Histologic Analysis of Idiopathic Pulmonary Fibrosis by Morphometric and Fractal Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Data
2.2. Histology and Histomorphometry
2.3. Statistics
3. Results
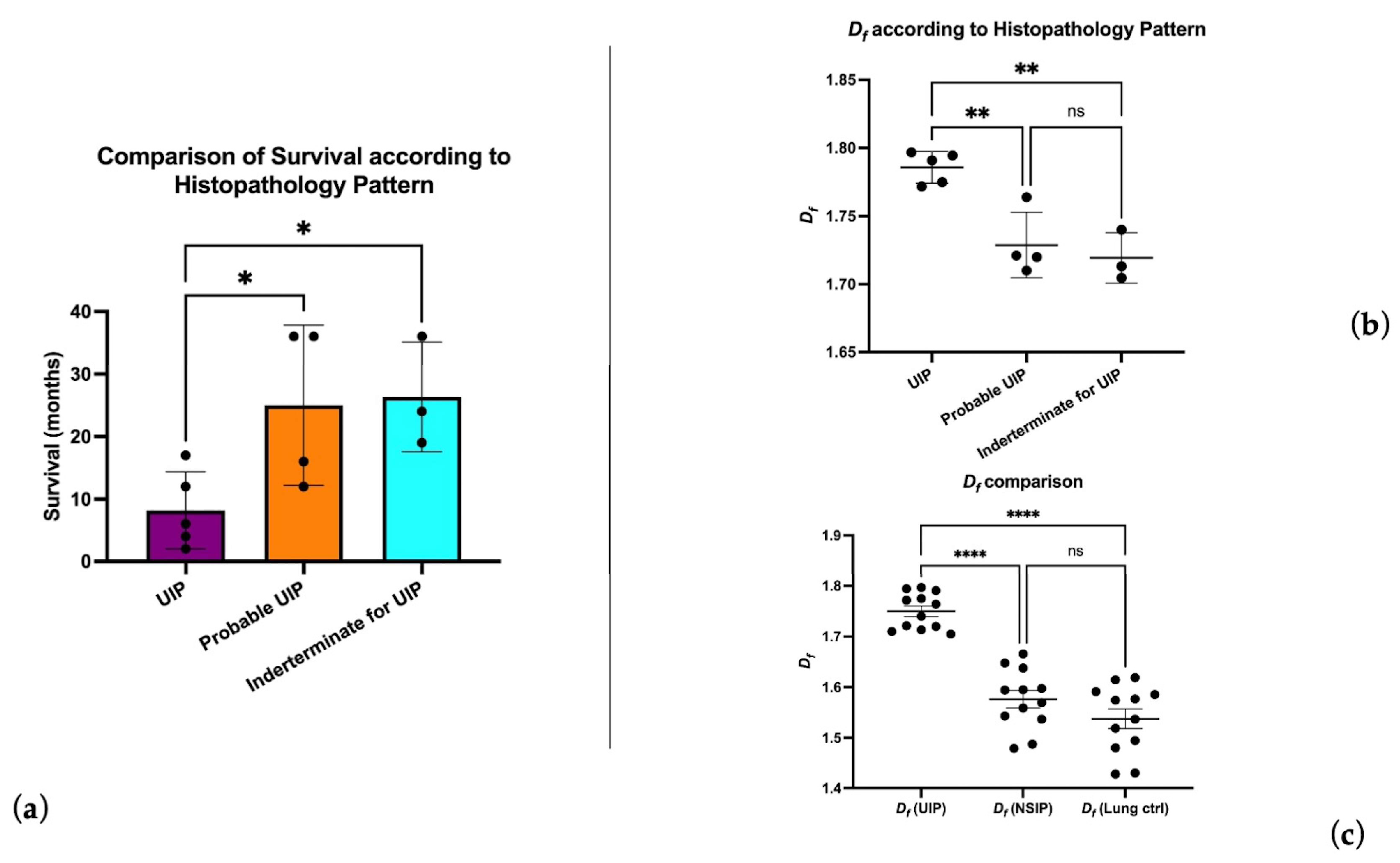
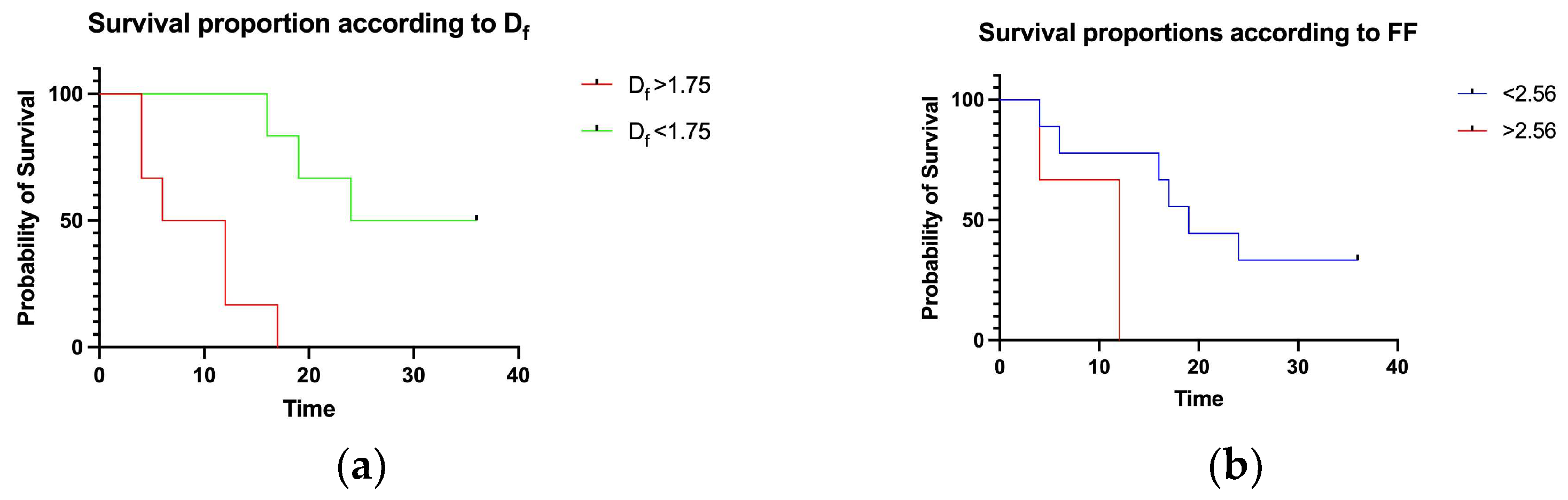
3.1. UIP and Df Analysis
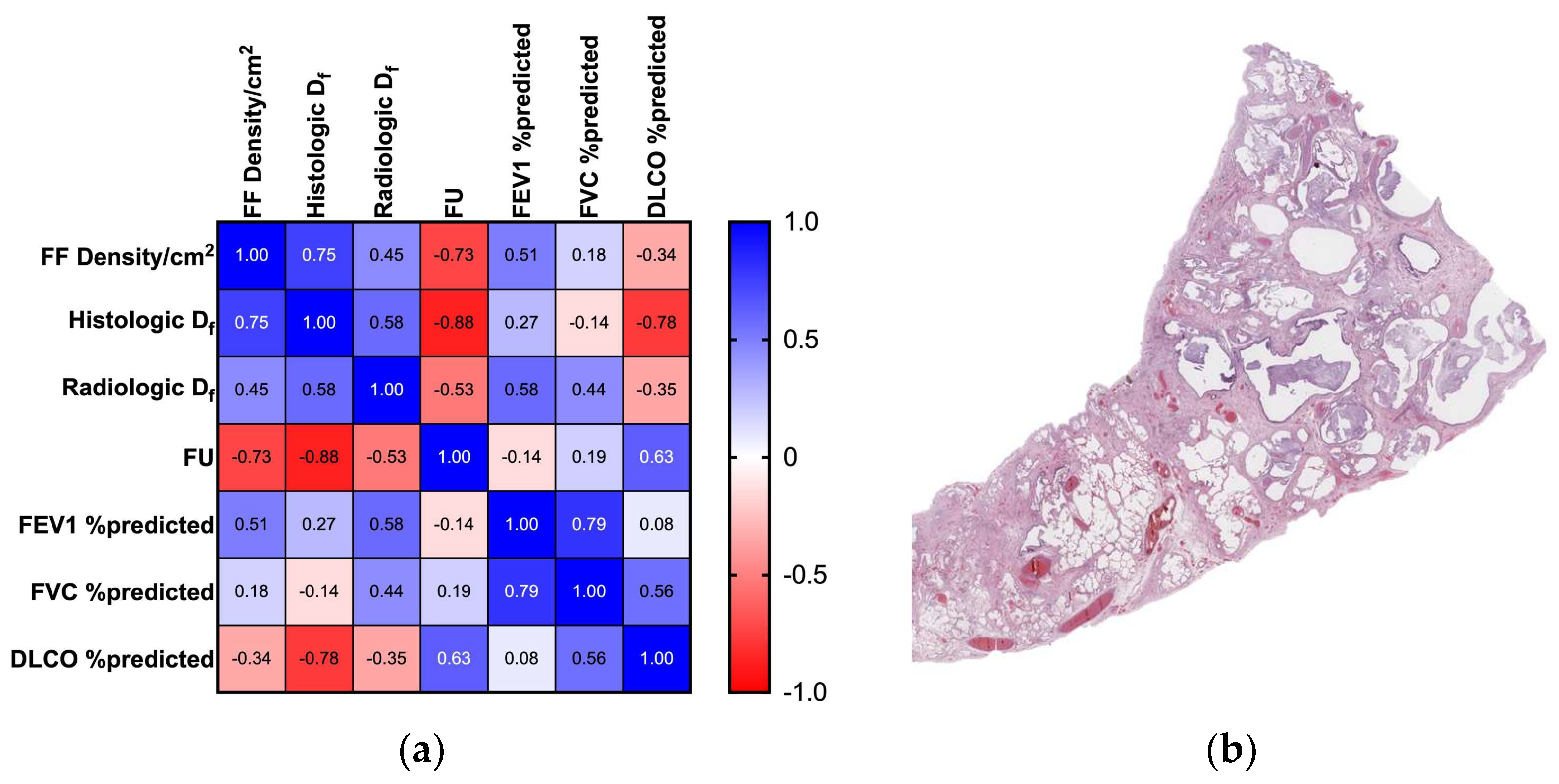
3.2. FF Density and Df Analysis
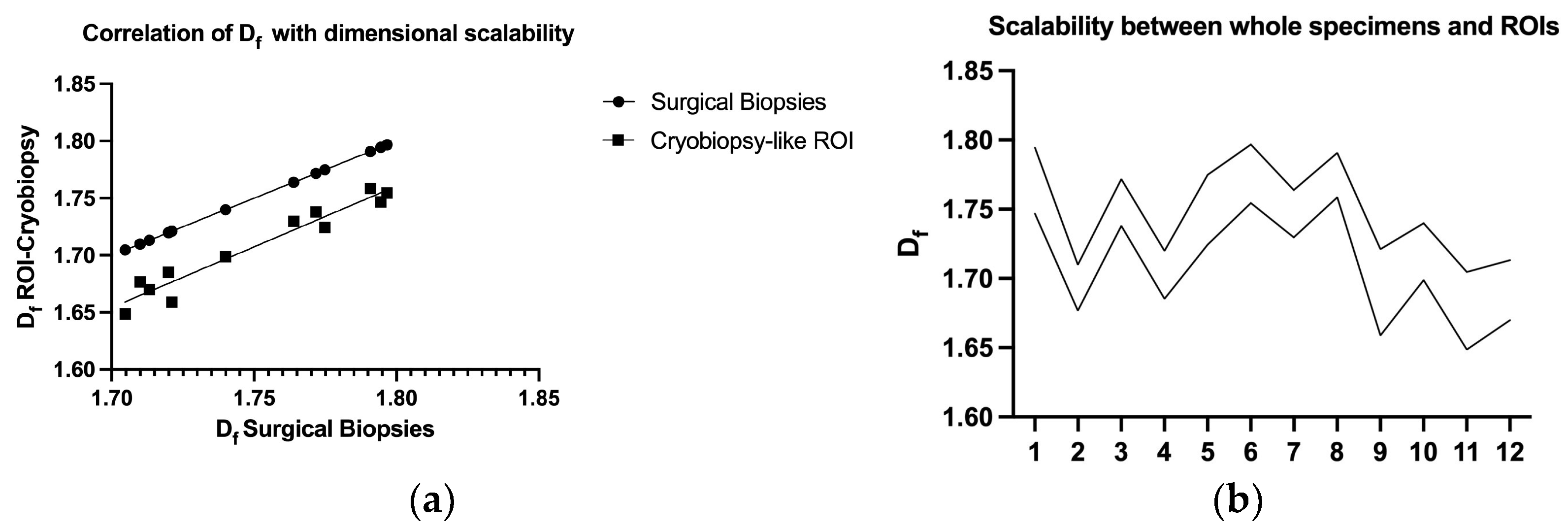
3.3. Df Measurements Scalability and Self-Similarity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Failla, M.; Crimi, N.; Raghu, G. Idiopathic Pulmonary Fibrosis: A Disease with Similarities and Links to Cancer Biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.; López-Otín, C.; Pardo, A. Age-Driven Developmental Drift in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2016, 48, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, W.; Xia, C.; Li, Z.; Zhao, W.; Xu, K.; Wang, N.; Lian, H.; Rosas, I.O.; Yu, G. TRIB3 Mediates Fibroblast Activation and Fibrosis Though Interaction with ATF4 in IPF. Int. J. Mol. Sci. 2022, 23, 15705. [Google Scholar] [CrossRef]
- Keow, J.; Cecchini, M.J.; Jayawardena, N.; Zompatori, M.; Joseph, M.G.; Mura, M. Digital Quantification of P16-Positive Foci in Fibrotic Interstitial Lung Disease Is Associated with a Phenotype of Idiopathic Pulmonary Fibrosis with Reduced Survival. Respir. Res. 2022, 23, 147. [Google Scholar] [CrossRef]
- De Vitis, C.; D’Ascanio, M.; Sacconi, A.; Pizzirusso, D.; Salvati, V.; Mancini, M.; Scafetta, G.; Cirombella, R.; Ascenzi, F.; Bruschini, S.; et al. B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 15040. [Google Scholar] [CrossRef]
- Poletti, V.; Ravaglia, C.; Buccioli, M.; Tantalocco, P.; Piciucchi, S.; Dubini, A.; Carloni, A.; Chilosi, M.; Tomassetti, S. Idiopathic Pulmonary Fibrosis: Diagnosis and Prognostic Evaluation. Respiration 2013, 86, 5–12. [Google Scholar] [CrossRef]
- Hansell, D.M.; Goldin, J.G.; King, T.E.; Lynch, D.A.; Richeldi, L.; Wells, A.U. CT Staging and Monitoring of Fibrotic Interstitial Lung Diseases in Clinical Practice and Treatment Trials: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2015, 3, 483–496. [Google Scholar] [CrossRef]
- Wu, X.; Kim, G.H.; Salisbury, M.L.; Barber, D.; Bartholmai, B.J.; Brown, K.K.; Conoscenti, C.S.; De Backer, J.; Flaherty, K.R.; Gruden, J.F.; et al. Computed Tomographic Biomarkers in Idiopathic Pulmonary Fibrosis. The Future of Quantitative Analysis. Am. J. Respir. Crit. Care Med. 2019, 199, 12–21. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Usual Interstitial Pneumonia (UIP): A Clinically Significant Pathologic Diagnosis. Mod. Pathol. 2022, 35, 580–588. [Google Scholar] [CrossRef]
- Tomassetti, S.; Ravaglia, C.; Puglisi, S.; Ryu, J.H.; Colby, T.V.; Cavazza, A.; Wells, A.U.; Pavone, M.; Vancheri, C.; Lavorini, F.; et al. Impact of Lung Biopsy Information on Treatment Strategy of Patients with Interstitial Lung Diseases. Ann. Am. Thorac. Soc. 2022, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Fulford, L.G.; Colby, T.V.; du Bois, R.M.; Hansell, D.M.; Wells, A.U. The Relationship between Individual Histologic Features and Disease Progression in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2002, 166, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Watanabe, K.; Nabeshima, K.; Hamasaki, M.; Iwasaki, H. Prognostic Significance of Fibroblastic Foci in Usual Interstitial Pneumonia and Non-Specific Interstitial Pneumonia. Respirology 2013, 18, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, K.; Mäyränpää, M.I.; Sihvo, H.-K.; Bergman, P.; Sutinen, E.; Ollila, H.; Kaarteenaho, R.; Myllärniemi, M. Artificial Intelligence Identifies Inflammation and Confirms Fibroblast Foci as Prognostic Tissue Biomarkers in Idiopathic Pulmonary Fibrosis. Hum. Pathol. 2021, 107, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez Aceves, G.A.; Celis López, M.A.; Alonso Vanegas, M.; Marrufo Meléndez, O.R.; Moreno Jiménez, S.; Pérez Cruz, J.C.; Díaz Peregrino, R.; González Aguilar, A.; Herrera González, J.A. Fractal Anatomy of the Hippocampal Formation. Surg. Radiol. Anat. 2018, 40, 1209–1215. [Google Scholar] [CrossRef]
- Leslie, K.O. My Approach to Interstitial Lung Disease Using Clinical, Radiological and Histopathological Patterns. J. Clin. Pathol. 2009, 62, 387–401. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Meregalli, V.; Alberti, F.; Madan, C.R.; Meneguzzo, P.; Miola, A.; Trevisan, N.; Sambataro, F.; Favaro, A.; Collantoni, E. Cortical Complexity Estimation Using Fractal Dimension: A Systematic Review of the Literature on Clinical and Nonclinical Samples. Eur. J. Neurosci. 2022, 55, 1547–1583. [Google Scholar] [CrossRef]
- Ternifi, R.; Wang, Y.; Gu, J.; Polley, E.C.; Carter, J.M.; Pruthi, S.; Boughey, J.C.; Fazzio, R.T.; Fatemi, M.; Alizad, A. Ultrasound High-Definition Microvasculature Imaging with Novel Quantitative Biomarkers Improves Breast Cancer Detection Accuracy. Eur. Radiol. 2022, 32, 7448–7462. [Google Scholar] [CrossRef]
- Lyu, X.; Jajal, P.; Tahir, M.Z.; Zhang, S. Fractal Dimension of Retinal Vasculature as an Image Quality Metric for Automated Fundus Image Analysis Systems. Sci. Rep. 2022, 12, 11868. [Google Scholar] [CrossRef]
- Lennon, F.E.; Cianci, G.C.; Cipriani, N.A.; Hensing, T.A.; Zhang, H.J.; Chen, C.-T.; Murgu, S.D.; Vokes, E.E.; Vannier, M.W.; Salgia, R. Lung Cancer—A Fractal Viewpoint. Nat. Rev. Clin. Oncol. 2015, 12, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.-L.A.; Mukhopadhyay, S.; Myers, J.L. Diagnosis of Usual Interstitial Pneumonia and Distinction from Other Fibrosing Interstitial Lung Diseases. Hum. Pathol. 2008, 39, 1275–1294. [Google Scholar] [CrossRef]
- Larsen, B.T. Usual Interstitial Pneumonia: A Clinically Significant Pattern, but Not the Final Word. Mod. Pathol. 2022, 35, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Korfei, M.; Mahavadi, P.; Guenther, A. Targeting Histone Deacetylases in Idiopathic Pulmonary Fibrosis: A Future Therapeutic Option. Cells 2022, 11, 1626. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T. Epigenetics Approaches toward Precision Medicine for Idiopathic Pulmonary Fibrosis: Focus on DNA Methylation. Biomedicines 2023, 11, 1047. [Google Scholar] [CrossRef]
- Lebel, M.; Cliche, D.O.; Charbonneau, M.; Adam, D.; Brochiero, E.; Dubois, C.M.; Cantin, A.M. Invadosome Formation by Lung Fibroblasts in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 24, 499. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Toews, G.B.; Travis, W.D.; Colby, T.V.; Kazerooni, E.A.; Gross, B.H.; Jain, A.; Strawderman, R.L.; Paine, R.; Flint, A.; et al. Clinical Significance of Histological Classification of Idiopathic Interstitial Pneumonia. Eur. Respir. J. 2002, 19, 275–283. [Google Scholar] [CrossRef]
- Enomoto, N.; Suda, T.; Kato, M.; Kaida, Y.; Nakamura, Y.; Imokawa, S.; Ida, M.; Chida, K. Quantitative Analysis of Fibroblastic Foci in Usual Interstitial Pneumonia. Chest 2006, 130, 22–29. [Google Scholar] [CrossRef]
- Troy, L.K.; Grainge, C.; Corte, T.J.; Williamson, J.P.; Vallely, M.P.; Cooper, W.A.; Mahar, A.; Myers, J.L.; Lai, S.; Mulyadi, E.; et al. Diagnostic Accuracy of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease Diagnosis (COLDICE): A Prospective, Comparative Study. Lancet Respir. Med. 2020, 8, 171–181. [Google Scholar] [CrossRef]
- Kheir, F.; Uribe Becerra, J.P.; Bissell, B.; Ghazipura, M.; Herman, D.; Hon, S.M.; Hossain, T.; Khor, Y.H.; Knight, S.L.; Kreuter, M.; et al. Transbronchial Lung Cryobiopsy in Patients with Interstitial Lung Disease: A Systematic Review. Ann. Am. Thorac. Soc. 2022, 19, 1193–1202. [Google Scholar] [CrossRef]
- Korevaar, D.A.; Colella, S.; Fally, M.; Camuset, J.; Colby, T.V.; Hagmeyer, L.; Hetzel, J.; Maldonado, F.; Morais, A.; Ravaglia, C.; et al. European Respiratory Society Guidelines on Transbronchial Lung Cryobiopsy in the Diagnosis of Interstitial Lung Diseases. Eur. Respir. J. 2022, 60, 2200425. [Google Scholar] [CrossRef] [PubMed]

| Clinical Data | UIP | Control |
|---|---|---|
| Age | 73.4 ± 5.07 | 35.7 ± 12.3 |
| Sex | M:11; F1 | M:7; F5 |
| Pack/years smoking | 35.7 ± 7.8 | 0/occasional |
| FEV1 %predicted | 73 ± 0.14 | na |
| FVC %predicted | 73 ± 0.17 | na |
| DLCO %predicted | 77 ± 0.04 | na |
| UIP Pattern | N= 5: Certain N= 4: Probable N = 3: Indeterminate |
|---|---|
| FF Density/cm2 | 2.538 ± 2.17 |
| Histologic Df | 1.75 ± 0.03 |
| Radiologic Df | 1.312 ± 0.09 |
| Follow Up | 18.33 ± 12.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, M.; Bargiacchi, L.; De Vitis, C.; D’Ascanio, M.; De Dominicis, C.; Ibrahim, M.; Rendina, E.A.; Ricci, A.; Di Napoli, A.; Mancini, R.; et al. Histologic Analysis of Idiopathic Pulmonary Fibrosis by Morphometric and Fractal Analysis. Biomedicines 2023, 11, 1483. https://doi.org/10.3390/biomedicines11051483
Mancini M, Bargiacchi L, De Vitis C, D’Ascanio M, De Dominicis C, Ibrahim M, Rendina EA, Ricci A, Di Napoli A, Mancini R, et al. Histologic Analysis of Idiopathic Pulmonary Fibrosis by Morphometric and Fractal Analysis. Biomedicines. 2023; 11(5):1483. https://doi.org/10.3390/biomedicines11051483
Chicago/Turabian StyleMancini, Massimiliano, Lavinia Bargiacchi, Claudia De Vitis, Michela D’Ascanio, Chiara De Dominicis, Mohsen Ibrahim, Erino Angelo Rendina, Alberto Ricci, Arianna Di Napoli, Rita Mancini, and et al. 2023. "Histologic Analysis of Idiopathic Pulmonary Fibrosis by Morphometric and Fractal Analysis" Biomedicines 11, no. 5: 1483. https://doi.org/10.3390/biomedicines11051483
APA StyleMancini, M., Bargiacchi, L., De Vitis, C., D’Ascanio, M., De Dominicis, C., Ibrahim, M., Rendina, E. A., Ricci, A., Di Napoli, A., Mancini, R., & Vecchione, A. (2023). Histologic Analysis of Idiopathic Pulmonary Fibrosis by Morphometric and Fractal Analysis. Biomedicines, 11(5), 1483. https://doi.org/10.3390/biomedicines11051483







