Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation
Abstract
:1. Introduction
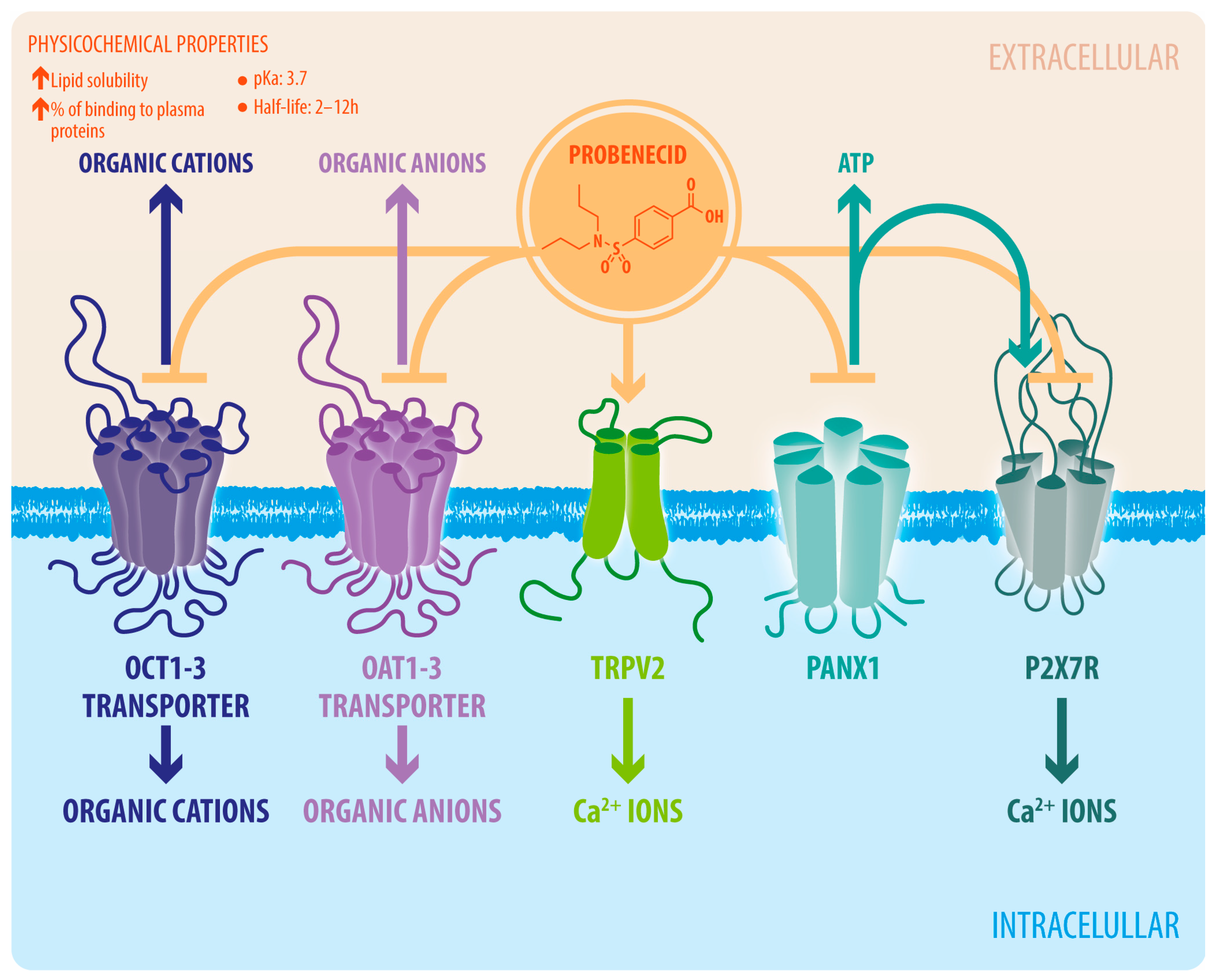
2. Probenecid: Pharmacology and Pharmacokinetics
3. Probenecid’s Pharmacological Targets
3.1. Organic Anion Transporter 1 and 3 (OAT1 and OAT3)
3.2. TRPV2 Channel
3.3. Pannexin-1 Channels
3.4. Other Targets
3.4.1. Purinergic Receptors
3.4.2. Organic Cation Transporters (OCTs)
4. Central Nervous System Diseases and the Role of Probenecid
4.1. Neuroinflammation
4.2. Epilepsy
4.3. Parkinson’s Disease
4.4. Alzheimer’s Disease (AD)
4.5. Other Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinney, S.E.; Peck, H.M.; Bochey, J.M.; Byham, B.B.; Schuchardt, G.S.; Beyer, K.H. Benemid, p-(DI-n-propylsulfamyl)-benzoic acid; toxicologic properties. J. Pharmacol. Exp. Ther. 1951, 102, 208–214. [Google Scholar] [PubMed]
- Colín-González, A.L.; Santamaría, A. Probenecid: An emerging tool for neuroprotection. CNS Neurol. Disord. Drug Targets 2013, 12, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Burnell, J.M.; Kirby, W.M.M. Effectiveness of a new compound, benemid, in elevating serum penicillin concentrations. J. Clin. Investig. 1951, 30, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, R.F.; Israili, Z.H.; Dayton, P.G. Clinical Pharmacokinetics of Probenecid. Clin. Pharmacokinet. 1981, 6, 135–151. [Google Scholar] [CrossRef]
- Talbott, J.H. Clinical and metabolic effects of benemid in gout. Bull. Rheum. Dis. 1951, 2, 1–2. [Google Scholar]
- Talbott, J.H.; Bishop, C.; Norcross, B.M.; Lockie, L.M. The clinical and metabolic effects of benemid in patients with gout. Trans. Assoc. Am. Physicians 1951, 64, 372–377. [Google Scholar]
- Robbins, N.; Koch, S.E.; Tranter, M.; Rubinstein, J. The History and Future of Probenecid. Cardiovasc. Toxicol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Sun, H.; Miller, N.W.; Elmquist, W.F. Effect of probenecid on fluorescein transport in the central nervous system using in vitro and in vivo models. Pharm. Res. 2001, 18, 1542–1549. [Google Scholar] [CrossRef]
- Syvänen, S.; Barletta, J.; Blomquist, G.; Långström, B.; Bergström, M. PET-evaluated transport of [11C]hydroxyurea across the rat blood-brain barrier--lack of influence of cyclosporin and probenecid. Drug Metab. Lett. 2007, 1, 189–194. [Google Scholar] [CrossRef]
- Tunblad, K.; Jonsson, E.N.; Hammarlund-Udenaes, M. Morphine blood-brain barrier transport is influenced by probenecid co-administration. Pharm. Res. 2003, 20, 618–623. [Google Scholar] [CrossRef]
- Bang, S.; Kim, K.Y.; Yoo, S.; Lee, S.-H.; Hwang, S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007, 425, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.D.; O’Brien, F.E.; Boylan, G.B.; Cryan, J.F.; Griffin, B.T. The effect of organic anion transporter 3 inhibitor probenecid on bumetanide levels in the brain: An integrated in vivo microdialysis study in the rat. J. Pharm. Pharmacol. 2015, 67, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.; Locovei, S.; Dahl, G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am. J. Physiol. Cell Physiol. 2008, 295, C761–C767. [Google Scholar] [CrossRef] [PubMed]
- Selen, A.; Amidon, G.L.; Wellingx, P. Pharmacokinetics of probenecid following oral doses to human volunteers. J. Pharm. Sci. 1982, 71, 1238–1242. [Google Scholar] [CrossRef]
- Boger, W.P.; Strickland, S.C. Probenecid (benemid); its uses and side-effects in 2502 patients. AMA Arch. Intern Med. 1955, 95, 83–92. [Google Scholar] [CrossRef]
- Takeda, M.; Narikawa, S.; Hosoyamada, M.; Cha, S.H.; Sekine, T.; Endou, H. Characterization of organic anion transport inhibitors using cells stably expressing human organic anion transporters. Eur. J. Pharmacol. 2001, 419, 113–120. [Google Scholar] [CrossRef]
- Arndt, P.; Volk, C.; Gorboulev, V.; Budiman, T.; Popp, C.; Ulzheimer-Teuber, I.; Akhoundova, A.; Koppatz, S.; Bamberg, E.; Nagel, G.; et al. Interaction of cations, anions, and weak base quinine with rat renal cation transporter rOCT2 compared with rOCT1. Am. J. Physiol. Ren. Physiol. 2001, 281, F454–F468. [Google Scholar] [CrossRef]
- Bhaskaracharya, A.; Dao-Ung, P.; Jalilian, I.; Spildrejorde, M.; Skarratt, K.; Fuller, S.J.; Sluyter, R.; Stokes, L. Probenecid Blocks Human P2X7 Receptor-Induced Dye Uptake via a Pannexin-1 Independent Mechanism. PLoS ONE 2014, 9, e93058. [Google Scholar] [CrossRef]
- Sweet, D.H. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol. Appl. Pharmacol. 2005, 204, 198–215. [Google Scholar] [CrossRef]
- Bahn, A.; Ljubojevic, M.; Lorenz, H.; Schultz, C.; Ghebremedhin, E.; Ugele, B.; Sabolic, I.; Burckhardt, G.; Hagos, Y. Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am. J. Physiol. Cell Physiol. 2005, 289, C1075–C1084. [Google Scholar] [CrossRef]
- Hagos, F.T.; Daood, M.J.; Ocque, J.A.; Nolin, T.D.; Bayir, H.; Poloyac, S.M.; Kochanek, P.M.; Clark, R.S.; Empey, P.E. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica 2017, 47, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Koepsell, H. Organic Cation Transporters in Health and Disease. Pharmacol. Rev. 2020, 72, 253–319. [Google Scholar] [CrossRef] [PubMed]
- Graves, W.K.; Neff, R.; Mark, P. Altered glucose metabolism in pregnancy: Its determination and fetal outcome. Trans. Pac. Coast Obstet. Gynecol. Soc. 1966, 34, 20–26. [Google Scholar] [PubMed]
- Neef, N.H.; Tozer, T.N.; Brodie, B.B. Application of seady-state kinetics to studies of the transfer of 5-hydroxyindoleacetic acid from brain to plasma. J. Pharmacol. Exp. Ther. 1967, 158, 214–218. [Google Scholar]
- Guldberg, H.; Ashcroft, G.; Crawford, T. Concentrations of 5-hydroxyindolylacetic acid and homovanillic acid in the cerebrospinal fluid of the dog before and during treatment with probenecid. Life Sci. 1966, 5, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Ahtee, L.; Sharman, D.F.; Vogt, M. Acid metabolites of monoamines in avian brain; effects of probenecid and reserpine. Br. J. Pharmacol. 1970, 38, 72–85. [Google Scholar] [CrossRef]
- Korf, J.; Van Praag, H.M. The intravenous probenecid test: A possible aid in evaluation of the serotonin hypothesis on the pathogenesis of depressions. Psychopharmacologia 1970, 18, 129–132. [Google Scholar] [CrossRef]
- van Praag, H.M.; Korf, J.; Schut, D. Cerebral monoamines and depression. An investigation with the Probenecid technique. Arch. Gen. Psychiatry 1973, 28, 827–831. [Google Scholar] [CrossRef]
- Moroni, F.; Russi, P.; Lombardi, G.; Beni, M.; Carlà, V. Presence of Kynurenic Acid in the Mammalian Brain. J. Neurochem. 1988, 51, 177–180. [Google Scholar] [CrossRef]
- Chauvel, V.; Vamos, E.; Pardutz, A.; Vecsei, L.; Schoenen, J.; Multon, S. Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. Exp. Neurol. 2012, 236, 207–214. [Google Scholar] [CrossRef]
- Miller, J.M.; MacGarvey, U.; Beal, M.F. The effect of peripheral loading with kynurenine and probenecid on extracellular striatal kynurenic acid concentrations. Neurosci. Lett. 1992, 146, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Peralvarez-Marin, A.; Donate-Macian, P.; Gaudet, R. What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 2013, 280, 5471–5487. [Google Scholar] [CrossRef] [PubMed]
- Shimosato, G.; Amaya, F.; Ueda, M.; Tanaka, Y.; Decosterd, I.; Tanaka, M. Peripheral inflammation induces up-regulation of TRPV2 expression in rat DRG. Pain 2005, 119, 225–232. [Google Scholar] [CrossRef]
- Shibasaki, K.; Ishizaki, Y.; Mandadi, S. Astrocytes express functional TRPV2 ion channels. Biochem. Biophys. Res. Commun. 2013, 441, 327–332. [Google Scholar] [CrossRef]
- Luo, H.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Smirnova, M.; Glacial, F.; Perrière, N.; Chaves, C.; Cisternino, S.; Declèves, X. Molecular and Functional Study of Transient Receptor Potential Vanilloid 1-4 at the Rat and Human Blood–Brain Barrier Reveals Interspecies Differences. Front. Cell Dev. Biol. 2020, 8, 578514. [Google Scholar] [CrossRef]
- Hassan, S.; Eldeeb, K.; Millns, P.J.; Bennett, A.J.; Alexander, S.P.H.; Kendall, D.A. Cannabidiol enhances microglial phagocytosis via transient receptor potential (TRP) channel activation. Br. J. Pharmacol. 2014, 171, 2426–2439. [Google Scholar] [CrossRef]
- Caterina, M.J. TRP Channel Cannabinoid Receptors in Skin Sensation, Homeostasis, and Inflammation. ACS Chem. Neurosci. 2014, 5, 1107–1116. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Farfariello, V.; Ricci-Vitiani, L.; Caprodossi, S.; Arcella, A.; Santoni, M.; Giangaspero, F.; De Maria, R.; et al. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis 2010, 31, 794–803. [Google Scholar] [CrossRef]
- Morelli, M.B.; Nabissi, M.; Amantini, C.; Farfariello, V.; Ricci-Vitiani, L.; di Martino, S.; Pallini, R.; LaRocca, L.M.; Caprodossi, S.; Santoni, M.; et al. The transient receptor potential vanilloid-2 cation channel impairs glioblastoma stem-like cell proliferation and promotes differentiation. Int. J. Cancer 2012, 131, E1067–E1077. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Nizamuddin, P.B.; Uddin, S.; Al-Thani, M.; Frenneaux, M.P.; Janahi, I.A.; Steinhoff, M.; Azizi, F. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis. Markers 2020, 2020, 8892312. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Amantini, C.; Maggi, F.; Marinelli, O.; Santoni, M.; Nabissi, M.; Morelli, M.B. The TRPV2 cation channels: From urothelial cancer invasiveness to glioblastoma multiforme interactome signature. Lab. Investig. 2020, 100, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol Increases Proliferation, Migration, Tubulogenesis, and Integrity of Human Brain Endothelial Cells through TRPV2 Activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef]
- Link, T.M.; Park, U.; Vonakis, B.M.; Raben, D.M.; Soloski, M.J.; Caterina, M.J. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat. Immunol. 2010, 11, 232–239. [Google Scholar] [CrossRef]
- Petitjean, H.; Hugel, S.; Barthas, F.; Bohren, Y.; Barrot, M.; Yalcin, I.; Schlichter, R. Activation of transient receptor potential vanilloid 2-expressing primary afferents stimulates synaptic transmission in the deep dorsal horn of the rat spinal cord and elicits mechanical hyperalgesia. Eur. J. Neurosci. 2014, 40, 3189–3201. [Google Scholar] [CrossRef]
- Jian, Z.; Ding, S.; Deng, H.; Wang, J.; Yi, W.; Wang, L.; Zhu, S.; Gu, L.; Xiong, X. Probenecid protects against oxygen–glucose deprivation injury in primary astrocytes by regulating inflammasome activity. Brain Res. 2016, 1643, 123–129. [Google Scholar] [CrossRef]
- Seo, J.H.; Dalal, M.S.; Contreras, J.E. Pannexin-1 channels as mediators of neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5189. [Google Scholar] [CrossRef]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. eLife 2020, 9, e54670. [Google Scholar] [CrossRef]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. 2020, 30, 446–448. [Google Scholar] [CrossRef]
- Yeung, A.K.; Patil, C.S.; Jackson, M.F. Pannexin-1 in the CNS: Emerging concepts in health and disease. J. Neurochem. 2020, 154, 468–485. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Grinspan, J.B.; Abrams, C.K.; Scherer, S.S. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 2007, 55, 46–56. [Google Scholar] [CrossRef]
- Iglesias, R.; Dahl, G.; Qiu, F.; Spray, D.C.; Scemes, E. Pannexin 1: The molecular substrate of astrocyte “hemichannels”. J. Neurosci. 2009, 29, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Montero, T.D.; von Bernhardi, R. Astrocytes inhibit nitric oxide-dependent Ca2+ dynamics in activated microglia: Involvement of ATP released via pannexin 1 channels. Glia 2013, 61, 2023–2037. [Google Scholar] [CrossRef] [PubMed]
- Swayne, L.A.; Sorbara, C.D.; Bennett, S.A. Pannexin 2 Is Expressed by Postnatal Hippocampal Neural Progenitors and Modulates Neuronal Commitment. J. Biol. Chem. 2010, 285, 24977–24986. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Hormuzdi, S.G.; Monyer, H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Mol. Brain Res. 2005, 141, 113–120. [Google Scholar] [CrossRef]
- Zappalà, A.; Volti, G.L.; Serapide, M.; Pellitteri, R.; Falchi, M.; La Delia, F.; Cicirata, V.; Cicirata, F. Expression of pannexin2 protein in healthy and ischemized brain of adult rats. Neuroscience 2007, 148, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Zoidl, G.; Petrasch-Parwez, E.; Ray, A.; Meier, C.; Bunse, S.; Habbes, H.-W.; Dahl, G.; Dermietzel, R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neuroscience 2007, 146, 9–16. [Google Scholar] [CrossRef]
- Ardiles, A.O.; Flores-Muñoz, C.; Toro-Ayala, G.; Cárdenas, A.M.; Palacios, A.G.; Munoz, P.; Fuenzalida, M.; Sáez, J.C.; Martinez, A.D. Pannexin 1 regulates bidirectional hippocampal synaptic plasticity in adult mice. Front. Cell. Neurosci. 2014, 8, 326. [Google Scholar] [CrossRef]
- Gajardo, I.; Salazar, C.S.; Lopez-Espíndola, D.; Estay, C.; Flores-Muñoz, C.; Elgueta, C.; González-Jamett, A.M.; Martínez, A.D.; Muñoz, P.; Ardiles, O. Lack of Pannexin 1 Alters Synaptic GluN2 Subunit Composition and Spatial Reversal Learning in Mice. Front. Mol. Neurosci. 2018, 11, 114. [Google Scholar] [CrossRef]
- Prochnow, N.; Abdulazim, A.; Kurtenbach, S.; Wildförster, V.; Dvoriantchikova, G.; Hanske, J.; Petrasch-Parwez, E.; Shestopalov, V.I.; Dermietzel, R.; Manahan-Vaughan, D.; et al. Pannexin1 Stabilizes Synaptic Plasticity and Is Needed for Learning. PLoS ONE 2012, 7, e51767. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Compan, V.; Zheng, W.; Martin, E.; North, R.A.; Verkhratsky, A.; Surprenant, A. Pannexin 1 forms an anion-selective channel. Pflügers Arch. 2012, 463, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Taruno, A.; Shiraishi, M.; Nakahari, T.; Inui, T.; Sokabe, M.; Eaton, D.C.; Marunaka, Y. Current-direction/amplitude-dependent single channel gating kinetics of mouse pannexin 1 channel: A new concept for gating kinetics. Sci. Rep. 2017, 7, 10512. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dahl, G. Pannexin1: A multifunction and multiconductance and/or permeability membrane channel. Am. J. Physiol. Cell Physiol. 2018, 315, C290–C299. [Google Scholar] [CrossRef]
- Romanov, R.A.; Bystrova, M.F.; Rogachevskaya, O.A.; Sadovnikov, V.B.; Shestopalov, V.I.; Kolesnikov, S.S. The ATP permeability of pannexin 1 channels in a heterologous system and in mammalian taste cells is dispensable. J. Cell Sci. 2012, 125, 5514–5523. [Google Scholar] [CrossRef]
- López, X.; Palacios-Prado, N.; Güiza, J.; Escamilla, R.; Fernández, P.; Vega, J.L.; Rojas, M.; Marquéz-Miranda, V.; Chamorro, E.; Cárdenas, A.M.; et al. A physiologic rise in cytoplasmic calcium ion signal increases pannexin1 channel activity via a C-terminus phosphorylation by CaMKII. Proc. Natl. Acad. Sci. USA 2021, 118, e2108967118. [Google Scholar] [CrossRef]
- Thompson, R.J.; Zhou, N.; MacVicar, B.A. Ischemia opens neuronal gap junction hemichannels. Science 2006, 312, 924–927. [Google Scholar] [CrossRef]
- Wang, J.; Ambrosi, C.; Qiu, F.; Jackson, D.G.; Sosinsky, G.; Dahl, G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 2014, 7, ra69. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Locovei, S.; Bao, L.; Dahl, G. Pannexin 1 in erythrocytes: Function without a gap. Proc. Natl. Acad. Sci. USA 2006, 103, 7655–7659. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; Jackson, M.F.; Olah, M.E.; Rungta, R.L.; Hines, D.J.; Beazely, M.A.; MacDonald, J.F.; MacVicar, B.A. Activation of pannexin-1 hemichannels augments aberrant bursting in the hippocampus. Science 2008, 322, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Lopatář, J.; Dale, N.; Frenguelli, B.G. Pannexin-1-mediated ATP release from area CA3 drives mGlu5-dependent neuronal oscillations. Neuropharmacology 2015, 93, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef]
- Weilinger, N.L.; Lohman, A.W.; Rakai, B.D.; Ma, E.M.; Bialecki, J.; Maslieieva, V.; Rilea, T.; Bandet, M.V.; Ikuta, N.T.; Scott, L.; et al. Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat. Neurosci. 2016, 19, 432–442. [Google Scholar] [CrossRef]
- Billaud, M.; Lohman, A.W.; Straub, A.C.; Looft-Wilson, R.; Johnstone, S.R.; Araj, C.A.; Best, A.K.; Chekeni, F.B.; Ravichandran, K.S.; Penuela, S.; et al. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ. Res. 2011, 109, 80–85. [Google Scholar] [CrossRef]
- Maldifassi, M.C.; Momboisse, F.; Guerra, M.J.; Vielma, A.H.; Maripillan, J.; Baez-Matus, X.; Flores-Munoz, C.; Cadiz, B.; Schmachtenberg, O.; Martinez, A.D.; et al. The interplay between alpha7 nicotinic acetylcholine receptors, pannexin-1 channels and P2X7 receptors elicit exocytosis in chromaffin cells. J. Neurochem. 2021, 157, 1789–1808. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ’find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef]
- Ma, W.; Hui, H.; Pelegrin, P.; Surprenant, A. Pharmacological Characterization of Pannexin-1 Currents Expressed in Mammalian Cells. J. Pharmacol. Exp. Ther. 2009, 328, 409–418. [Google Scholar] [CrossRef]
- Sahu, G.; Sukumaran, S.; Bera, A.K. Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins. Sci. Rep. 2014, 4, 4955. [Google Scholar] [CrossRef]
- Zarrinmayeh, H.; Territo, P.R. Purinergic Receptors of the Central Nervous System: Biology, PET Ligands, and Their Applications. Mol. Imaging 2020, 19, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Physiology and Pathophysiology of Purinergic Neurotransmission. Physiol. Rev. 2007, 87, 659–797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Arias, J.C.; van der Slagt, E.; Vecchiarelli, H.A.; Candlish, R.C.; York, N.; Young, P.A.; Shevtsova, O.; Juma, A.; Tremblay, M.; Swayne, L.A. Purinergic signaling in nervous system health and disease: Focus on pannexin 1. Pharmacol. Ther. 2021, 225, 107840. [Google Scholar] [CrossRef]
- Li, S.; Bjelobaba, I.; Stojilkovic, S.S. Interactions of Pannexin1 channels with purinergic and NMDA receptor channels. Biochim. Biophys. Acta Biomembr. 2018, 1860, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gisclon, L.G.; Boyd, R.A.; Williams, R.L.; Giacomini, K.M. The effect of probenecid on the renal elimination of cimetidine. Clin. Pharmacol. Ther. 1989, 45, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Boom, S.P.; Gribnau, F.W.; Russel, F. Organic cation transport and cationic drug interactions in freshly isolated proximal tubular cells of the rat. J. Pharmacol. Exp. Ther. 1992, 263, 445–450. [Google Scholar] [PubMed]
- Inotsume, N.; Nishimura, M.; Nakano, M.; Fujiyama, S.; Sato, T. The Inhibitory Effect of Probenecid on Renal Excretion of Famotidine in Young, Healthy Volunteers. J. Clin. Pharmacol. 1990, 30, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Couroussé, T.; Gautron, S. Role of organic cation transporters (OCTs) in the brain. Pharmacol. Ther. 2015, 146, 94–103. [Google Scholar] [CrossRef]
- McKinney, T.D.; Myers, P.; Speeg, K.V., Jr. Cimetidine secretion by rabbit renal tubules in vitro. Am. J. Physiol. Ren. Physiol. 1981, 241, F69–F76. [Google Scholar] [CrossRef]
- Hsyu, P.H.; Gisclon, L.G.; Hui, A.C.; Giacomini, K.M. Interactions of organic anions with the organic cation transporter in renal BBMV. Am. J. Physiol. Ren. Physiol. 1988, 254, F56–F61. [Google Scholar] [CrossRef]
- Koepsell, H. General Overview of Organic Cation Transporters in Brain. In Organic Cation Transporters in the Central Nervous System; Daws, L.C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–39. [Google Scholar]
- Shayanfar, A.; Gharekhani, A.; Ghasemian, E. Cimetidine is critical in CNS disorders. Biosci. Hypotheses 2009, 2, 180–181. [Google Scholar] [CrossRef]
- Werdinius, B. Effect of Probenecid on the Levels of Monoamine Metabolites in the Rat Brain. Acta Pharmacol. Toxicol. 1967, 25, 18–23. [Google Scholar] [CrossRef]
- Venero, J.; Machado, A.; Cano, J. Effect of ageing on monoamine turnover in the prefrontal cortex of rats. Mech. Ageing Dev. 1993, 72, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cumming, P.; Brown, E.; Damsma, G.; Fibiger, H. Formation and Clearance of Interstitial Metabolites of Dopamine and Serotonin in the Rat Striatum: An In Vivo Microdialysis Study. J. Neurochem. 1992, 59, 1905–1914. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Lang, Y.; Chu, F.; Shen, D.; Zhang, W.; Zheng, C.; Zhu, J.; Cui, L. Role of Inflammasomes in Neuroimmune and Neurodegenerative Diseases: A Systematic Review. Mediat. Inflamm. 2018, 2018, 1549549. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Gao, W.; Li, L.; Li, P.; Zhang, L.; Gong, Y.-N.; Peng, X.; Xi, J.J.; Chen, S.; et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 2014, 513, 237–241. [Google Scholar] [CrossRef]
- Bennett, M.V.; Garré, J.M.; Orellana, J.A.; Bukauskas, F.F.; Nedergaard, M.; Giaume, C.; Sáez, J.C. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012, 1487, 3–15. [Google Scholar] [CrossRef]
- Walev, I.; Reske, K.; Palmer, M.; Valeva, A.; Bhakdi, S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995, 14, 1607–1614. [Google Scholar] [CrossRef]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X7receptor-Pannexin1 complex: Pharmacology and signaling. Am. J. Physiol. Cell Physiol. 2008, 295, C752–C760. [Google Scholar] [CrossRef] [PubMed]
- Adamson, S.E.; Leitinger, N. The role of pannexin1 in the induction and resolution of inflammation. FEBS Lett. 2014, 588, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Gombault, A.; Baron, L.; Couillin, I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front. Immunol. 2012, 3, 414. [Google Scholar] [CrossRef]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Giaume, C.; Sáez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef]
- Orellana, J.A.; Shoji, K.F.; Abudara, V.; Ezan, P.; Amigou, E.; Sáez, P.J.; Jiang, J.X.; Naus, C.C.; Sáez, J.C.; Giaume, C. Amyloid beta-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 2011, 31, 4962–4977. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Delalio, L.J.; Best, A.K.; Macal, E.; Milstein, J.; Donnelly, I.; Miller, A.M.; McBride, M.; Shu, X.; Koval, M.; et al. Endothelial Pannexin 1 Channels Control Inflammation by Regulating Intracellular Calcium. J. Immunol. 2020, 204, 2995–3007. [Google Scholar] [CrossRef]
- Zheng, Y.; Tang, W.; Zeng, H.; Peng, Y.; Yu, X.; Yan, F.; Cao, S. Probenecid-Blocked Pannexin-1 Channel Protects against Early Brain Injury via Inhibiting Neuronal AIM2 Inflammasome Activation after Subarachnoid Hemorrhage. Front. Neurol. 2022, 13, 854671. [Google Scholar] [CrossRef]
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Moshe, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Rugg-Gunn, F.; Miserocchi, A.; McEvoy, A. Epilepsy surgery. Pract. Neurol. 2020, 20, 4–14. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, C.; Bravo-Tobar, I.D.; Duarte, Y.; Barrio, L.C.; Sáez, J.C. Contribution of non-selective membrane channels and receptors in epilepsy. Pharmacol. Ther. 2022, 231, 107980. [Google Scholar] [CrossRef] [PubMed]
- Aquilino, M.S.; Whyte-Fagundes, P.; Zoidl, G.; Carlen, P.L. Pannexin-1 channels in epilepsy. Neurosci. Lett. 2019, 695, 71–75. [Google Scholar] [CrossRef]
- Santiago, M.F.; Veliskova, J.; Patel, N.K.; Lutz, S.E.; Caille, D.; Charollais, A.; Meda, P.; Scemes, E. Targeting Pannexin1 Improves Seizure Outcome. PLoS ONE 2011, 6, e25178. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Blauwblomme, T.; Moulard, J.; Chever, O.; Vasile, F.; Guinard, E.; Le Bert, M.; Couillin, I.; Pallud, J.; Capelle, L.; et al. Pannexin-1 channels contribute to seizure generation in human epileptic brain tissue and in a mouse model of epilepsy. Sci. Transl. Med. 2018, 10, eaar3796. [Google Scholar] [CrossRef]
- Aquilino, M.S.; Whyte-Fagundes, P.; Lukewich, M.K.; Zhang, L.; Bardakjian, B.L.; Zoidl, G.R.; Carlen, P.L. Pannexin-1 Deficiency Decreases Epileptic Activity in Mice. Int. J. Mol. Sci. 2020, 21, 7510. [Google Scholar] [CrossRef]
- Steinhäuser, C.; Seifert, G.; Bedner, P. Astrocyte dysfunction in temporal lobe epilepsy: K + channels and gap junction coupling. Glia 2012, 60, 1192–1202. [Google Scholar] [CrossRef]
- Volnova, A.; Tsytsarev, V.; Ganina, O.; Vélez-Crespo, G.E.; Alves, J.M.; Ignashchenkova, A.; Inyushin, M. The Anti-Epileptic Effects of Carbenoxolone In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 663. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Z.; Ni, Y.; Dai, Z. Carbenoxolone pretreatment and treatment of posttraumatic epilepsy. Neural Regen. Res. 2013, 8, 169–176. [Google Scholar]
- Michalski, K.; Kawate, T. Carbenoxolone inhibits Pannexin1 channels through interactions in the first extracellular loop. J. Gen. Physiol. 2016, 147, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Biju, K.; Evans, R.C.; Shrestha, K.; Carlisle, D.C.; Gelfond, J.; Clark, R.A. Methylene Blue Ameliorates Olfactory Dysfunction and Motor Deficits in a Chronic MPTP/Probenecid Mouse Model of Parkinson’s Disease. Neuroscience 2018, 380, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Méndez-Cuesta, L.A.; Perez-De La Cruz, V.; Fortoul-van Der Goes, T.I.; Santamaría, A. Protective effect of systemic l-kynurenine and probenecid administration on behavioural and morphological alterations induced by toxic soluble amyloid beta (25–35) in rat hippocampus. Behav. Brain Res. 2010, 210, 240–250. [Google Scholar] [CrossRef]
- Flores-Muñoz, C.; Gómez, B.; Mery, E.; Mujica, P.; Gajardo, I.; Córdova, C.; Lopez-Espíndola, D.; Durán-Aniotz, C.; Hetz, C.; Muñoz, P.; et al. Acute Pannexin 1 Blockade Mitigates Early Synaptic Plasticity Defects in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2020, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Hainz, N.; Wolf, S.; Tschernig, T.; Meier, C. Probenecid Application Prevents Clinical Symptoms and Inflammation in Experimental Autoimmune Encephalomyelitis. Inflammation 2016, 39, 123–128. [Google Scholar] [CrossRef]
- Hainz, N.; Wolf, S.; Beck, A.; Wagenpfeil, S.; Tschernig, T.; Meier, C. Probenecid arrests the progression of pronounced clinical symptoms in a mouse model of multiple sclerosis. Sci. Rep. 2017, 7, 17214. [Google Scholar] [CrossRef]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading Depression Triggers Headache by Activating Neuronal Panx1 Channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef]
- Shao, Q.-H.; Chen, Y.; Li, F.-F.; Wang, S.; Zhang, X.-L.; Yuan, Y.-H.; Chen, N.-H. TLR4 deficiency has a protective effect in the MPTP/probenecid mouse model of Parkinson’s disease. Acta Pharmacol. Sin. 2019, 40, 1503–1512. [Google Scholar] [CrossRef]
- Silva-Adaya, D.; Perez-De La Cruz, V.; Villeda-Hernández, J.; Carrillo-Mora, P.; González-Herrera, I.G.; García, E.; Colín-Barenque, L.; Pedraza-Chaverrí, J.; Santamaría, A. Protective effect of l-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy. Neurotoxicology Teratol. 2011, 33, 303–312. [Google Scholar] [CrossRef]
- Wei, R.; Wang, J.; Xu, Y.; Yin, B.; He, F.; Du, Y.; Peng, G.; Luo, B. Probenecid protects against cerebral ischemia/reperfusion injury by inhibiting lysosomal and inflammatory damage in rats. Neuroscience 2015, 301, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Lin, S.G.; Chen, X.; Zhou, Z.W.; Liang, J.; Duan, W.; Chowbay, B.; Wen, J.Y.; Chan, E.; Cao, J.; et al. Transport of cryptotanshinone, a major active triterpenoid in Salvia miltiorrhiza Bunge widely used in the treatment of stroke and Alzheimer’s disease, across the blood-brain barrier. Curr. Drug Metab. 2007, 8, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, Y.; Yan, C.; Mei, X.; Jiang, T.; Ma, Z.; Wang, Q. Probenecid Relieves Cerebral Dysfunction of Sepsis by Inhibiting Pannexin 1-Dependent ATP Release. Inflammation 2019, 42, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Pajares, M.; IRojo, A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Samiei, M.; Maleki Dizaj, S.; Vinken, M. The role and therapeutic potential of connexins, pannexins and their channels in Parkinson’s disease. Cell. Signal. 2019, 58, 111–118. [Google Scholar] [CrossRef]
- Díaz, E.F.; Labra, V.C.; Alvear, T.F.; Mellado, L.A.; Inostroza, C.A.; Oyarzún, J.E.; Salgado, N.; Quintanilla, R.A.; Orellana, J.A. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes. Glia 2019, 67, 1598–1619. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Weintraub, S.; Wicklund, A.H.; Salmon, D.P. The Neuropsychological Profile of Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006171. [Google Scholar] [CrossRef]
- Masliah, E.; Mallory, M.; Alford, M.; DeTeresa, R.; Hansen, L.; McKeel, D., Jr.; Morris, J. Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 2001, 56, 127–129. [Google Scholar] [CrossRef]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef]
- Sheng, M.; Sabatini, B.L.; Sudhof, T.C. Synapses and Alzheimer’s disease. Cold Spring Harb. Perspect Biol. 2012, 4, a005777. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect Med. 2012, 2, a006213. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Floyd, R.A.; Zheng, N.Y.; Nael, R.; Robinson, K.A.; Nguyen, X.; Pye, Q.N.; Stewart, C.A.; Geddes, J.; Markesbery, W.R.; et al. p38 kinase is activated in the Alzheimer’s disease brain. J. Neurochem. 1999, 72, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, a024240. [Google Scholar] [CrossRef]
- Vamos, E.; Voros, K.; Zadori, D.; Vecsei, L.; Klivenyi, P. Neuroprotective effects of probenecid in a transgenic animal model of Huntington’s disease. J. Neural Transm. 2009, 116, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- Zhang, X.; Levy, D.; Noseda, R.; Kainz, V.; Jakubowski, M.; Burstein, R. Activation of Meningeal Nociceptors by Cortical Spreading Depression: Implications for Migraine with Aura. J. Neurosci. 2010, 30, 8807–8814. [Google Scholar] [CrossRef]
- Singh, M.B.; White, J.A.; McKimm, E.J.; Milosevic, M.; Antic, S.D. Mechanisms of Spontaneous Electrical Activity in the Developing Cerebral Cortex—Mouse Subplate Zone. Cereb. Cortex 2019, 29, 3363–3379. [Google Scholar] [CrossRef] [PubMed]

| Target | IC50/EC50 | Assay | Reference |
|---|---|---|---|
| OAT1 | IC50 12.3 μM 1 | [14C]PAH uptake | Takeda et al., 2001 [16] |
| OAT3 | IC50 4.93 μM 1 | [3H]ES uptake | Takeda et al., 2001 [16] |
| TRPV2 | EC50 31.9 μM 2 | Calcium currents | Bang et al., 2007 [11] |
| Panx1 | IC50 150 μM 3 | Ionic currents | Silverman et al., 2008 [13] |
| OCT1 | IC50 1640 μM 4 | [14C]TEA uptake | Arndt et al., 2001 [17] |
| OCT2 | IC50 1700 μM 4 | [14C]TEA uptake | Arndt et al., 2001 [17] |
| P2X7R | IC50 203 μM 5 | Ethidium uptake | Bhaskaracharya et al., 2014 [18] |
| Study | Type of Study | Study Model | Doses or Concentration | CNS Pathology | Probenecid Effect |
|---|---|---|---|---|---|
| Aquilino et al., 2020 [119] | In vivo | Mice pretreated with PBN are exposed to 80 mg/kg de PTZ | 250 mg/kg i.p. | Epilepsy | Decrease in seizures severity. |
| Biju et al., 2018 [124] | In vivo, ex vivo | MPTP mice/PBN and behavioral assessment and tyrosine hydroxylase (TH) neuron analysis | 250 mg/kg i.p. | PD | Low-dose methylene blue has neuroprotective actions in PD. |
| Carrillo-Mora et al., 2010 [125] | In vivo, ex vivo | Coadministration of kynurenic acid and PBN in beta-amyloid peptide rats. Evaluation by locomotor, memory, and morphological tests | 50 mg/kg i.h. or i.p. | AD | Improvements in spatial memory and decrease in neurodegenerative events. |
| Dossi et al., 2018 [118] | Ex vivo, in vivo | Postoperative samples of human tissue in patients with epilepsy Mouse model with kainic acid of temporal lobe epilepsy (TLE) | 1 mM 200 mg/kg i.p. | Epilepsy | Significant decrease in epileptic discharges. |
| Flores-Muñoz et al., 2020 [126] | Ex vivo | Transgenic APP/PS1 mice were dissected in different histological sections, to which PBN was administered | 100 μM | EA | Decrease in synaptic plasticity deficits and improvement in dendritic spine density and dendritic arborization. |
| Hainz et al., 2016 [127] | In vivo | Experimental autoimmune encephalomyelitis (EAE) mouse model–multiple sclerosis (MS) mouse model | 200 mg/kg i.p. | EAE/MS | Significant decrease in inflammation and infiltrating T cells in the CNS. |
| Hainz et al., 2017 [128] | In vivo | Experimental autoimmune encephalomyelitis (EAE) mouse model–multiple sclerosis (MS) mouse model | 200 mg/kg i.p. | EAE/MS | Decrease in inflammation and T-cell infiltration and increase in oligodendrocyte number. |
| Jian et al., 2016 [47] | In vitro | Primary neuron and astrocyte culture from newborn mice exposed to oxygen–glucose deprivation/reoxygenation (OGD/RX) | 5–10 μM | Ischemia | Inhibition of inflammasome and caspase 1 activities. |
| Karatas et al., 2013 [129] | In vivo | Experimental mice model of cortical spreading depression (CSD) induced by pinprick or KCl | 60 μg i.c.v. | Migraine/headache | Suppression of trigeminovascular activation, dural mast cell degranulation, inflammation, and headache. |
| Shao et al., 2019 [130] | Ex vivo | MPTP mice/PBN and subsequent substantia nigra and striatum analysis | 250 mg/kg i.p. | PD | Verification of the neuroprotective role of TLR4 in PD. |
| Silva-Adaya et al., 2011 [131] | In vivo | 6-OHDA-induced PD model mice, coadministration of PBN with L-kineurin | 70 mg/kg i.p. | PD | Increase in CNS kynurenic acid levels. |
| Silverman et al., 2009 [74] | In vitro | Primary neuron and astrocyte culture. Culture of oocytes absent from follicular cells of Xenopus laevis frogs | 2 mM | NA | Blockade of inflammasome activation and PANX1 currents. |
| Sun et al., 2001 [8] | In vitro | Analysis of fluorescein passage in bovine brain micro vessel endothelial cells (BBMEC) | 100 μM | NA | Increase in the passage of fluorescein in BBMEC. |
| Tunblad et al., 2003 [10] | In vivo | PBN is administered using micro dialysis probes to evaluate its influence on the passage of morphine to the CNS in rats | 20 mg/kg e.f.b. 20 mg/kg/h i.f. | NA | Increase in morphine half-life by almost twice in rat brain. |
| Wei et al., 2015 [132] | In vivo | Cerebral ischemia/reperfusion (I/R) rat model | 2 mg/kg i.p. 5 mg/kg gavage 0.1–1–10 mg/mL i.v. | Cerebral ischemia | Reduction in CA1 neuron loss and inflammation. |
| Yu X. Y. et al., 2007 [133] | Ex vivo | Administration of cytosine with PBN in rats and evaluation of its role | 200 μM | AD | Decreases in cytosine output. |
| Zhang et al., 2019 [134] | In vivo | Sepsis-associated encephalopathy (SAE) mouse model | 50 mg/kg i.p. | SAE | Attenuation in neuroinflammatory response and cognitive impairments. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rodríguez, C.; Mujica, P.; Illanes-González, J.; López, A.; Vargas, C.; Sáez, J.C.; González-Jamett, A.; Ardiles, Á.O. Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation. Biomedicines 2023, 11, 1516. https://doi.org/10.3390/biomedicines11061516
García-Rodríguez C, Mujica P, Illanes-González J, López A, Vargas C, Sáez JC, González-Jamett A, Ardiles ÁO. Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation. Biomedicines. 2023; 11(6):1516. https://doi.org/10.3390/biomedicines11061516
Chicago/Turabian StyleGarcía-Rodríguez, Claudia, Paula Mujica, Javiera Illanes-González, Araceli López, Camilo Vargas, Juan C. Sáez, Arlek González-Jamett, and Álvaro O. Ardiles. 2023. "Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation" Biomedicines 11, no. 6: 1516. https://doi.org/10.3390/biomedicines11061516
APA StyleGarcía-Rodríguez, C., Mujica, P., Illanes-González, J., López, A., Vargas, C., Sáez, J. C., González-Jamett, A., & Ardiles, Á. O. (2023). Probenecid, an Old Drug with Potential New Uses for Central Nervous System Disorders and Neuroinflammation. Biomedicines, 11(6), 1516. https://doi.org/10.3390/biomedicines11061516









