Developing In Vitro Models to Define the Role of Direct Mitochondrial Toxicity in Frequently Reported Drug-Induced Rhabdomyolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Data Source, Case Identification, and Accuracy of Data
2.3. Cell Culture
2.4. Cell Plating and Induction of Differentiated Myotubes of L6 and HSKMDC
2.5. Acute Metabolic Switch on Differentiated Myotubes
2.6. Measurement of Cellular ATP Content Following Drug Exposure on L6 and HSKMDC Differentiated Myotubes
2.7. Assessment of Cell Death Using Lactate Dehydrogenase (LDH) Content on (HSKMDC) Differentiated Myotubes
2.8. Mitochondrial Stress Test in Differentiated Myotubes Using Seahorse Flux Analyser (XFe96)
2.9. Statistical Analysis
3. Results
3.1. The Most Common Drugs Suspected to Induce Rhabdomyolysis, as Reported to FAERS, Were Identified
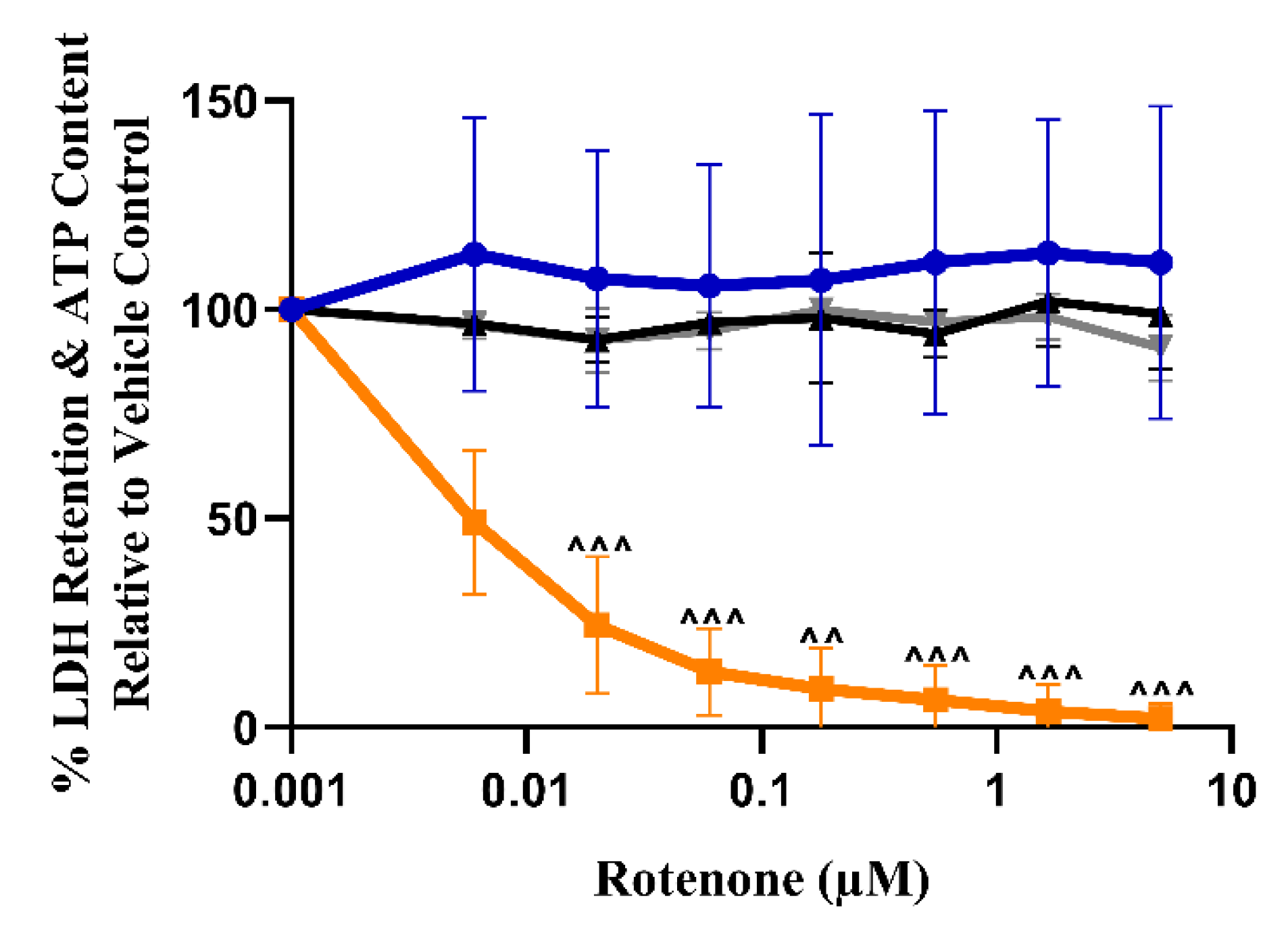
3.2. The Measurement of Cellular ATP Content Can Detect the Early Onset of Mitochondrial Dysfunction in L6 Cells
3.3. Respirometry Reveals That the Suspect Drugs Identified as Mitotoxins Inhibit Electron Transport Chain (ETC) Activity in L6 Cells
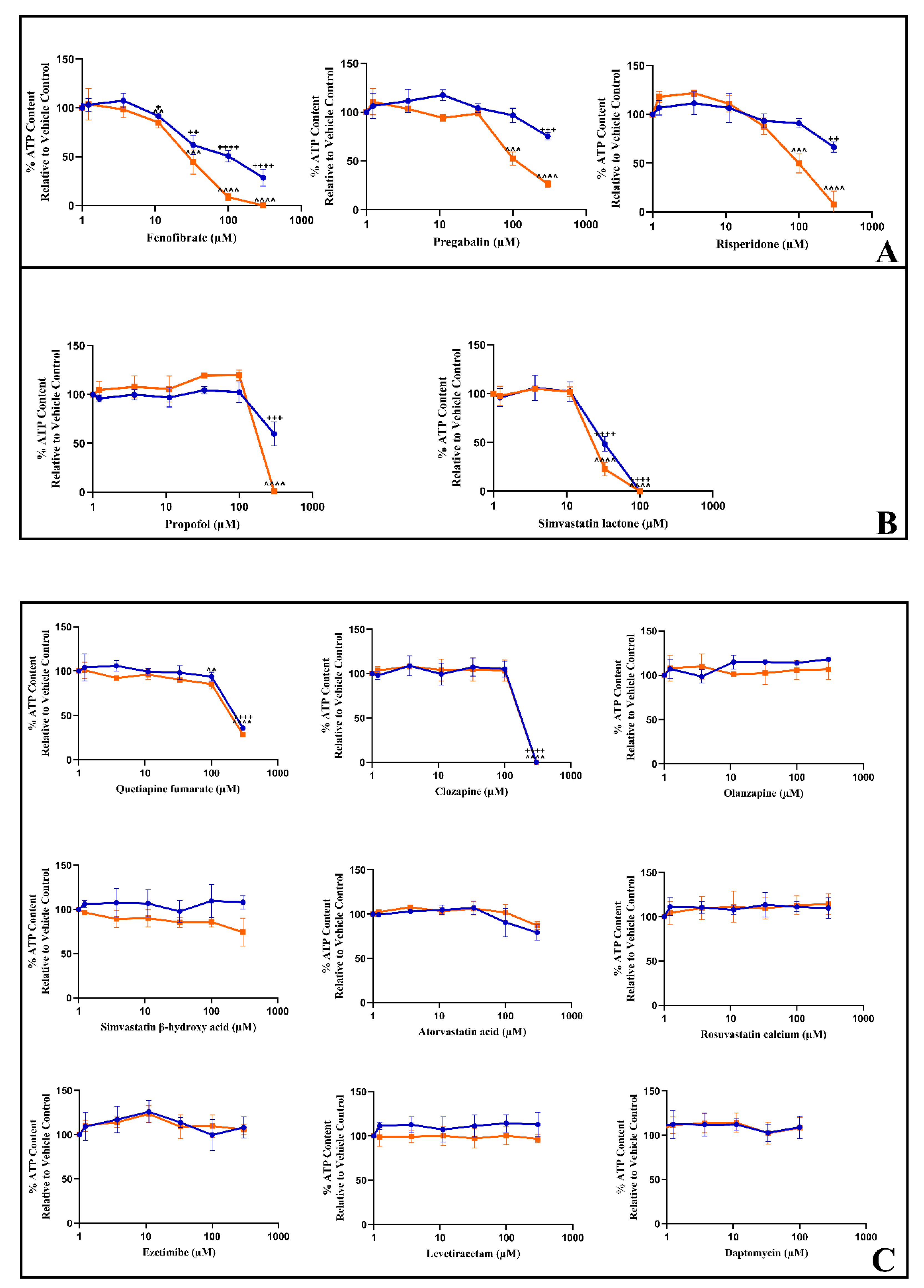
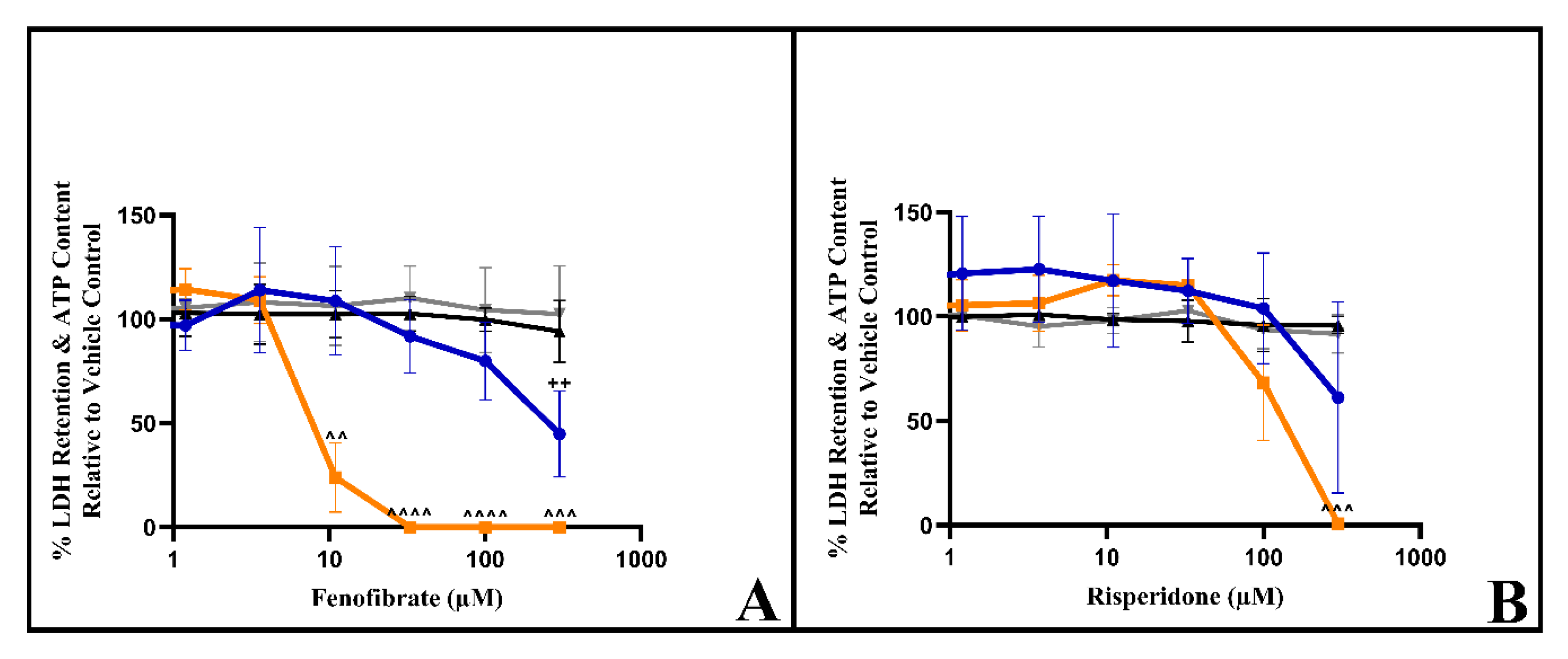
3.4. The Dual Assessment of Cellular ATP Content and Cytotoxicity Can Identify the Early Onset of Mitochondrial Dysfunction before Cell Death in HSKMDC Cells
3.5. Respirometry Reveals That Fenofibrate and Risperidone Inhibit Electron Transport Chain Activity in HSKMDC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sauret, J.M.; Marinides, G.; Wang, G.K. Rhabdomyolysis. Am. Fam. Physician 2002, 65, 907–912. [Google Scholar] [PubMed]
- Criner, J.A.; Appelt, M.; Coker, C.; Conrad, S.; Holliday, J. Rhabdomyolysis: The hidden killer. Medsurg. Nurs. 2002, 11, 138–143. [Google Scholar] [PubMed]
- Pasternak, R.C.; Smith, S.C.; Merz, C.N.B.; Grundy, S.M.; Cleeman, J.I.; Lenfant, C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke 2002, 33, 2337–2341. [Google Scholar] [CrossRef]
- Antons, K.A.; Williams, C.D.; Baker, S.K.; Phillips, P.S. Clinical Perspectives of Statin-Induced Rhabdomyolysis. Am. J. Med. 2006, 119, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.L.; Baldea, A.; Luchette, F. Rhabdomyolysis in the Intensive Care Unit. J. Intensiv. Care Med. 2012, 27, 335–342. [Google Scholar] [CrossRef]
- Huerta-Alardín, A.L.; Varon, J.; Marik, P.E. Bench-to-bedside review: Rhabdomyolysis—An overview for clinicians. Crit. Care 2005, 9, 158–169. [Google Scholar] [CrossRef]
- Khan, F.Y. Rhabdomyolysis: A review of the literature. Neth. J. Med. 2009, 67, 272–283. [Google Scholar]
- Cervellin, G.; Comelli, I.; Lippi, G. Rhabdomyolysis: Historical background, clinical, diagnostic and therapeutic features. Clin. Chem. Lab. Med. 2010, 48, 749–756. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Misirli, G.; Hatzitolios, A.I.; Giannoglou, G.D. The syndrome of rhabdomyolysis: Complications and treatment. Eur. J. Intern. Med. 2008, 19, 568–574. [Google Scholar] [CrossRef]
- Bagley, W.H.; Yang, H.; Shah, K.H. Rhabdomyolysis. Intern. Emerg. Med. 2007, 2, 210–218. [Google Scholar] [CrossRef]
- Holt, S.; Moore, K.P. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensiv. Care Med. 2001, 27, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.E.; Kenney, K.; Deuster, P.; Campbell, W. Exertional rhabdomyolysis: A clinical review with a focus on genetic influences. J. Clin. Neuromuscul. Dis. 2012, 13, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Chakravartty, S.; Sarma, D.R.; Patel, A.G. Rhabdomyolysis in Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1333–1340. [Google Scholar] [CrossRef]
- Iwere, R.B.; Hewitt, J. Myopathy in older people receiving statin therapy: A systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2015, 80, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Pariser, J.J.; Pearce, S.M.; Patel, S.G.; Anderson, B.B.; Packiam, V.T.; Shalhav, A.L.; Bales, G.T.; Smith, N.D. Rhabdomyolysis After Major Urologic Surgery: Epidemiology, Risk Factors, and Outcomes. Urology 2015, 85, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Rees, A. Effects of HMG-CoA reductase inhibitors on skeletal muscle: Are all statins the same? Drug Saf. 2002, 25, 649–663. [Google Scholar] [CrossRef]
- Chander, V.; Chopra, K. Molsidomine, a nitric oxide donor and l-arginine protects against rhabdomyolysis-induced myoglobinuric acute renal failure. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2005, 1723, 208–214. [Google Scholar] [CrossRef]
- Larbi, E.B. Drug-induced rhabdomyolysis. Ann. Saudi Med. 1998, 18, 525–530. [Google Scholar] [CrossRef]
- Ko, A.; Song, J.; Golovko, G.; El Ayadi, A.; Ozhathil, D.K.; Wermine, K.; Africa, R.E.; Gotewal, S.; Reynolds, S.; Wolf, S.E. Higher risk of acute kidney injury and death with rhabdomyolysis in severely burned patients. Surgery 2022, 171, 1412–1416. [Google Scholar] [CrossRef]
- Schlander, M.; Hernandez-Villafuerte, K.; Cheng, C.-Y.; Mestre-Ferrandiz, J.; Baumann, M. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. Pharmacoeconomics 2021, 39, 1243–1269. [Google Scholar] [CrossRef]
- Marwick, C. Bayer is forced to release documents over withdrawal of cerivastatin. BMJ 2003, 326, 518. [Google Scholar] [CrossRef] [PubMed]
- Angelmar, R. The Rise and Fall of Baycol/Lipobay. J. Med. Mark. 2007, 7, 77–88. [Google Scholar] [CrossRef]
- Huybrechts, K.F.; Desai, R.J.; Park, M.; Gagne, J.J.; Najafzadeh, M.; Avorn, J. The Potential Return on Public Investment in Detecting Adverse Drug Effects. Med. Care 2017, 55, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Haskins, N. Rhabdomyolysis and acute renal failure in intensive care. Nurs. Crit. Care 1998, 3, 283–288. [Google Scholar]
- Knochel, J.P. Mechanisms of rhabdomyolysis. Curr. Opin. Rheumatol. 1993, 5, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Jones, D.A.; Edwards, R.H.T. Experimental skeletal muscle damage: The nature of the calcium-activated degenerative processes. Eur. J. Clin. Investig. 1984, 14, 369–374. [Google Scholar] [CrossRef]
- Rubin, B.B.; Liauw, S.; Tittley, J.; Romaschin, A.D.; Walker, P.M. Prolonged adenine nucleotide resynthesis and reperfusion injury in postischemic skeletal muscle. Am. J. Physiol. Circ. Physiol. 1992, 262, H1538–H1547. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.B.; Warren, G.L.; Warren, J.A. Mechanisms of Exercise-Induced Muscle Fibre Injury. Sport. Med. 1991, 12, 184–207. [Google Scholar] [CrossRef]
- Galtier, F.; Mura, T.; de Mauverger, E.R.; Chevassus, H.; Farret, A.; Gagnol, J.-P.; Costa, F.; Dupuy, A.; Petit, P.; Cristol, J.; et al. Effect of a high dose of simvastatin on muscle mitochondrial metabolism and calcium signaling in healthy volunteers. Toxicol. Appl. Pharmacol. 2012, 263, 281–286. [Google Scholar] [CrossRef]
- Sirvent, P.; Mercier, J.; Vassort, G.; Lacampagne, A. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 329, 1067–1075. [Google Scholar] [CrossRef]
- Allard, N.A.E.; Schirris, T.; Verheggen, R.J.; Russel, F.; Rodenburg, R.; Smeitink, J.A.M.; Thompson, P.D.; Hopman, M.T.E.; Timmers, S. Statins Affect Skeletal Muscle Performance: Evidence for Disturbances in Energy Metabolism. J. Clin. Endocrinol. Metab. 2018, 103, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.-B.; Thalacker-Mercer, A.; Anderson, E.J.; Lin, C.-T.; Kane, D.A.; Lee, N.-S.; Cortright, R.N.; Bamman, M.M.; Neufer, P.D. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free. Radic. Biol. Med. 2012, 52, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Singh, F.; Charles, A.-L.; Schlagowski, A.-I.; Bonifacio, A.; Echaniz-Laguna, A.; Geny, B.; Krähenbühl, S.; Zoll, J. Statins Trigger Mitochondrial Reactive Oxygen Species-Induced Apoptosis in Glycolytic Skeletal Muscle. Antioxid. Redox Signal. 2016, 24, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Dranka, B.P.; Benavides, G.A.; Diers, A.R.; Giordano, S.; Zelickson, B.R.; Reily, C.; Zou, L.; Chatham, J.C.; Hill, B.G.; Zhang, J.; et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free. Radic. Biol. Med. 2011, 51, 1621–1635. [Google Scholar] [CrossRef]
- Muntean, D.M.; Thompson, P.D.; Catapano, A.L.; Stasiolek, M.; Fabis, J.; Muntner, P.; Serban, M.-C.; Banach, M. Statin-associated myopathy and the quest for biomarkers: Can we effectively predict statin-associated muscle symptoms? Drug Discov. Today 2017, 22, 85–96. [Google Scholar] [CrossRef]
- Mammen, A.L. Toxic myopathies. Contin. Lifelong Learn. Neurol. 2013, 19, 1634–1649. [Google Scholar] [CrossRef]
- Scruggs, E.R.; Naylor, A.J.D. Mechanisms of Zidovudine-Induced Mitochondrial Toxicity and Myopathy. Pharmacology 2008, 82, 83–88. [Google Scholar] [CrossRef]
- Chawla, J. Stepwise Approach to Myopathy in Systemic Disease. Front. Neurol. 2011, 2, 49. [Google Scholar] [CrossRef]
- Dykens, J.A.; Will, Y. Drug-Induced Mitochondrial Dysfunction; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- De Vriese, A.S.; Van Coster, R.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.-J.; Groote, C.C.-D.; Vandecasteele, S.; et al. Linezolid-Induced Inhibition of Mitochondrial Protein Synthesis. Clin. Infect. Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- FAERS Public Dashboard. Available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed on 1 April 2020).
- Hill, B.G.; Benavides, G.A.; Lancaster, J.R.; Ballinger, S.; Dell’Italia, L.; Zhang, J.; Darley-Usmar, V.M. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012, 393, 1485–1512. [Google Scholar] [CrossRef]
- Kamalian, L.; Douglas, O.; Jolly, C.E.; Snoeys, J.; Simic, D.; Monshouwer, M.; Williams, D.P.; Park, B.K.; Chadwick, A.E. The utility of HepaRG cells for bioenergetic investigation and detection of drug-induced mitochondrial toxicity. Toxicol. Vitr. 2018, 53, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Furberg, C.D.; Pitt, B. Withdrawal of cerivastatin from the world market. Curr. Control Trials Cardiovasc. Med. 2001, 2, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.A.; Wilson, J.P. FDA adverse event reports on statin-associated rhabdomyolysis. Ann. Pharmacother. 2002, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, L.; Chadwick, A.E.; Bayliss, M.; French, N.S.; Monshouwer, M.; Snoeys, J.; Park, B.K. The utility of HepG2 cells to identify direct mitochondrial dysfunction in the absence of cell death. Toxicol. Vitr. 2015, 29, 732–740. [Google Scholar] [CrossRef]
- Swiss, R.; Niles, A.; Cali, J.J.; Nadanaciva, S.; Will, Y. Validation of a HTS-amenable assay to detect drug-induced mitochondrial toxicity in the absence and presence of cell death. Toxicol. Vitr. 2013, 27, 1789–1797. [Google Scholar] [CrossRef]
- Wu, M.; Neilson, A.; Swift, A.L.; Moran, R.; Tamagnine, J.; Parslow, D.; Armistead, S.; Lemire, K.; Orrell, J.; Teich, J.; et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Physiol. 2007, 292, C125–C136. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Medical Dictionary for Regulatory Activities. Available online: http://www.meddramsso.com/index.asp (accessed on 1 April 2020).
- Rodriguez, E.M.; Staffa, J.A.; Graham, D.J. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol. Drug Saf. 2001, 10, 407–410. [Google Scholar] [CrossRef]
- Wysowski, D.K.; Swartz, L. Adverse Drug Event Surveillance and Drug Withdrawals in the United States, 1969–2002. Arch. Intern. Med. 2005, 165, 1363–1369. [Google Scholar] [CrossRef]
- FAERS Electronic Submissions. Available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-electronic-submissions (accessed on 1 April 2020).
- FAERS Data Limitations. Available online: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers (accessed on 1 April 2020).
- Welch, H.K.; Kellum, J.A.; Kane-Gill, S.L. Drug-Associated Acute Kidney Injury Identified in the United States Food and Drug Administration Adverse Event Reporting System Database. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 785–793. [Google Scholar] [CrossRef]
- Downing, N.S.; Shah, N.D.; Aminawung, J.A.; Pease, A.M.; Zeitoun, J.-D.; Krumholz, H.M.; Ross, J.S. Postmarket Safety Events Among Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010. JAMA 2017, 317, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef]
- Magee, C.N.; Medani, S.A.; Leavey, S.F.; Conlon, P.J.; Clarkson, M.R. Severe Rhabdomyolysis as a Consequence of the Interaction of Fusidic Acid and Atorvastatin. Am. J. Kidney Dis. 2010, 56, e11–e15. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, A.M.; Puig, L.S.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef] [PubMed]
- Will, Y.; Dykens, J. Mitochondrial toxicity assessment in industry—A decade of technology development and insight. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.V.; Oliveira, P.J.; Will, Y.; Nadanaciva, S. Mitochondrial bioenergetics and drug-induced toxicity in a panel of mouse embryonic fibroblasts with mitochondrial DNA single nucleotide polymorphisms. Toxicol. Appl. Pharmacol. 2012, 264, 167–181. [Google Scholar] [CrossRef]
- Diaz-Ruiz, R.; Rigoulet, M.; Devin, A. The Warburg and Crabtree effects: On the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, L.D.; Hynes, J.; Dykens, J.A.; Jamieson, J.D.; Will, Y. Circumventing the Crabtree Effect: Replacing Media Glucose with Galactose Increases Susceptibility of HepG2 Cells to Mitochondrial Toxicants. Toxicol. Sci. 2007, 97, 539–547. [Google Scholar] [CrossRef]
- Swiss, R.; Will, Y. Assessment of Mitochondrial Toxicity in HepG2 Cells Cultured in High-Glucose- or Galactose-Containing Media. Curr. Protoc. Toxicol. 2011, 49, 2.20.1–2.20.14. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Jones, S.W.; Penman, S.L.; French, N.S.; Park, B.K.; Chadwick, A.E. Investigating dihydroorotate dehydrogenase inhibitor mediated mitochondrial dysfunction in hepatic in vitro models. Toxicol. Vitr. 2021, 72, 105096. [Google Scholar] [CrossRef] [PubMed]
- Brunmair, B.; Lest, A.; Staniek, K.; Gras, F.; Scharf, N.; Roden, M.; Nohl, H.; Waldhäusl, W.; Fürnsinn, C. Fenofibrate Impairs Rat Mitochondrial Function by Inhibition of Respiratory Complex I. J. Pharmacol. Exp. Ther. 2004, 311, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Török, M.; Zahno, A.; Waldhauser, K.M.; Brecht, K.; Krähenbühl, S. Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. CMLS 2006, 63, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Vanlander, A.V.; Okun, J.G.; de Jaeger, A.; Smet, J.; De Latter, E.; De Paepe, B.; Dacremont, G.; Wuyts, B.; Vanheel, B.; De Paepe, P.; et al. Possible Pathogenic Mechanism of Propofol Infusion Syndrome Involves Coenzyme Q. Anesthesiology 2015, 122, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Urban, T.; Waldauf, P.; Krajčová, A.; Jiroutková, K.; Halačová, M.; Džupa, V.; Janoušek, L.; Pokorná, E.; Duška, F. Kinetic characteristics of propofol-induced inhibition of electron-transfer chain and fatty acid oxidation in human and rodent skeletal and cardiac muscles. PLoS ONE 2019, 14, e0217254. [Google Scholar] [CrossRef]
- Murakami, Y.; Ueki, R.; Tachikawa, T.; Hirose, M. The Basic Study of the Mechanism of Propofol-Related Infusion Syndrome Using a Murine Skeletal Muscle Injury Model. Anesthesiol. Pain Med. 2019, 9, e89417. [Google Scholar] [CrossRef]
- Burkhardt, C.; Kelly, J.P.; Lim, Y.-H.; Filley, C.M.; Parker, W.D. Neuroleptic medications inhibit complex I of the electron transport chain. Ann. Neurol. 1993, 33, 512–517. [Google Scholar] [CrossRef]
- Sidaway, J.; Wang, Y.; Marsden, A.M.; Orton, T.C.; Westwood, F.R.; Azuma, C.T.; Scott, R.C. Statin-induced myopathy in the rat: Relationship between systemic exposure, muscle exposure and myopathy. Xenobiotica 2009, 39, 90–98. [Google Scholar] [CrossRef]
- Sanuki, Y.; Araki, T.; Nakazono, O.; Tsurui, K. A rapid mitochondrial toxicity assay utilizing rapidly changing cell energy metabolism. J. Toxicol. Sci. 2017, 42, 349–358. [Google Scholar] [CrossRef]



| Suspected Drug | Number of Rhabdomyolysis Cases | (%) | ROR | 95% CI | Suspected Drug | Number of Rhabdomyolysis Cases | (%) | ROR | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Simvastatin | 4705 | 17.3 | 10.3 | 10.0–10.7 | Gemfibrozil | 442 | 1.6 | 15.0 | 13.5–16.6 |
| Atorvastatin | 3307 | 12.2 | 4.3 | 4.1–4.4 | Propofol | 434 | 1.6 | 2.2 | 2.0–2.4 |
| Rosuvastatin | 2008 | 7.4 | 2.9 | 2.7–3.0 | Clarithromycin | 427 | 1.6 | 1.2 | 1.1–1.4 |
| Quetiapine | 761 | 2.8 | 0.5 | 0.5–0.6 | Venlafaxine | 425 | 1.6 | 0.5 | 0.5–0.6 |
| Risperidone | 654 | 2.4 | 0.5 | 0.5–0.6 | Aripiprazole | 419 | 1.5 | 0.4 | 0.4–0.4 |
| Olanzapine | 648 | 2.4 | 0.9 | 0.8–0.9 | Amlodipine | 405 | 1.5 | 0.5 | 0.5–0.6 |
| Levetiracetam | 588 | 2.2 | 0.9 | 0.9–1.0 | Daptomycin | 380 | 1.4 | 3.2 | 2.9–3.6 |
| Ezetimibe/Simvastatin | 541 | 2.0 | 8.0 | 7.3–8.8 | Omeprazole | 363 | 1.3 | 0.5 | 0.4–0.5 |
| Cyclosporine | 505 | 1.9 | 0.5 | 0.5–0.6 | Sertraline | 347 | 1.3 | 0.4 | 0.3–0.4 |
| Ezetimibe | 501 | 1.9 | 2.6 | 2.4–2.9 | Mirtazapine | 318 | 1.2 | 0.8 | 0.7–0.9 |
| Furosemide | 480 | 1.8 | 0.8 | 0.7–0.9 | Lamotrigine | 314 | 1.2 | 0.3 | 0.3–0.4 |
| Metformin | 478 | 1.8 | 0.4 | 0.4–0.4 | Aspirin | 313 | 1.2 | 0.2 | 0.2–0.2 |
| Acetaminophen | 449 | 1.7 | 0.3 | 0.3–0.3 | Haloperidol | 310 | 1.1 | 1.3 | 1.1–1.4 |
| Fenofibrate | 449 | 1.7 | 3.8 | 3.4–4.2 | Alprazolam | 290 | 1.1 | 0.4 | 0.4–0.5 |
| Pregabalin | 442 | 1.6 | 0.2 | 0.2–0.3 | Clozapine | 286 | 1.1 | 0.2 | 0.2–0.3 |
| Fusidic Acid | 285 | 1.1 | 54.4 | 46.3–63.9 |
| Suspected Drug Reported as a Single Drug Used | Number of Rhabdomyolysis Cases (n = 6583) | (%) | Number of Death Cases (n = 576) |
|---|---|---|---|
| Simvastatin | 1815 | 27.6 | 161 |
| Atorvastatin | 1386 | 21.1 | 169 |
| Rosuvastatin | 1122 | 17.0 | 47 |
| Levetiracetam | 386 | 5.9 | 8 |
| Ezetimibe/Simvastatin | 350 | 5.3 | 10 |
| Olanzapine | 258 | 3.9 | 23 |
| Daptomycin | 227 | 3.5 | 18 |
| Quetiapine | 199 | 3.0 | 26 |
| Propofol | 179 | 2.7 | 70 |
| Ezetimibe | 166 | 2.5 | 6 |
| Pregabalin | 132 | 2.0 | 13 |
| Fenofibrate | 126 | 1.9 | 6 |
| Risperidone | 121 | 1.8 | 7 |
| Clozapine | 116 | 1.8 | 12 |
| ATP IC50 (μM) ± S.D. Glucose Galactose | IC50-ATPglu/IC50-ATPgal (p-Value) | ||
|---|---|---|---|
| Control compound: Rotenone | >5 | 0.036 ± 0.008 | >138.9 (<0.0001) |
Tested reported drugs:
| |||
| Fenofibrate | 95.8 ± 27.6 | 30.0 ± 19.4 | 3.2 (0.0330) |
| Pregabalin | >300 | 116.0 ± 2 2.5 | >2.6 (0.0049) |
| Risperidone | >261.0 ± 67.5 | 102.7 ± 28.2 | >2.5 (0.0401) |
| |||
| Propofol | >300 | 241.6 ± 13.4 | >1.2 (0.0172) |
| Simvastatin Lactone | 40.8 ± 20.7 | 28.1 ± 9.4 | 1.5 (0.4116) |
| |||
| Quetiapine | 265.2 ± 4.9 | 216.5 ± 57.8 | 1.2 (0.2814) (ns) |
| Clozapine | 218.3 ± 13.2 | 213.2 ± 10.1 | 1 (0.6270) (ns) |
| Olanzapine | >300 | >300 | ~1 (n/d) |
| Simvastatin acid | >300 | >300 | ~1 (n/d) |
| Atorvastatin acid | >300 | >300 | ~1 (n/d) |
| Rosuvastatin | >300 | >300 | ~1 (n/d) |
| Ezetimibe | >300 | >300 | ~1 (n/d) |
| Levetiracetam | >300 | >300 | ~1 (n/d) |
| Daptomycin | >100 | >100 | ~1 (n/d) |
| ATP IC50 (μM) ± S.D. Glucose Galactose | IC50-ATPglu/ IC50-ATPgal (p Value) | LDH IC50 (μM) ± S.D. Glucose Galactose | IC50-LDH gal/ IC50-ATP al | |||
|---|---|---|---|---|---|---|
| Rotenone | >5 | 0.016 ± 0.007 | >318.4 (<0.0001) | >5 | >5 | >312.5 |
| Fenofibrate | >300 | 9.7 ± 1.1 | >24.0 (0.028) | >300 | >300 | >30.9 |
| Risperidone | >300 | 143.4 ± 51.5 | >1.9 (0.0419) | >300 | >300 | >2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Dayel, F.F.; Alfirevic, A.; Chadwick, A.E. Developing In Vitro Models to Define the Role of Direct Mitochondrial Toxicity in Frequently Reported Drug-Induced Rhabdomyolysis. Biomedicines 2023, 11, 1485. https://doi.org/10.3390/biomedicines11051485
Bin Dayel FF, Alfirevic A, Chadwick AE. Developing In Vitro Models to Define the Role of Direct Mitochondrial Toxicity in Frequently Reported Drug-Induced Rhabdomyolysis. Biomedicines. 2023; 11(5):1485. https://doi.org/10.3390/biomedicines11051485
Chicago/Turabian StyleBin Dayel, Faten F., Ana Alfirevic, and Amy E. Chadwick. 2023. "Developing In Vitro Models to Define the Role of Direct Mitochondrial Toxicity in Frequently Reported Drug-Induced Rhabdomyolysis" Biomedicines 11, no. 5: 1485. https://doi.org/10.3390/biomedicines11051485
APA StyleBin Dayel, F. F., Alfirevic, A., & Chadwick, A. E. (2023). Developing In Vitro Models to Define the Role of Direct Mitochondrial Toxicity in Frequently Reported Drug-Induced Rhabdomyolysis. Biomedicines, 11(5), 1485. https://doi.org/10.3390/biomedicines11051485








