.; Chlouverakis, G.I.; Vardas, P.E. Effect of sinus rhythm restoration after electrical cardioversion on apelin and brain natriuretic Peptide prohormone levels in patients with persistent atrial fibrillation. Am. J. Cardiol. 2010, 105, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Tveit, A.; Seljeflot, I.; Grundvold, I.; Abdelnoor, M.; Arnesen, H.; Smith, P. Candesartan, NT-proBNP and recurrence of atrial fibrillation after electrical cardioversion. Int. J. Cardiol. 2009, 131, 234–239. [Google Scholar] [CrossRef]
- Mabuchi, N.; Tsutamoto, T.; Maeda, K.; Kinoshita, M. Plasma cardiac natriuretic peptides as biochemical markers of recurrence of atrial fibrillation in patients with mild congestive heart failure. Jpn. Circ. J. 2000, 64, 765–771. [Google Scholar] [CrossRef]
- Lewicka, E.; Dudzińska-Gehrmann, J.; Dąbrowska-Kugacka, A.; Zagożdżon, P.; Stepnowska, E.; Liżewska, A.; Kozłowski, D.; Raczak, G. Plasma biomarkers as predictors of recurrence of atrial fibrillation. Pol. Arch. Med. Wewn. 2015, 125, 424–433. [Google Scholar] [CrossRef]
- Buccelletti, F.; Gilardi, E.; Marsiliani, D.; Carroccia, A.; Silveri, N.G.; Franceschi, F. Predictive value of NT-proBNP for cardioversion in a new onset atrial fibrillation. Eur. J. Emerg. Med. 2011, 18, 157–161. [Google Scholar] [CrossRef]
- Koniari, I.; Artopoulou, E.; Velissaris, D.; Ainslie, M.; Mplani, V.; Karavasili, G.; Kounis, N.; Tsigkas, G. Biomarkers in the clinical management of patients with atrial fibrillation and heart failure. J. Geriatr. Cardiol. 2021, 18, 908–951. [Google Scholar] [CrossRef]
- Scalise, R.F.M.; De Sarro, R.; Caracciolo, A.; Lauro, R.; Squadrito, F.; Carerj, S.; Bitto, A.; Micari, A.; Bella, G.D.; Costa, F.; et al. Fibrosis after Myocardial Infarction: An Overview on Cellular Processes, Molecular Pathways, Clinical Evaluation and Prognostic Value. Med. Sci. 2021, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Ito, H.; Onuki, T.; Miyoshi, F.; Watanabe, N.; Asano, T.; Tanno, K.; Kobayashi, Y. Candesartan decreases type III procollagen-N-peptide levels and inflammatory marker levels and maintains sinus rhythm in patients with atrial fibrillation. J. Cardiovasc. Pharmacol. 2010, 55, 511–517. [Google Scholar] [CrossRef]
- Clementy, N.; Piver, E.; Bisson, A.; Andre, C.; Bernard, A.; Pierre, B.; Fauchier, L.; Babuty, D. Galectin-3 in Atrial Fibrillation: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 976. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Yin, X.; Levy, D.; Vasan, R.S.; Magnani, J.W.; Ellinor, P.T.; McManus, D.D.; Lubitz, S.A.; Larson, M.G.; Benjamin, E.J. Galectin 3 and incident atrial fibrillation in the community. Am. Heart J. 2014, 167, 729–734.e1. [Google Scholar] [CrossRef]
- Takemoto, Y.; Ramirez, R.J.; Yokokawa, M.; Kaur, K.; Ponce-Balbuena, D.; Sinno, M.C.; Willis, B.C.; Ghanbari, H.; Ennis, S.R.; Guerrero-Serna, G.; et al. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic Transl. Sci. 2016, 1, 143–154. [Google Scholar] [CrossRef]
- Yalcin, M.U.; Gurses, K.M.; Kocyigit, D.; Canpinar, H.; Canpolat, U.; Evranos, B.; Yorgun, H.; Sahiner, M.L.; Kaya, E.B.; Hazirolan, T.; et al. The Association of Serum Galectin-3 Levels with Atrial Electrical and Structural Remodeling. J. Cardiovasc. Electrophysiol. 2015, 26, 635–640. [Google Scholar] [CrossRef]
- Gürses, K.M.; Yalçın, M.U.; Koçyiğit, D.; Canpınar, H.; Ateş, A.H.; Canpolat, U.; Yorgun, H.; Güç, D.; Aytemir, K. Serum galectin-3 level predicts early recurrence following successful direct-current cardioversion in persistent atrial fibrillation patients. Turk. Kardiyol. Dern. Ars. 2019, 47, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Wałek, P.; Grabowska, U.; Cieśla, E.; Sielski, J.; Roskal-Wałek, J.; Wożakowska-Kapłon, B. Analysis of the Correlation of Galectin-3 Concentration with the Measurements of Echocardiographic Parameters Assessing Left Atrial Remodeling and Function in Patients with Persistent Atrial Fibrillation. Biomolecules 2021, 11, 1108. [Google Scholar] [CrossRef]
- Gong, M.; Cheung, A.; Wang, Q.S.; Li, G.; Goudis, C.A.; Bazoukis, G.; Lip, G.Y.H.; Baranchuk, A.; Korantzopoulos, P.; Letsas, K.P.; et al. Galectin-3 and risk of atrial fibrillation: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23104. [Google Scholar] [CrossRef]
- Cichoń, M.; Mizia-Szubryt, M.; Olszanecka-Glinianowicz, M.; Bożentowicz-Wikarek, M.; Owczarek, A.J.; Michalik, R.; Mizia-Stec, K. Biomarkers of left atrial overload in obese and nonobese patients with atrial fibrillation qualified for electrical cardioversion. Kardiol. Pol. 2021, 79, 269–276. [Google Scholar] [CrossRef]
- Pauklin, P.; Zilmer, M.; Eha, J.; Tootsi, K.; Kals, M.; Kampus, P. Markers of Inflammation, Oxidative Stress, and Fibrosis in Patients with Atrial Fibrillation. Oxid. Med. Cell. Longev. 2022, 2022, 4556671. [Google Scholar] [CrossRef] [PubMed]
- Saez-Maleta, R.; Merino-Merino, A.; Gundin-Menendez, S.; Salgado-Aranda, R.; Al Kassam-Martinez, D.; Pascual-Tejerina, V.; Martin-Gonzalez, J.; Garcia-Fernandez, J.; Perez-Rivera, J.A. sST2 and Galectin-3 genotyping in patients with persistent atrial fibrillation. Mol. Biol. Rep. 2021, 48, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Han, Q.F.; Mo, D.G. The progress of the soluble suppression of tumorigenicity 2 (sST2) in atrial fibrillation. J. Interv. Card. Electrophysiol. 2022, 65, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Li, Y.; Yan, Q.; Wu, W.; Xu, P.; Liu, L.; Luan, C.; Zhang, J.; Zheng, Q.; Xue, J. Higher serum sST2 is associated with increased left atrial low-voltage areas and atrial fibrillation recurrence in patients undergoing radiofrequency ablation. J. Interv. Card. Electrophysiol. 2022, 64, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, W.; Shao, Y.; Zhang, M.; Li, Z.; Wang, Z.; Lu, Y. Association of Soluble Suppression of Tumorigenicity 2 with New-Onset Atrial Fibrillation in Acute Myocardial Infarction. Cardiology 2022, 147, 381–388. [Google Scholar] [CrossRef]
- Tseng, C.C.S.; Huibers, M.M.H.; van Kuik, J.; de Weger, R.A.; Vink, A.; de Jonge, N. The interleukin-33/ST2 pathway is expressed in the failing human heart and associated with pro-fibrotic remodeling of the myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 15–21. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Zhang, C.; Tian, J.; Lu, A.; Bai, R.; An, J.; Greiser, A.; Huang, J.; Ma, X. Cardiovascular magnetic resonance-determined left ventricular myocardium impairment is associated with C-reactive protein and ST2 in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Magn. Reson. 2021, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.P.; Du, X.; Chen, J.J. Biomarkers for Predicting the Occurrence and Progression of Atrial Fibrillation: Soluble Suppression of Tumorigenicity 2 Protein and Tissue Inhibitor of Matrix Metalloproteinase-1. Int. J. Clin. Pract. 2022, 2022, 6926510. [Google Scholar] [CrossRef] [PubMed]
- Okar, S.; Kaypakli, O.; Şahin, D.Y.; Koç, M. Fibrosis Marker Soluble ST2 Predicts Atrial Fibrillation Recurrence after Cryoballoon Catheter Ablation of Nonvalvular Paroxysmal Atrial Fibrillation. Korean Circ. J. 2018, 48, 920–929. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Lin, Y.; Liang, X.; Zhao, S.; Li, M.; Chen, M. Role of sST2 in predicting recurrence of atrial fibrillation after radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 2020, 43, 1235–1241. [Google Scholar] [CrossRef]
- Wałek, P.; Gorczyca, I.; Grabowska, U.; Spałek, M.; Wożakowska-Kapłon, B. The prognostic value of soluble suppression of tumourigenicity 2 and galectin-3 for sinus rhythm maintenance after cardioversion due to persistent atrial fibrillation in patients with normal left ventricular systolic function. Europace 2020, 22, 1470–1479. [Google Scholar] [CrossRef]
- Begg, G.A.; Lip, G.Y.; Plein, S.; Tayebjee, M.H. Circulating biomarkers of fibrosis and cardioversion of atrial fibrillation: A prospective, controlled cohort study. Clin. Biochem. 2017, 50, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Munetsugu, Y.; Kawasaki, S.; Onishi, K.; Onuma, Y.; Kikuchi, M.; Tanno, K.; Kobayashi, Y. Type III procollagen-N-peptide as a predictor of persistent atrial fibrillation recurrence after cardioversion. Europace 2012, 14, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Çöteli, C.; Hazırolan, T.; Aytemir, K.; Erdemir, A.G.; Bakır, E.N.; Canpolat, U.; Yorgun, H.; Ateş, A.H.; Kaya, E.B.; Dikmen, Z.G.; et al. Evaluation of atrial fibrosis in atrial fibrillation patients with three different methods. Turk. J. Med. Sci. 2022, 52, 175–187. [Google Scholar] [CrossRef]
- Dong, Q.; Li, S.; Wang, W.; Han, L.; Xia, Z.; Wu, Y.; Tang, Y.; Li, J.; Cheng, X. FGF23 regulates atrial fibrosis in atrial fibrillation by mediating the STAT3 and SMAD3 pathways. J. Cell. Physiol. 2019, 234, 19502–19510. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Zhong, Y.T.; Tu, C.; Lan, J. Significance of serum fibroblast growth factor-23 and miR-208b in pathogenesis of atrial fibrillation and their relationship with prognosis. World J. Clin. Cases 2020, 8, 3458–3464. [Google Scholar] [CrossRef]
- Mizia-Stec, K.; Wieczorek, J.; Polak, M.; Wybraniec, M.T.; Woźniak-Skowerska, I.; Hoffmann, A.; Nowak, S.; Wikarek, M.; Wnuk-Wojnar, A.; Chudek, J.; et al. Lower soluble Klotho and higher fibroblast growth factor 23 serum levels are associated with episodes of atrial fibrillation. Cytokine 2018, 111, 106–111. [Google Scholar] [CrossRef]
- Chua, W.; Purmah, Y.; Cardoso, V.R.; Gkoutos, G.V.; Tull, S.P.; Neculau, G.; Thomas, M.R.; Kotecha, D.; Lip, G.Y.H.; Kirchhof, P.; et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur. Heart J. 2019, 40, 1268–1276. [Google Scholar] [CrossRef]
- Tan, Z.; Song, T.; Huang, S.; Liu, M.; Ma, J.; Zhang, J.; Yu, P.; Liu, X. Relationship between serum growth differentiation factor 15, fibroblast growth factor-23 and risk of atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 899667. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
- Breit, S.N.; Brown, D.A.; Tsai, V.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Perrone, M.A.; Aimo, A.; Bernardini, S.; Clerico, A. Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. Int. J. Mol. Sci. 2023, 24, 844. [Google Scholar] [CrossRef]
- Aulin, J.; Hijazi, Z.; Lindbäck, J.; Alexander, J.H.; Gersh, B.J.; Granger, C.B.; Hanna, M.; Horowitz, J.; Lopes, R.D.; McMurray, J.J.V.; et al. Biomarkers and heart failure events in patients with atrial fibrillation in the ARISTOTLE trial evaluated by a multi-state model. Am. Heart J. 2022, 251, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Wallentin, L.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Granger, C.B.; Lopes, R.D.; Pol, T.; et al. Screening of Multiple Biomarkers Associated with Ischemic Stroke in Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e018984. [Google Scholar] [CrossRef]
- Charafeddine, K.; Zakka, P.; Bou Dargham, B.; Abdulhai, F.; Zakka, K.; Zouein, F.A.; Refaat, M. Potential Biomarkers in Atrial Fibrillation: Insight into Their Clinical Significance. J. Cardiovasc. Pharmacol. 2021, 78, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Liu, H.; Ng, C.Y.; Xu, G.; Liu, E.; Li, G.; Liu, T. Circulating serum levels of growth differentiation factor-15 and neuregulin-1 in patients with paroxysmal non-valvular atrial fibrillation. Int. J. Cardiol. 2014, 172, e311–e313. [Google Scholar] [CrossRef]
- Eddy, A.C.; Trask, A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Cytokine Growth Factor Rev. 2021, 57, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lyngbakken, M.N.; Rønningen, P.S.; Solberg, M.G.; Berge, T.; Brynildsen, J.; Aagaard, E.N.; Kvisvik, B.; Røsjø, H.; Steine, K.; Tveit, A.; et al. Prediction of incident atrial fibrillation with cardiac biomarkers and left atrial volumes. Heart 2023, 109, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.T.; Małecka, B.; Lelakowski, J.; Undas, A. Association of NT-proBNP and GDF-15 with markers of a prothrombotic state in patients with atrial fibrillation off anticoagulation. Clin. Res. Cardiol. 2020, 109, 426–434. [Google Scholar] [CrossRef]
- Ding, B.; Liu, P.; Zhang, F.; Hui, J.; He, L. Predicting Values of Neutrophil-to-Lymphocyte Ratio (NLR), High-Sensitivity C-Reactive Protein (hs-CRP), and Left Atrial Diameter (LAD) in Patients with Nonvalvular Atrial Fibrillation Recurrence After Radiofrequency Ablation. Med. Sci. Monit. 2022, 28, e934569. [Google Scholar] [CrossRef]
- Li, X.; Peng, S.; Wu, X.; Guan, B.; Tse, G.; Chen, S.; Zhou, G.; Wei, Y.; Gong, C.; Lu, X.; et al. C-reactive protein and atrial fibrillation: Insights from epidemiological and Mendelian randomization studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1519–1527. [Google Scholar] [CrossRef]
- Fu, Y.; Pan, Y.; Gao, Y.; Yang, X.; Chen, M. Predictive value of CHA2DS2-VASc score combined with hs-CRP for new-onset atrial fibrillation in elderly patients with acute myocardial infarction. BMC Cardiovasc. Disord. 2021, 21, 175. [Google Scholar] [CrossRef]
- Sinning, C.; Kempf, T.; Schwarzl, M.; Lanfermann, S.; Ojeda, F.; Schnabel, R.B.; Zengin, E.; Wild, P.S.; Lackner, K.J.; Munzel, T.; et al. Biomarkers for characterization of heart failure–Distinction of heart failure with preserved and reduced ejection fraction. Int. J. Cardiol. 2017, 227, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, C.; Vlachopoulos, C.; Lazaros, G.; Tousoulis, D. Biomarkers of Atrial Fibrillation in Metabolic Syndrome. Curr. Med. Chem. 2019, 26, 898–908. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, Q. Expression and predictive value of NLRP3 in patients with atrial fibrillation and stroke. Am. J. Transl. Res. 2022, 14, 3104–3112. [Google Scholar] [PubMed]
- Loricchio, M.L.; Cianfrocca, C.; Pasceri, V.; Bianconi, L.; Auriti, A.; Calo, L.; Lamberti, F.; Castro, A.; Pandozi, C.; Palamara, A.; et al. Relation of C-reactive protein to long-term risk of recurrence of atrial fibrillation after electrical cardioversion. Am. J. Cardiol. 2007, 99, 1421–1424. [Google Scholar] [CrossRef]
- Lombardi, F.; Tundo, F.; Belletti, S.; Mantero, A.; Melzi D’eril, G.V. C-reactive protein but not atrial dysfunction predicts recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. J. Cardiovasc. Med. 2008, 9, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Barassi, A.; Pezzilli, R.; Morselli-Labate, A.M.; Lombardi, F.; Belletti, S.; Dogliotti, G.; Corsi, M.M.; Merlini, G.; Melzi d’Eril, G.V. Serum amyloid a and C-reactive protein independently predict the recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. Can. J. Cardiol. 2012, 28, 537–541. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Kalantzi, K.; Siogas, K.; Goudevenos, J.A. Long-term prognostic value of baseline C-reactive protein in predicting recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin. Electrophysiol. 2008, 31, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Arakawa, T.; Uchiyama, T.; Kodama, I.; Hishida, H. High-sensitivity C-reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int. J. Cardiol. 2006, 108, 346–353. [Google Scholar] [CrossRef]
- Henningsen, K.M.; Therkelsen, S.K.; Bruunsgaard, H.; Krabbe, K.S.; Pedersen, B.K.; Svendsen, J.H. Prognostic impact of hs-CRP and IL-6 in patients with persistent atrial fibrillation treated with electrical cardioversion. Scand. J. Clin. Lab. Investig. 2009, 69, 425–432. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Korantzopoulos, P.; Goudevenos, J.A.; Li, G. Meta-analysis of association between C-reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am. J. Cardiol. 2008, 101, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Yo, C.H.; Lee, S.H.; Chang, S.S.; Lee, M.C.; Lee, C.C. Value of high-sensitivity C-reactive protein assays in predicting atrial fibrillation recurrence: A systematic review and meta-analysis. BMJ Open 2014, 4, e004418. [Google Scholar] [CrossRef]
- Celebi, O.O.; Celebi, S.; Canbay, A.; Ergun, G.; Aydogdu, S.; Diker, E. The effect of sinus rhythm restoration on high-sensitivity C-reactive protein levels and their association with long-term atrial fibrillation recurrence after electrical cardioversion. Cardiology 2011, 118, 168–174. [Google Scholar] [CrossRef]
- Krishnan, A.; Chilton, E.; Raman, J.; Saxena, P.; McFarlane, C.; Trollope, A.F.; Kinobe, R.; Chilton, L. Are Interactions between Epicardial Adipose Tissue, Cardiac Fibroblasts and Cardiac Myocytes Instrumental in Atrial Fibrosis and Atrial Fibrillation? Cells 2021, 10, 2501. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and Heart Failure: Challenging Role in Adverse Cardiac Remodeling, Myopathy, and Clinical Outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef] [PubMed]
- Suffee, N.; Moore-Morris, T.; Jagla, B.; Mougenot, N.; Dilanian, G.; Berthet, M.; Proukhnitzky, J.; Le Prince, P.; Tregouet, D.A.; Pucéat, M.; et al. Reactivation of the Epicardium at the Origin of Myocardial Fibro-Fatty Infiltration During the Atrial Cardiomyopathy. Circ. Res. 2020, 126, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Maeda, N.; Sonoda, M.; Ohashi, K.; Hibuse, T.; Nishizawa, H.; Nishida, M.; Hiuge, A.; Kurata, A.; Kihara, S.; et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 863–870. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.H.; Kim, E.N.; Hong, Y.A.; Park, H.J.; Chung, S.; Choi, B.S.; Kim, Y.S.; Park, J.Y.; Kim, H.W.; et al. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell Death Dis. 2022, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaundal, R.K.; Zhao, B.; Bouchareb, R.; Lebeche, D. Resistin induces cardiac fibroblast-myofibroblast differentiation through JAK/STAT3 and JNK/c-Jun signaling. Pharmacol. Res. 2021, 167, 105414. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Jurado-López, R.; Valero-Muñoz, M.; Bartolomé, M.V.; Ballesteros, S.; Luaces, M.; Briones, A.M.; López-Andrés, N.; Miana, M.; Cachofeiro, V. Leptin induces cardiac fibrosis through galectin-3, mTOR and oxidative stress: Potential role in obesity. J. Hypertens. 2014, 32, 1104–1114; discussion 1114. [Google Scholar] [CrossRef]
- Rienstra, M.; Sun, J.X.; Lubitz, S.A.; Frankel, D.S.; Vasan, R.S.; Levy, D.; Magnani, J.W.; Sullivan, L.M.; Meigs, J.B.; Ellinor, P.T.; et al. Plasma resistin, adiponectin, and risk of incident atrial fibrillation: The Framingham Offspring Study. Am. Heart J. 2012, 163, 119–124.e1. [Google Scholar] [CrossRef]
- Peller, M.; Kapłon-Cieślicka, A.; Rosiak, M.; Tymińska, A.; Ozierański, K.; Eyileten, C.; Kondracka, A.; Mirowska-Guzel, D.; Opolski, G.; Postuła, M.; et al. Are adipokines associated with atrial fibrillation in type 2 diabetes? Endokrynol. Pol. 2020, 71, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Velliou, M.; Sanidas, E.; Papadopoulos, D.; Iliopoulos, D.; Mantzourani, M.; Toutouzas, K.; Barbetseas, J. Adipokines and atrial fibrillation: The important role of apelin. Hell. J. Cardiol. 2021, 62, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Agbaedeng, T.A.; Zacharia, A.L.; Iroga, P.E.; Rathnasekara, V.M.; Munawar, D.A.; Bursill, C.; Noubiap, J.J. Associations between adipokines and atrial fibrillation: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Antushevich, H.; Wójcik, M. Review: Apelin in disease. Clin. Chim. Acta 2018, 483, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ilaghi, M.; Soltanizadeh, A.; Amiri, S.; Kohlmeier, K.A.; Shabani, M. The apelin/APJ signaling system and cytoprotection: Role of its cross-talk with kappa opioid receptor. Eur. J. Pharmacol. 2022, 936, 175353. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Chen, L. Novel pathogenesis: Regulation of apoptosis by Apelin/APJ system. Acta Biochim. Biophys. Sin. 2017, 49, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wu, D.; Li, L.; Chen, L. Apelin/APJ system: A bifunctional target for cardiac hypertrophy. Int. J. Cardiol. 2017, 230, 164–170. [Google Scholar] [CrossRef]
- Rikitake, Y. The apelin/APJ system in the regulation of vascular tone: Friend or foe? J. Biochem. 2021, 169, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, H.; Adhikari, B.K.; Wang, S.; Yang, N.; Liu, W.; Sun, J.; Wang, Y. The Role of Apelin-APJ System in Diabetes and Obesity. Front. Endocrinol. 2022, 13, 820002. [Google Scholar] [CrossRef]
- Parikh, V.N.; Liu, J.; Shang, C.; Woods, C.; Chang, A.C.; Zhao, M.; Charo, D.N.; Grunwald, Z.; Huang, Y.; Seo, K.; et al. Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H348–H356. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; O’Rourke, S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol. Ther. 2018, 190, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.J. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 1–10. [Google Scholar] [CrossRef]
- Askin, L.; Askin, H.S.; Tanrıverdi, O.; Ozyildiz, A.G.; Duman, H. Serum apelin levels and cardiovascular diseases. North Clin. Istanb. 2022, 9, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Riazian, M.; Khorrami, E.; Alipoor, E.; Moradmand, S.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Assessment of Apelin Serum Levels in Persistent Atrial Fibrillation and Coronary Artery Disease. Am. J. Med. Sci. 2016, 352, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Hosseini-Bensenjan, M.; Salahi, S.; Moazzen, F.; Aria, H.; Manafi, A.; Hosseini, S.; Niknam, M.; Asadikaram, G. Apelin and its ratio to lipid factors are associated with cardiovascular diseases: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0271899. [Google Scholar] [CrossRef]
- Bohm, A.; Urban, L.; Tothova, L.; Bezak, B.; Uher, T.; Musil, P.; Kyselovic, J.; Lipton, J.; Olejnik, P.; Hatala, R. Concentration of apelin inversely correlates with atrial fibrillation burden. Bratisl. Lek. Listy 2021, 122, 165–171. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Fan, J.; Zhong, B.; Xu, Q. Apelin: A novel prognostic predictor for atrial fibrillation recurrence after pulmonary vein isolation. Medicine 2018, 97, e12580. [Google Scholar] [CrossRef]
- Falcone, C.; Buzzi, M.P.; D’Angelo, A.; Schirinzi, S.; Falcone, R.; Rordorf, R.; Capettini, A.C.; Landolina, M.; Storti, C.; Pelissero, G. Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation. Int. J. Immunopathol. Pharmacol. 2010, 23, 917–925. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lakin, R.; Zhang, H.; Liu, J.; Sachedina, A.; Singh, M.; Wilson, E.; Perez, M.; Verma, S.; Quertermous, T.; et al. Apelin increases atrial conduction velocity, refractoriness, and prevents inducibility of atrial fibrillation. JCI Insight 2020, 5, e126525. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Zhang, L.; Cheng, X.; Wang, H.; Qin, W.; Zhou, X.; Tang, B. Apelin Inhibits Angiotensin II-Induced Atrial Fibrosis and Atrial Fibrillation via TGF-β1/Smad2/α-SMA Pathway. Front. Physiol. 2020, 11, 583570. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, Y.; Li, X.; Chen, F.; Zhao, J.; Hu, J.; Shan, A.; Qiao, S.; Wei, Z.; He, G.; et al. Involvement of Apelin/APJ Axis in Thrombogenesis in Valve Heart Disease Patients with Atrial Fibrillation. Int. Heart J. 2019, 60, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Yoshida, K.; Ieda, M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int. J. Mol. Sci. 2019, 20, 2824. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and musculoskeletal health. Ann. New York Acad. Sci. 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef]
- Gamas, L.; Matafome, P.; Seiça, R. Irisin and Myonectin Regulation in the Insulin Resistant Muscle: Implications to Adipose Tissue: Muscle Crosstalk. J. Diabetes Res. 2015, 2015, 359159. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, X.; Chen, Y.; Zhao, Q. Decreased irisin secretion contributes to muscle insulin resistance in high-fat diet mice. Int. J. Clin. Exp. Pathol. 2015, 8, 6490–6497. [Google Scholar] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef]
- Shoukry, A.; Shalaby, S.M.; El-Arabi Bdeer, S.; Mahmoud, A.A.; Mousa, M.M.; Khalifa, A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life 2016, 68, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Wang, C.Y. Role of Irisin in Myocardial Infarction, Heart Failure, and Cardiac Hypertrophy. Cells 2021, 10, 2103. [Google Scholar] [CrossRef]
- Luna-Ceron, E.; González-Gil, A.M.; Elizondo-Montemayor, L. Current Insights on the Role of Irisin in Endothelial Dysfunction. Curr. Vasc. Pharmacol. 2022, 20, 205–220. [Google Scholar] [CrossRef]
- Hsieh, I.C.; Ho, M.Y.; Wen, M.S.; Chen, C.C.; Hsieh, M.J.; Lin, C.P.; Yeh, J.K.; Tsai, M.L.; Yang, C.H.; Wu, V.C.; et al. Serum irisin levels are associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Int. J. Cardiol. 2018, 261, 12–17. [Google Scholar] [CrossRef]
- Abd El-Mottaleb, N.A.; Galal, H.M.; El Maghraby, K.M.; Gadallah, A.I. Serum irisin level in myocardial infarction patients with or without heart failure. Can. J. Physiol. Pharmacol. 2019, 97, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Fushtey, I.M.; Berezin, A.E. Serum Levels of Irisin Predict Cumulative Clinical Outcomes in Heart Failure Patients with Type 2 Diabetes Mellitus. Front. Physiol. 2022, 13, 922775. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Stöhr, E.; Berezin, A.E. Discriminative Value of Serum Irisin in Prediction of Heart Failure with Different Phenotypes among Patients with Type 2 Diabetes Mellitus. Cells 2022, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, J.; Straus, L.; Novaković, M.; Košuta, D.; Božič Mijovski, M.; Tasič, J.; Jug, B. HFpEF and Atrial Fibrillation: The Enigmatic Interplay of Dysmetabolism, Biomarkers, and Vascular Endothelial Dysfunction. Dis. Markers 2022, 2022, 9539676. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. Osteopontin in Cardiovascular Diseases. Biomolecules 2021, 11, 1047. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A. Circulating osteopontin as a marker of early coronary vascular calcification in type two diabetes mellitus patients with known asymptomatic coronary artery disease. Atherosclerosis 2013, 229, 475–481. [Google Scholar] [CrossRef]
- Rewiuk, K.; Grodzicki, T. Osteoprotegerin and TRAIL in Acute Onset of Atrial Fibrillation. Biomed. Res. Int. 2015, 2015, 259843. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bao, Y.; Du, Z.; Zhou, Y.; Zhang, N.; Lin, C.; Xie, Y.; Zhang, R.; Li, Q.; Quan, J.; et al. Plasma protein profiling analysis in patients with atrial fibrillation before and after three different ablation techniques. Front. Cardiovasc. Med. 2023, 9, 1077992. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Kremzer, A.A.; Martovitskaya, Y.V.; Samura, T.A.; Berezina, T.A.; Zulli, A.; Klimas, J.; Kruzliak, P. The utility of biomarker risk prediction score in patients with chronic heart failure. Int. J. Clin. Exp. Med. 2015, 8, 18255–18264. [Google Scholar] [CrossRef]
- Lo, Y.M.D.; Han, D.S.C.; Jiang, P.; Chiu, R.W.K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 2021, 372, eaaw3616. [Google Scholar] [CrossRef]
- Luo, H.; Wei, W.; Ye, Z.; Zheng, J.; Xu, R.H. Liquid Biopsy of Methylation Biomarkers in Cell-Free DNA. Trends Mol. Med. 2021, 27, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.M.; Blume, N.; Abu-Fares, O.; Götz, F.; Ernst, J.; Leotescu, A.; Gabriel, M.M.; van Gemmeren, T.; Worthmann, H.; Lichtinghagen, R.; et al. Endogenous Deoxyribonuclease Activity and Cell-Free Deoxyribonucleic Acid in Acute Ischemic Stroke: A Cohort Study. Stroke 2022, 53, 1235–1244. [Google Scholar] [CrossRef]
- Berezina, T.A.; Kopytsya, M.P.; Petyunina, O.V.; Berezin, A.A.; Obradovic, Z.; Schmidbauer, L.; Lichtenauer, M.; Berezin, A.E. Lower Circulating Cell-Free Mitochondrial DNA Is Associated with Heart Failure in Type 2 Diabetes Mellitus Patients. Cardiogenetics 2023, 13, 15–30. [Google Scholar] [CrossRef]
- Li, X.; Hu, R.; Luo, T.; Peng, C.; Gong, L.; Hu, J.; Yang, S.; Li, Q. Serum cell-free DNA and progression of diabetic kidney disease: A prospective study. BMJ Open Diabetes Res. Care 2020, 8, e001078. [Google Scholar] [CrossRef]
- Gianni, C.; Palleschi, M.; Merloni, F.; Di Menna, G.; Sirico, M.; Sarti, S.; Virga, A.; Ulivi, P.; Cecconetto, L.; Mariotti, M.; et al. Cell-Free DNA Fragmentomics: A Promising Biomarker for Diagnosis, Prognosis and Prediction of Response in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 14197. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis with Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front. Immunol. 2022, 12, 801897. [Google Scholar] [CrossRef]
- Yamazoe, M.; Sasano, T.; Ihara, K.; Takahashi, K.; Nakamura, W.; Takahashi, N.; Komuro, H.; Hamada, S.; Furukawa, T. Sparsely methylated mitochondrial cell free DNA released from cardiomyocytes contributes to systemic inflammatory response accompanied by atrial fibrillation. Sci. Rep. 2021, 11, 5837. [Google Scholar] [CrossRef]
- Wiersma, M.; van Marion, D.M.S.; Bouman, E.J.; Li, J.; Zhang, D.; Ramos, K.S.; Lanters, E.A.H.; de Groot, N.M.S.; Brundel, B.J.J.M. Cell-Free Circulating Mitochondrial DNA: A Potential Blood-Based Marker for Atrial Fibrillation. Cells 2020, 9, 1159. [Google Scholar] [CrossRef]
- Soltész, B.; Urbancsek, R.; Pös, O.; Hajas, O.; Forgács, I.N.; Szilágyi, E.; Nagy-Baló, E.; Szemes, T.; Csanádi, Z.; Nagy, B. Quantification of peripheral whole blood, cell-free plasma and exosome encapsulated mitochondrial DNA copy numbers in patients with atrial fibrillation. J. Biotechnol. 2019, 299, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Geurts, S.; Mens, M.M.J.; Bos, M.M.; Ikram, M.A.; Ghanbari, M.; Kavousi, M. Circulatory MicroRNAs in Plasma and Atrial Fibrillation in the General Population: The Rotterdam Study. Genes 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Elias, A.; Tajes, M.; Yañez-Bisbe, L.; Enjuanes, C.; Comín-Colet, J.; Serra, S.A.; Fernández-Fernández, J.M.; Aguilar-Agon, K.W.; Reilly, S.; Martí-Almor, J.; et al. Atrial Fibrillation in Heart Failure Is Associated with High Levels of Circulating microRNA-199a-5p and 22-5p and a Defective Regulation of Intracellular Calcium and Cell-to-Cell Communication. Int. J. Mol. Sci. 2021, 22, 10377. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Zhang, Y.L.; Yang, X.C. Relationship between circulating miRNA-21, atrial fibrosis, and atrial fibrillation in patients with atrial enlargement. Ann. Palliat. Med. 2021, 10, 12742–12749. [Google Scholar] [CrossRef] [PubMed]
- Benito, B.; García-Elías, A.; Ois, Á.; Tajes, M.; Vallès, E.; Ble, M.; Yáñez Bisbe, L.; Giralt-Steinhauer, E.; Rodríguez-Campello, A.; Cladellas Capdevila, M.; et al. Plasma levels of miRNA-1-3p are associated with subclinical atrial fibrillation in patients with cryptogenic stroke. Rev. Esp. Cardiol. (Engl. Ed). 2022, 75, 717–726, (In English and Spanish). [Google Scholar] [CrossRef]
- Park, H.; Park, H.; Park, J. Circulating microRNA-423 attenuates the phosphorylation of calcium handling proteins in atrial fibrillation. Mol. Med. Rep. 2022, 25, 186. [Google Scholar] [CrossRef]
- Arroyo, A.B.; de Los Reyes-García, A.M.; Rivera-Caravaca, J.M.; Valledor, P.; García-Barberá, N.; Roldán, V.; Vicente, V.; Martínez, C.; González-Conejero, R. MiR-146a Regulates Neutrophil Extracellular Trap Formation That Predicts Adverse Cardiovascular Events in Patients with Atrial Fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.M.G.; de Araújo, J.N.G.; de Oliveira, K.M.; Novaes, A.E.M.; Lopes, M.B.; de Sousa, J.C.V.; Filho, A.A.A.; Luchessi, A.D.; de Rezende, A.A.; Hirata, M.H.; et al. Circulating miRNAs in acute new-onset atrial fibrillation and their target mRNA network. J. Cardiovasc. Electrophysiol. 2018, 29, 1159–1166. [Google Scholar] [CrossRef]
- Zhou, Q.; Maleck, C.; von Ungern-Sternberg, S.N.I.; Neupane, B.; Heinzmann, D.; Marquardt, J.; Duckheim, M.; Scheckenbach, C.; Stimpfle, F.; Gawaz, M.; et al. Circulating MicroRNA-21 Correlates with Left Atrial Low-Voltage Areas and Is Associated with Procedure Outcome in Patients Undergoing Atrial Fibrillation Ablation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006242. [Google Scholar] [CrossRef]
- Rochette, L.; Lorin, J.; Zeller, M.; Guilland, J.C.; Lorgis, L.; Cottin, Y.; Vergely, C. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: Possible therapeutic targets? Pharmacol. Ther. 2013, 140, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Büttner, P.; Bahls, M.; Böger, R.H.; Hindricks, G.; Thiele, H.; Schwedhelm, E.; Kornej, J. Arginine derivatives in atrial fibrillation progression phenotypes. J. Mol. Med. 2020, 98, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Ramuschkat, M.; Appelbaum, S.; Atzler, D.; Zeller, T.; Bauer, C.; Ojeda, F.M.; Sinning, C.R.; Hoffmann, B.; Lackner, K.J.; Böger, R.H.; et al. ADMA, subclinical changes and atrial fibrillation in the general population. Int. J. Cardiol. 2016, 203, 640–646. [Google Scholar] [CrossRef]
- Horowitz, J.D.; De Caterina, R.; Heresztyn, T.; Alexander, J.H.; Andersson, U.; Lopes, R.D.; Steg, P.G.; Hylek, E.M.; Mohan, P.; Hanna, M.; et al. Asymmetric and Symmetric Dimethylarginine Predict Outcomes in Patients with Atrial Fibrillation: An ARISTOTLE Substudy. J. Am. Coll. Cardiol. 2018, 72, 721–733. [Google Scholar] [CrossRef]
- Goette, A.; Hammwöhner, M.; Bukowska, A.; Scalera, F.; Martens-Lobenhoffer, J.; Dobrev, D.; Ravens, U.; Weinert, S.; Medunjanin, S.; Lendeckel, U.; et al. The impact of rapid atrial pacing on ADMA and endothelial NOS. Int. J. Cardiol. 2012, 154, 141–146. [Google Scholar] [CrossRef]
- Goni, L.; Razquin, C.; Toledo, E.; Guasch-Ferré, M.; Clish, C.B.; Babio, N.; Wittenbecher, C.; Atzeni, A.; Li, J.; Liang, L.; et al. Arginine catabolism metabolites and atrial fibrillation or heart failure risk: 2 case-control studies within the Prevención con Dieta Mediterránea (PREDIMED) trial. Am. J. Clin. Nutr. 2022, 116, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, W.; Feng, W.; Qu, X. Effects of rosuvastatin on serum asymmetric dimethylarginine levels and atrial structural remodeling in atrial fibrillation dogs. Pacing Clin. Electrophysiol. 2012, 35, 456–464. [Google Scholar] [CrossRef]
- Lao, M.C.; Liu, L.J.; Luo, C.F.; Lu, G.H.; Zhai, Y.S.; Chen, X.L.; Gao, X.R. Effect of asymmetrical dimethylarginine for predicting pro-thrombotic risk in atrial fibrillation. Zhonghua Yi Xue Za Zhi 2016, 96, 2059–2063. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Lu, T.M.; Lin, Y.J.; Tsao, H.M.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Hsieh, M.H.; Chen, S.A. Plasma asymmetric dimethylarginine and adverse events in patients with atrial fibrillation referred for coronary angiogram. PLoS ONE 2013, 8, e71675. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Qu, X.; Yu, Y.; Zhang, X.; Feng, W.; Song, Y. Asymmetric dimethylarginine concentration and early recurrence of atrial fibrillation after electrical cardioversion. Pacing Clin. Electrophysiol. 2008, 31, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Tveit, A.; Arnesen, H.; Smith, P.; Bratseth, V.; Seljeflot, I. L-arginine, asymmetric dimethylarginine and rhythm outcome after electrical cardioversion for atrial fibrillation. Cardiology 2010, 117, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Maas, R.; Wang, N.; Yin, X.; Larson, M.G.; Levy, D.; Ellinor, P.T.; Lubitz, S.A.; McManus, D.D.; Magnani, J.W.; et al. Asymmetric dimethylarginine, related arginine derivatives, and incident atrial fibrillation. Am. Heart J. 2016, 176, 100–106. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, O.; Berezin, A.E.; Mirna, M.; Boxhammer, E.; Gharibeh, S.X.; Hoppe, U.C.; Lichtenauer, M. Biomarkers of Atrial Fibrillation Recurrence in Patients with Paroxysmal or Persistent Atrial Fibrillation Following External Direct Current Electrical Cardioversion. Biomedicines 2023, 11, 1452. https://doi.org/10.3390/biomedicines11051452
Demirel O, Berezin AE, Mirna M, Boxhammer E, Gharibeh SX, Hoppe UC, Lichtenauer M. Biomarkers of Atrial Fibrillation Recurrence in Patients with Paroxysmal or Persistent Atrial Fibrillation Following External Direct Current Electrical Cardioversion. Biomedicines. 2023; 11(5):1452. https://doi.org/10.3390/biomedicines11051452
Chicago/Turabian StyleDemirel, Ozan, Alexander E. Berezin, Moritz Mirna, Elke Boxhammer, Sarah X. Gharibeh, Uta C. Hoppe, and Michael Lichtenauer. 2023. "Biomarkers of Atrial Fibrillation Recurrence in Patients with Paroxysmal or Persistent Atrial Fibrillation Following External Direct Current Electrical Cardioversion" Biomedicines 11, no. 5: 1452. https://doi.org/10.3390/biomedicines11051452
APA StyleDemirel, O., Berezin, A. E., Mirna, M., Boxhammer, E., Gharibeh, S. X., Hoppe, U. C., & Lichtenauer, M. (2023). Biomarkers of Atrial Fibrillation Recurrence in Patients with Paroxysmal or Persistent Atrial Fibrillation Following External Direct Current Electrical Cardioversion. Biomedicines, 11(5), 1452. https://doi.org/10.3390/biomedicines11051452









