CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. In Vivo Experiments
2.3. Magnetic Resonance Imaging (MRI)
2.4. Immunohistochemistry
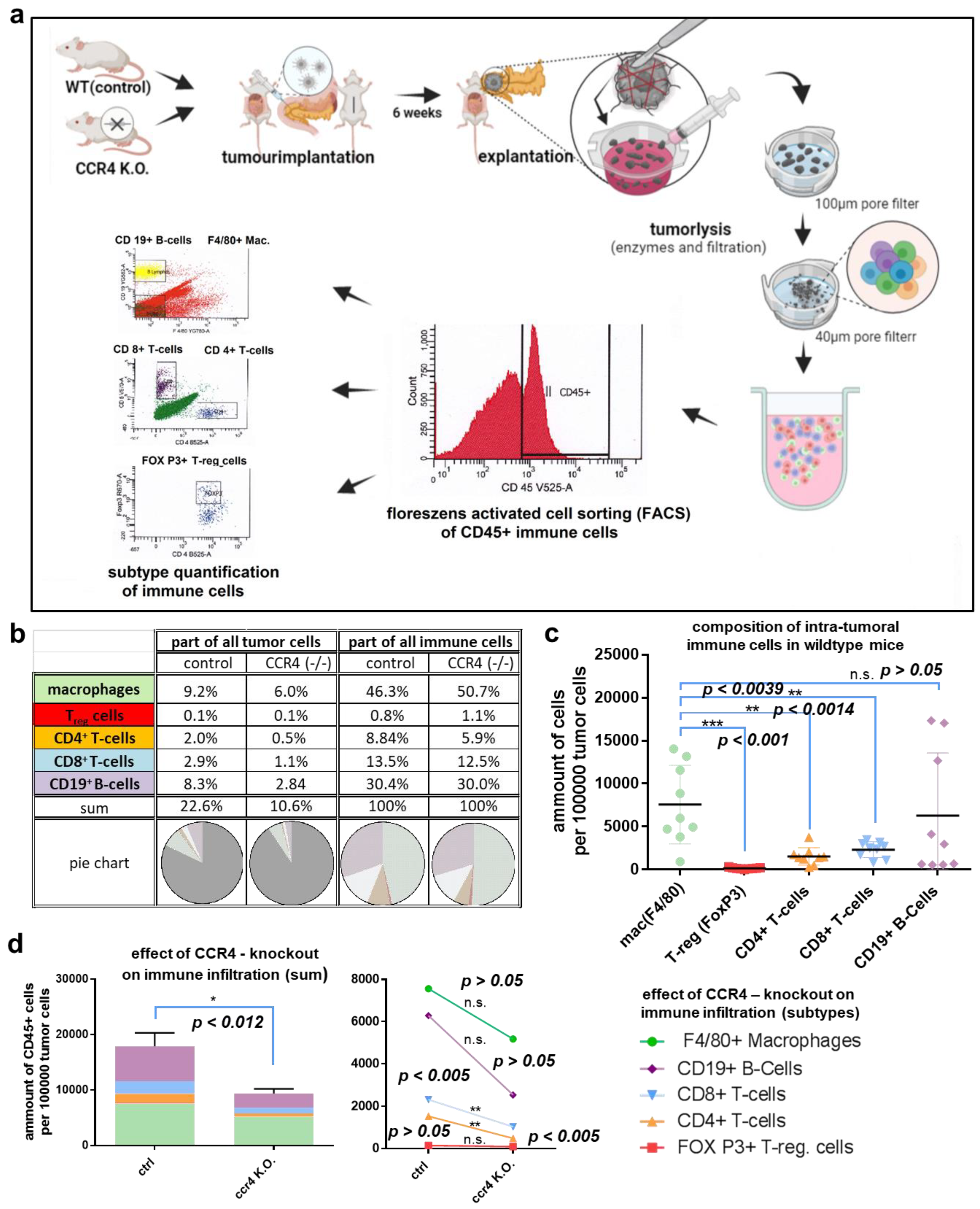
2.5. Flow Cytometry
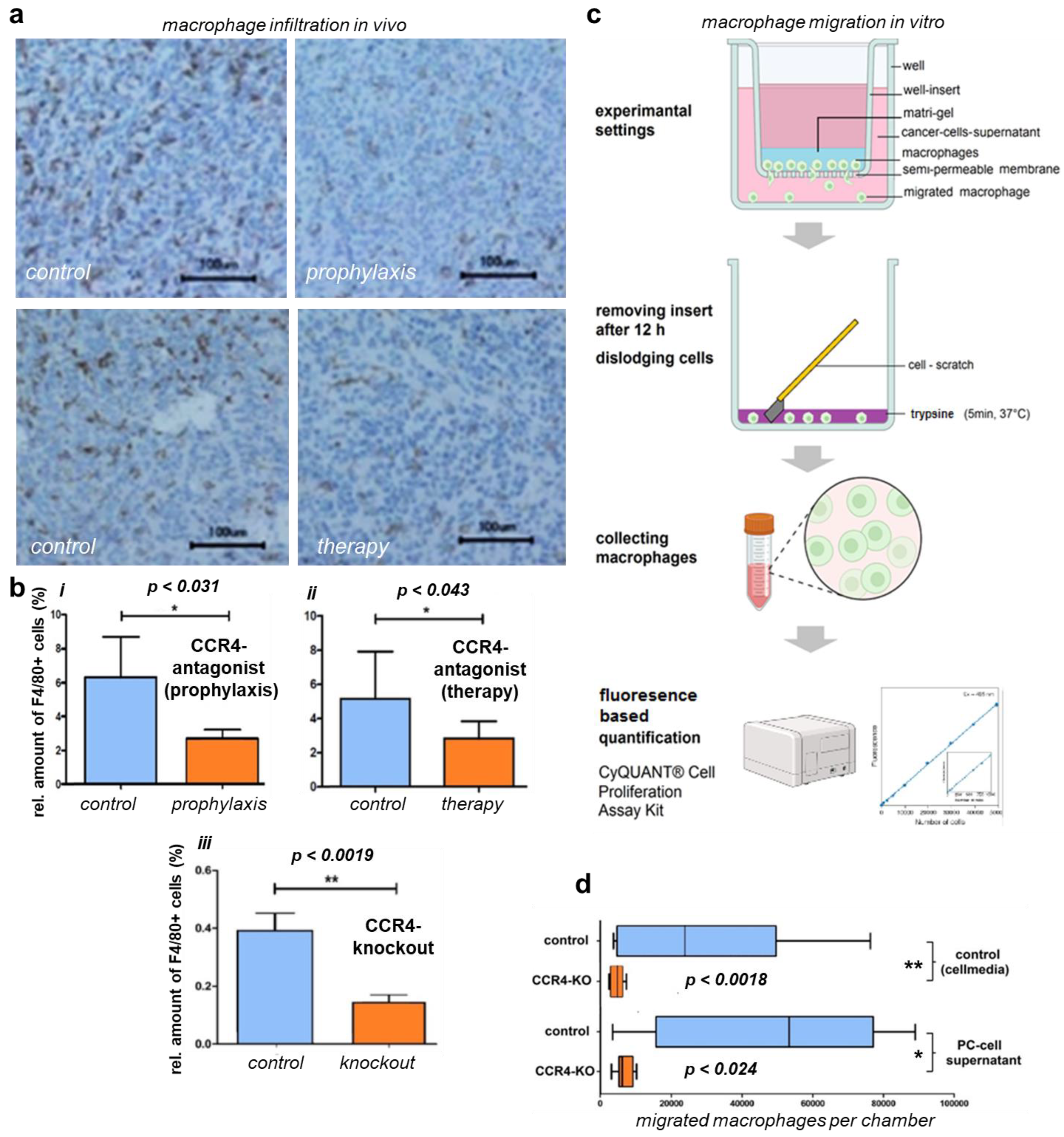
2.6. Migration Analysis
2.7. Statistical Analysis
3. Results
3.1. CCR4 Inhibition and Depletion Were Associated with Lower Tumor Burden In Vivo
3.2. CCR4 Inhibition and Depletion Were Linked to Improved Tumor Survival In Vivo
3.3. Loss of CCR4 Reduced Immune Cell Infiltration
3.4. CCR4 Inhibition and Depletion Affected Macrophage Migration and Infiltration
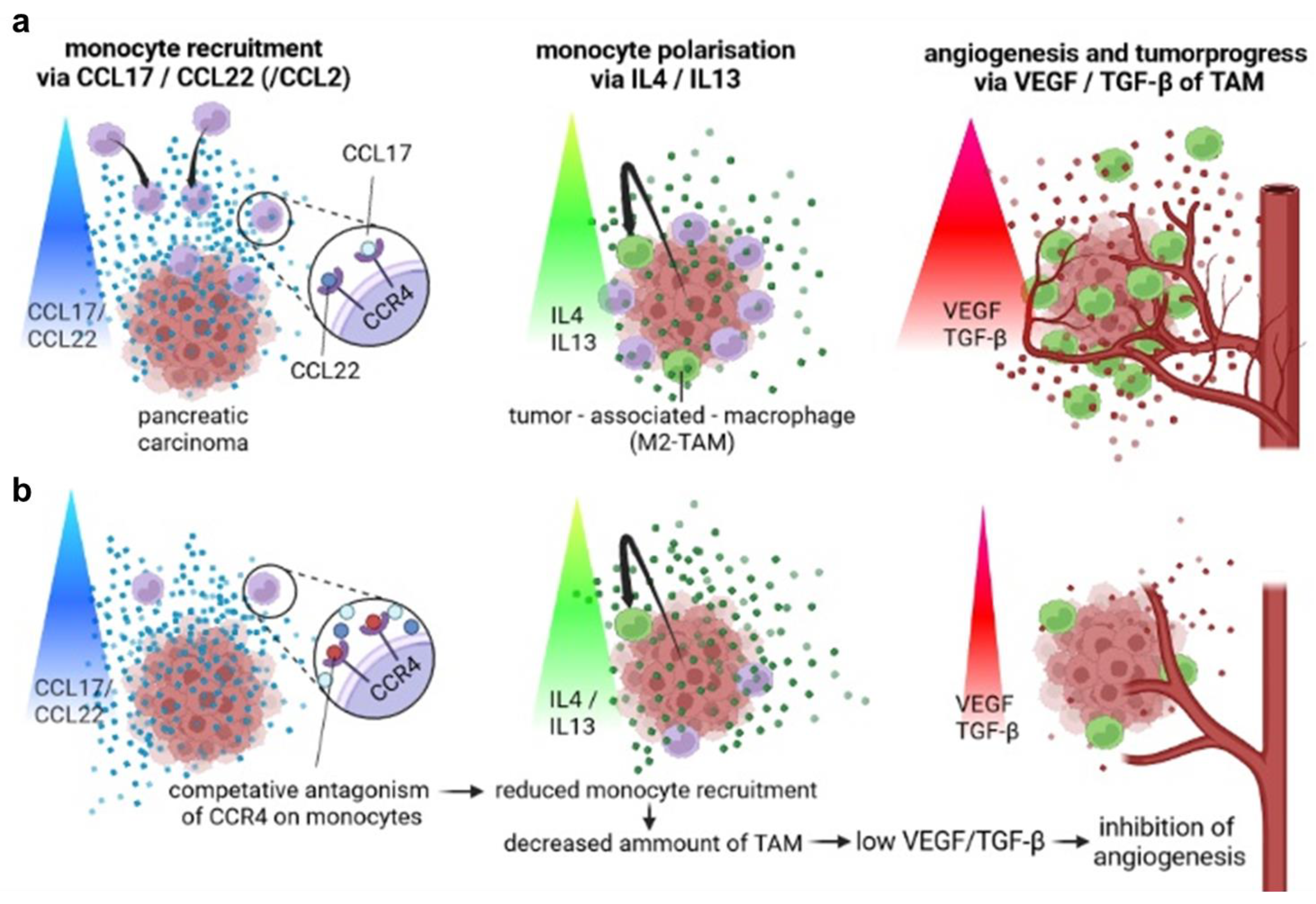
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef] [PubMed]
- Ness, T.L.; Ewing, J.L.; Hogaboam, C.M.; Kunkel, S.L. Ccr4 Is a Key Modulator of Innate Immune Responses. J. Immunol. 2006, 177, 7531–7539. [Google Scholar] [CrossRef] [PubMed]
- Al-Banna, N.A.; Vaci, M.; Slauenwhite, D.; Johnston, B.; Issekutz, T.B. Ccr4 and Cxcr3 Play Different Roles in the Migration of T Cells to Inflammation in Skin, Arthritic Joints, and Lymph Nodes. Eur. J. Immunol. 2014, 44, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Bian, Y.; Gao, P.; Yashiro-Ohtani, Y.; Zhou, X.Y.; Ono, S.; Nakahara, H.; Kogo, M.; Hamaoka, T.; Fujiwara, H. Induction of Surface Ccr4 and Its Functionality in Mouse Th2 Cells Is Regulated Differently During Th2 Development. J. Leukoc. Biol. 2005, 78, 753–761. [Google Scholar] [CrossRef]
- Wang, Z.; Louras, N.J.; Lellouch, A.G.; Pratts, S.G.; Zhang, H.; Wang, H.; Huang, C.A.; Cetrulo, C.L., Jr.; Madsen, J.C.; Sachs, D.H.; et al. Dosing Optimization of Ccr4 Immunotoxin for Improved Depletion of Ccr4(+) Treg in Nonhuman Primates. Mol. Oncol. 2018, 12, 1374–1382. [Google Scholar] [CrossRef]
- Ketcham, J.M.; Marshall, L.A.; Talay, O. Ccr4 Antagonists Inhibit Treg Trafficking into the Tumor Microenvironment. ACS Med. Chem. Lett. 2018, 9, 953–955. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Tumour Microenvironment of Pancreatic Cancer: Immune Landscape Is Dictated by Molecular and Histopathological Features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic Cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic Analyses Identify Molecular Subtypes of Pancreatic Cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hruban, R.H.; Goggins, M.; Parsons, J.; Kern, S.E. Progression Model for Pancreatic Cancer. Clin. Cancer Res. 2000, 6, 2969–2972. [Google Scholar] [PubMed]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Vonlaufen, A.; Joshi, S.; Qu, C.; Phillips, P.A.; Xu, Z.; Parker, N.R.; Toi, C.S.; Pirola, R.C.; Wilson, J.S.; Goldstein, D.; et al. Pancreatic Stellate Cells: Partners in Crime with Pancreatic Cancer Cells. Cancer Res. 2008, 68, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. Emt and Dissemination Precede Pancreatic Tumor Formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; Ruggieri, S.; Ribatti, D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers 2019, 11, 381. [Google Scholar] [CrossRef]
- Cui, R.; Yue, W.; Lattime, E.C.; Stein, M.N.; Xu, Q.; Tan, X.L. Targeting Tumor-Associated Macrophages to Combat Pancreatic Cancer. Oncotarget 2016, 7, 50735–50754. [Google Scholar] [CrossRef]
- Kurahara, H.; Shinchi, H.; Mataki, Y.; Maemura, K.; Noma, H.; Kubo, F.; Sakoda, M.; Ueno, S.; Natsugoe, S.; Takao, S. Significance of M2-Polarized Tumor-Associated Macrophage in Pancreatic Cancer. J. Surg. Res. 2009, 167, e211–e219. [Google Scholar] [CrossRef]
- Mielgo, A.; Schmid, M.C. Impact of Tumour Associated Macrophages in Pancreatic Cancer. BMB Rep. 2013, 46, 131–138. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Liu, Q.; Dou, R.; Xiong, B. Crosstalk between Cancer Cells and Tumor Associated Macrophages Is Required for Mesenchymal Circulating Tumor Cell-Mediated Colorectal Cancer Metastasis. Mol. Cancer 2019, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Algul, H.; Tuveson, D.A.; Gress, T.M. Stromal Biology and Therapy in Pancreatic Cancer: A Changing Paradigm. Gut 2015, 64, 1476–1484. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Sung, G.H.; Chang, H.; Lee, J.Y.; Song, S.Y.; Kim, H.S. Pancreatic-Cancer-Cell-Derived Trefoil Factor 2 Impairs Maturation and Migration of Human Monocyte-Derived Dendritic Cells in Vitro. Anim. Cells Syst. 2018, 22, 368–381. [Google Scholar] [CrossRef]
- Khabipov, A.; Kading, A.; Liedtke, K.R.; Freund, E.; Partecke, L.I.; Bekeschus, S. Raw 264.7 Macrophage Polarization by Pancreatic Cancer Cells—A Model for Studying Tumour-Promoting Macrophages. Anticancer Res. 2019, 39, 2871–2882. [Google Scholar] [CrossRef]
- Ren, B.; Cui, M.; Yang, G.; Wang, H.; Feng, M.; You, L.; Zhao, Y. Tumor Microenvironment Participates in Metastasis of Pancreatic Cancer. Mol. Cancer 2018, 17, 108. [Google Scholar] [CrossRef]
- Shu, Y.; Cheng, P. Targeting Tumor-Associated Macrophages for Cancer Immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188434. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-Associated Macrophages: A Promising Target for a Cancer Immunotherapeutic Strategy. Pharmacol. Res. 2020, 161, 105111. [Google Scholar] [CrossRef]
- Leonard, E.J.; Yoshimura, T. Human Monocyte Chemoattractant Protein-1 (Mcp-1). Immunol. Today 1990, 11, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. Mcp-1: Function, Regulation, and Involvement in Disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the Ccl2-Ccr2 Signaling Axis in Cancer Metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef]
- Brummer, G.; Fang, W.; Smart, C.; Zinda, B.; Alissa, N.; Berkland, C.; Miller, D.; Cheng, N. Ccr2 Signaling in Breast Carcinoma Cells Promotes Tumor Growth and Invasion by Promoting Ccl2 and Suppressing Cd154 Effects on the Angiogenic and Immune Microenvironments. Oncogene 2019, 39, 2275–2289. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, X.; Chen, X.; Xia, J.; Cheng, B.; Tao, X. Ccl2 Promotes Cell Migration by Inducing Epithelial-Mesenchymal Transition in Oral Squamous Cell Carcinoma. J. Oral Pathol. Med. 2019, 48, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.; Huang, M.; Kosonen, R.; Lee, J.E. Ccr4 and Ccr5 Involvement in Monocyte-Derived Macrophage Migration in Neuroinflammation. Front. Immunol. 2022, 13, 876033. [Google Scholar] [CrossRef]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Pienta, K.J.; Machiels, J.P.; Schrijvers, D.; Alekseev, B.; Shkolnik, M.; Crabb, S.J.; Li, S.; Seetharam, S.; Puchalski, T.A.; Takimoto, C.; et al. Phase 2 Study of Carlumab (Cnto 888), a Human Monoclonal Antibody against Cc-Chemokine Ligand 2 (Ccl2), in Metastatic Castration-Resistant Prostate Cancer. Investig. New Drugs 2012, 31, 760–768. [Google Scholar] [CrossRef]
- Bonapace, L.; Coissieux, M.M.; Wyckoff, J.; Mertz, K.D.; Varga, Z.; Junt, T.; Bentires-Alj, M. Cessation of Ccl2 Inhibition Accelerates Breast Cancer Metastasis by Promoting Angiogenesis. Nature 2014, 515, 130–133. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. Ccr4 and Its Ligands: From Bench to Bedside. Int. Immunol. 2014, 27, 11–20. [Google Scholar] [CrossRef]
- Power, C.A.; Meyer, A.; Nemeth, K.; Bacon, K.B.; Hoogewerf, A.J.; Proudfoot, A.E.; Wells, T.N. Molecular Cloning and Functional Expression of a Novel Cc Chemokine Receptor Cdna from a Human Basophilic Cell Line. J. Biol. Chem. 1995, 270, 19495–19500. [Google Scholar] [CrossRef] [PubMed]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue Tolerable Plasma (Ttp) Induces Apoptosis in Pancreatic Cancer Cells in Vitro and in Vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Partecke, L.I.; Sendler, M.; Kaeding, A.; Weiss, F.U.; Mayerle, J.; Dummer, A.; Nguyen, T.D.; Albers, N.; Speerforck, S.; Lerch, M.M.; et al. A Syngeneic Orthotopic Murine Model of Pancreatic Adenocarcinoma in the C57/Bl6 Mouse Using the Panc02 and 6606pda Cell Lines. Eur. Surg. Res. 2011, 47, 98–107. [Google Scholar] [CrossRef]
- Partecke, L.I.; Speerforck, S.; Kading, A.; Seubert, F.; Kuhn, S.; Lorenz, E.; Schwandke, S.; Sendler, M.; Kessler, W.; Trung, D.N.; et al. Chronic Stress Increases Experimental Pancreatic Cancer Growth, Reduces Survival and Can Be Antagonised by Beta-Adrenergic Receptor Blockade. Pancreatology 2016, 16, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Chen, S.; Zeng, Q.; Zhao, Y.; Luo, F. Tumor-Associated Macrophage or Chemokine Ligand Ccl17 Positively Regulates the Tumorigenesis of Hepatocellular Carcinoma. Med. Oncol. 2016, 33, 17. [Google Scholar] [CrossRef]
- Maolake, A.; Izumi, K.; Shigehara, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Takezawa, Y.; Machioka, K.; Narimoto, K.; Namiki, M.; et al. Tumor-Associated Macrophages Promote Prostate Cancer Migration through Activation of the Ccl22-Ccr4 Axis. Oncotarget 2016, 8, 9739–9751. [Google Scholar] [CrossRef]
- Cheng, X.; Wu, H.; Jin, Z.J.; Ma, D.; Yuen, S.; Jing, X.Q.; Shi, M.M.; Shen, B.Y.; Peng, C.H.; Zhao, R.; et al. Up-Regulation of Chemokine Receptor Ccr4 Is Associated with Human Hepatocellular Carcinoma Malignant Behavior. Sci. Rep. 2017, 7, 12362. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, D.; Zhang, L.; Zhang, J.; Xiao, Y.; Wu, Q.; Wang, Y.; Zhan, Q. Tumor-Associated Macrophage (Tam)-Derived Ccl22 Induces Fak Addiction in Esophageal Squamous Cell Carcinoma (Escc). Cell. Mol. Immunol. 2022, 19, 1054–1066. [Google Scholar] [CrossRef]
- Budczies, J.; Kirchner, M.; Kluck, K.; Kazdal, D.; Glade, J.; Allgauer, M.; Kriegsmann, M.; Heussel, C.P.; Herth, F.J.; Winter, H.; et al. Deciphering the Immunosuppressive Tumor Microenvironment in Alk- and Egfr-Positive Lung Adenocarcinoma. Cancer Immunol. Immunother. 2021, 71, 251–265. [Google Scholar] [CrossRef]
- Berlato, C.; Khan, M.N.; Schioppa, T.; Thompson, R.; Maniati, E.; Montfort, A.; Jangani, M.; Canosa, M.; Kulbe, H.; Hagemann, U.B.; et al. A Ccr4 Antagonist Reverses the Tumor-Promoting Microenvironment of Renal Cancer. J. Clin. Investig. 2017, 127, 801–813. [Google Scholar] [CrossRef]
- Gautam, S.K.; Basu, S.; Aithal, A.; Dwivedi, N.V.; Gulati, M.; Jain, M. Regulation of Pancreatic Cancer Therapy Resistance by Chemokines. Semin. Cancer Biol. 2022, 86, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, F.R.; Goulart, M.R.; Fincham, R.E.A.; Bombadieri, M.; Kocher, H.M. B Cells in Pancreatic Cancer Stroma. World J. Gastroenterol. 2022, 28, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, F.R.; Fincham, R.E.A.; Spear, S.; Clear, A.; Roy-Luzarraga, M.; Balkwill, F.R.; Gribben, J.G.; Bombardieri, M.; Hodivala-Dilke, K.; Capasso, M.; et al. Pancreatic Cancer Chemotherapy Is Potentiated by Induction of Tertiary Lymphoid Structures in Mice. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1543–1565. [Google Scholar] [CrossRef]
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells in Cancer. Annu. Rev. Cancer Biol. 2020, 4, 459–477. [Google Scholar] [CrossRef]
- Oleinika, K.; Nibbs, R.J.; Graham, G.J.; Fraser, A.R. Suppression, Subversion and Escape: The Role of Regulatory T Cells in Cancer Progression. Clin. Exp. Immunol. 2013, 171, 36–45. [Google Scholar] [CrossRef]
- Mota Reyes, C.; Demir, E.; Cifcibasi, K.; Istvanffy, R.; Friess, H.; Demir, I.E. Regulatory T Cells in Pancreatic Cancer: Of Mice and Men. Cancers 2022, 14, 4582. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Targeting Treg Cells in Cancer Immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Peterson, E.; Sun, J.; Goudie, C.; Drapkin, R.I.; Liu, J.F.; Matulonis, U.; Zhu, Q.; Marasco, W.A. Anti-Ccr4 Monoclonal Antibody Enhances Antitumor Immunity by Modulating Tumor-Infiltrating Tregs in an Ovarian Cancer Xenograft Humanized Mouse Model. Oncoimmunology 2015, 5, e1090075. [Google Scholar] [CrossRef]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-Cc Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef]
- Shevchenko, I.; Karakhanova, S.; Soltek, S.; Link, J.; Bayry, J.; Werner, J.; Umansky, V.; Bazhin, A.V. Low-Dose Gemcitabine Depletes Regulatory T Cells and Improves Survival in the Orthotopic Panc02 Model of Pancreatic Cancer. Int. J. Cancer 2012, 133, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Hamid, O.; Nayak-Kapoor, A.; Sahebjam, S.; Sznol, M.; Collaku, A.; Fox, F.E.; Marshall, M.A.; Hong, D.S. Mogamulizumab in Combination with Durvalumab or Tremelimumab in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. 2020, 26, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Fong, L. Cytotoxic Cd4(+) T Cells in Cancer: Expanding the Immune Effector Toolbox. Immunity 2021, 54, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic Cd8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Shen, B.; Fang, Y.; Deng, X.; Chen, H.; Jin, J.; Peng, C.; Li, H. Drug-Eluting Scaffold Inhibited in Vivo Pancreatic Tumorigenesis by Engaging Murine Ccr4(+)Cd8(+) T Cells. Colloids Surfaces B Biointerfaces 2017, 158, 469–473. [Google Scholar] [CrossRef]
- Rapp, M.; Grassmann, S.; Chaloupka, M.; Layritz, P.; Kruger, S.; Ormanns, S.; Rataj, F.; Janssen, K.P.; Endres, S.; Anz, D.; et al. C-C Chemokine Receptor Type-4 Transduction of T Cells Enhances Interaction with Dendritic Cells, Tumor Infiltration and Therapeutic Efficacy of Adoptive T Cell Transfer. Oncoimmunology 2015, 5, e1105428. [Google Scholar] [CrossRef]
- Pawluczuk, E.; Lukaszewicz-Zajac, M.; Mroczko, B. The Role of Chemokines in the Development of Gastric Cancer—Diagnostic and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 8456. [Google Scholar] [CrossRef]
- Ling, Z.; Li, W.; Hu, J.; Li, Y.; Deng, M.; Zhang, S.; Ren, X.; Wu, T.; Xia, J.; Cheng, B.; et al. Targeting Ccl2-Ccr4 Axis Suppress Cell Migration of Head and Neck Squamous Cell Carcinoma. Cell Death Dis. 2022, 13, 158. [Google Scholar] [CrossRef]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of Cancer Immunity and the Cancer-Immune Set Point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Burns, W.R.; Frankel, T.L.; Cho, C.S.; Nathan, H. Validation of the American Joint Commission on Cancer (Ajcc) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: A Surveillance, Epidemiology and End Results (Seer) Analysis. Ann. Surg. Oncol. 2017, 24, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Zegadlo, A.; Zabicka, M.; Kania-Pudlo, M.; Maliborski, A.; Rozyk, A.; Sosnicki, W. Assessment of Solitary Pulmonary Nodules Based on Virtual Monochrome Images and Iodine-Dependent Images Using a Single-Source Dual-Energy Ct with Fast Kvp Switching. J. Clin. Med. 2020, 9, 2514. [Google Scholar] [CrossRef]
- Bhanumathy, K.K.; Balagopal, A.; Vizeacoumar, F.S.; Vizeacoumar, F.J.; Freywald, A.; Giambra, V. Protein Tyrosine Kinases: Their Roles and Their Targeting in Leukemia. Cancers 2021, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T Helper Type 2 Cell Infiltrate Correlates with Cancer-Associated Fibroblast Thymic Stromal Lymphopoietin Production and Reduced Survival in Pancreatic Cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Pamer, E.G. Monocyte Recruitment During Infection and Inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory Monocyte Mobilization Decreases Patient Survival in Pancreatic Cancer: A Role for Targeting the Ccl2/Ccr2 Axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [CrossRef]
- Mukaida, N.; Sasaki, S.I.; Baba, T. Ccl4 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 23–32. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. Ccr5/Ccl5 Axis Interaction Promotes Migratory and Invasiveness of Pancreatic Cancer Cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic Cells, Monocytes and Macrophages: A Unified Nomenclature Based on Ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13 . [Google Scholar] [CrossRef]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune Checkpoint Therapy-Current Perspectives and Future Directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef] [PubMed]
- Mucileanu, A.; Chira, R.; Mircea, P.A. Pd-1/Pd-L1 Expression in Pancreatic Cancer and Its Implication in Novel Therapies. Med. Pharm. Rep. 2021, 94, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.J.; Jaffee, E.M.; Zheng, L. The Tumour Microenvironment in Pancreatic Cancer—Clinical Challenges and Opportunities. Nat. Rev. Clin. Oncol. 2020, 17, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.J.; Blaydorn, L.; Beck, J.; Bornemann-Kolatzki, K.; Urnovitz, H.; Schutz, E.; Khemka, V. Phase Ib/Ii Study of Gemcitabine, Nab-Paclitaxel, and Pembrolizumab in Metastatic Pancreatic Adenocarcinoma. Investig. New Drugs 2017, 36, 96–102. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic Developments in Pancreatic Cancer: Current and Future Perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J., 3rd; Weekes, C.; et al. Results from a Phase Iib, Randomized, Multicenter Study of Gvax Pancreas and Crs-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (Eclipse Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Lohr, J.M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.L.; Heinemann, V. Addressing the Challenges of Pancreatic Cancer: Future Directions for Improving Outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef]
- Beatty, G.L.; Winograd, R.; Evans, R.A.; Long, K.B.; Luque, S.L.; Lee, J.W.; Clendenin, C.; Gladney, W.L.; Knoblock, D.M.; Guirnalda, P.D.; et al. Exclusion of T Cells from Pancreatic Carcinomas in Mice Is Regulated by Ly6c(Low) F4/80(+) Extratumoral Macrophages. Gastroenterology 2015, 149, 201–210. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Nathanson, K.L. Immunotherapy at Large: The Road to Personalized Cancer Vaccines. Nat. Med. 2013, 19, 1098–1100. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabipov, A.; Trung, D.N.; van der Linde, J.; Miebach, L.; Lenz, M.; Erne, F.; von Bernstorff, W.; Schulze, T.; Kersting, S.; Bekeschus, S.; et al. CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice. Biomedicines 2023, 11, 1517. https://doi.org/10.3390/biomedicines11061517
Khabipov A, Trung DN, van der Linde J, Miebach L, Lenz M, Erne F, von Bernstorff W, Schulze T, Kersting S, Bekeschus S, et al. CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice. Biomedicines. 2023; 11(6):1517. https://doi.org/10.3390/biomedicines11061517
Chicago/Turabian StyleKhabipov, Aydar, Dung Nguyen Trung, Julia van der Linde, Lea Miebach, Maik Lenz, Felix Erne, Wolfram von Bernstorff, Tobias Schulze, Stephan Kersting, Sander Bekeschus, and et al. 2023. "CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice" Biomedicines 11, no. 6: 1517. https://doi.org/10.3390/biomedicines11061517
APA StyleKhabipov, A., Trung, D. N., van der Linde, J., Miebach, L., Lenz, M., Erne, F., von Bernstorff, W., Schulze, T., Kersting, S., Bekeschus, S., & Partecke, L. I. (2023). CCR4 Blockade Diminishes Intratumoral Macrophage Recruitment and Augments Survival of Syngeneic Pancreatic Cancer-Bearing Mice. Biomedicines, 11(6), 1517. https://doi.org/10.3390/biomedicines11061517






