Establishment of a Rodent Glioblastoma Partial Resection Model for Chemotherapy by Local Drug Carriers—Sharing Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Animal Care
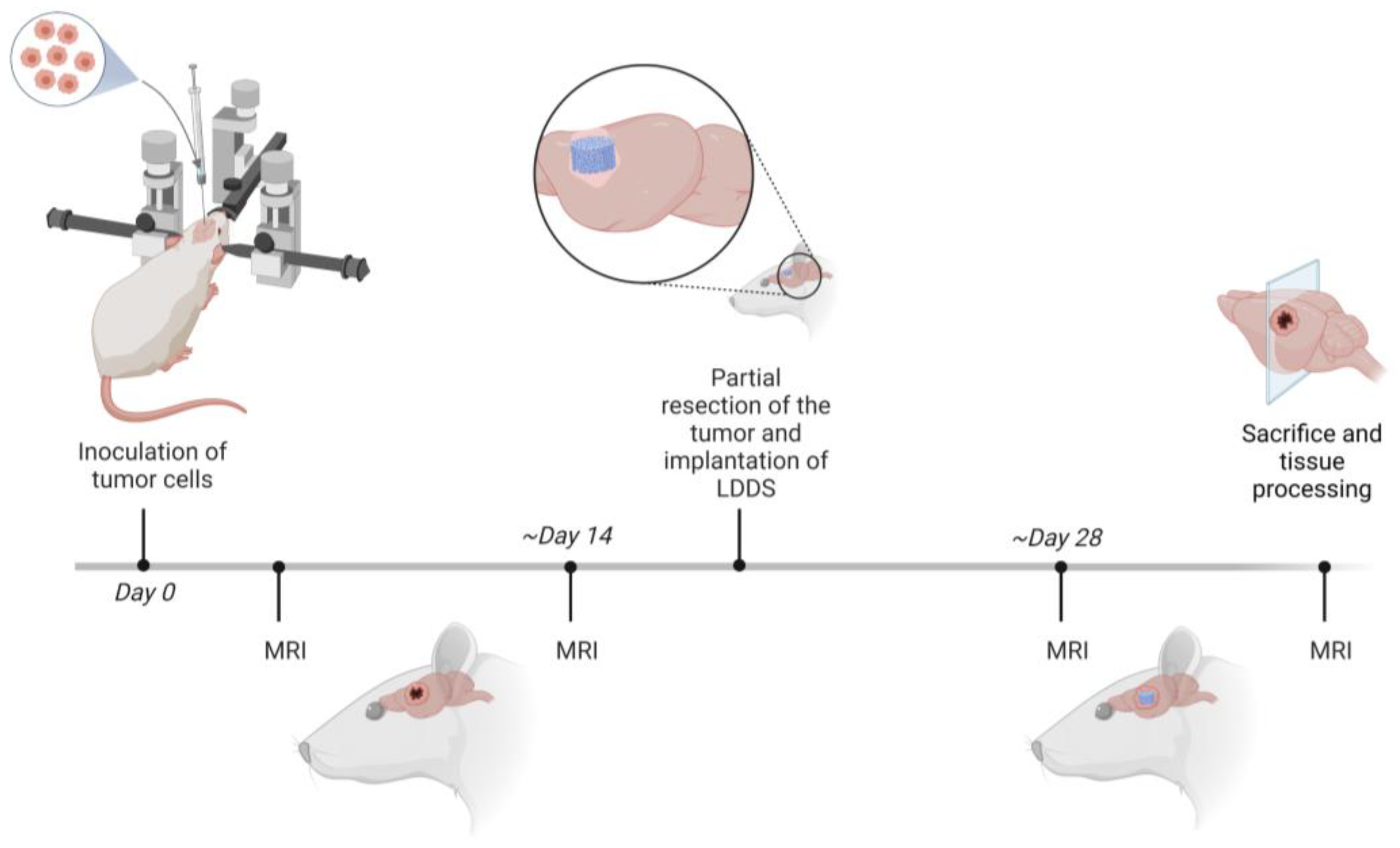
- 1A Establishment of the model (n = 20). Aim: Technical practice, determination of optimal cell amount for inoculation; no survival after partial tumor resection and implantation of the LDDS; one animal of this group was used for the Fluorescence Molecular Tomography (FMT) experiment.
- 1B Examination of the tumor progression after partial resection (n = 12). Aim: Observing the clinical outcome after partial resection and implantation of the LDDS as well as monitoring the tumor progression around the implant.
- 1C Establishment in athymic rats (n = 8; including nU87 = 6; nGSCs (glioma stem-like cells) = 2). Aim: Proving the feasibility in athymic rats using human GBM cells.
2.2. Cell Culture
2.3. Surgical Procedures
2.4. MRI Measurements
2.5. Release Measurements of an Exemplary LDDS In Vitro
2.6. Release Measurements of an Exemplary LDDS In Vivo
2.7. Statistical Analysis
3. Results
3.1. Selection of a Suitable Animal Model and Cell Lines
3.2. Tumor Cells
3.2.1. Creation of Standardized Conditions in the Context of Cell Culture
3.2.2. Standardized Inoculation Procedure
3.3. Laboratory Animals
3.3.1. Creation of Standardized Conditions for Laboratory Animals
3.3.2. GBM Partial Resection and Perioperative Management
3.4. Testing the Release of Local Drug Delivery Systems
3.4.1. Release of an Exemplary Local Drug Delivery System In Vitro
3.4.2. Release of an Exemplary Local Drug Delivery System In Vivo
3.5. Monitoring of the Animal Experiment Using MRI
3.6. Further Tissue Processing
4. Discussion
4.1. Selection of Suitable Animal Models and Cell Lines
4.2. Tumor Growth Behavior
4.3. GBM Partial Resection and Perioperative Management
4.4. Using of Imaging Techniques
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Birzu, C.; French, P.; Caccese, M.; Cerretti, G.; Idbaih, A.; Zagonel, V.; Lombardi, G. Recurrent Glioblastoma: From Molecular Landscape to New Treatment Perspectives. Cancers 2020, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef]
- Hauck, M.; Hellmold, D.; Kubelt, C.; Synowitz, M.; Adelung, R.; Schütt, F.; Held-Feindt, J. Localized Drug Delivery Systems in High-Grade Glioma Therapy—From Construction to Application. Adv. Ther. 2022, 5, 2200013. [Google Scholar] [CrossRef]
- Ceña, V.; Játiva, P. Nanoparticle crossing of blood–brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Wait, S.D.; Prabhu, R.S.; Burri, S.H.; Atkins, T.G.; Asher, A.L. Polymeric drug delivery for the treatment of glioblastoma. Neuro-Oncology 2015, 17 (Suppl. S2), ii9–ii23. [Google Scholar] [CrossRef]
- Attenello, F.J.; Mukherjee, D.; Datoo, G.; McGirt, M.J.; Bohan, E.; Weingart, J.D.; Olivi, A.; Quinones-Hinojosa, A.; Brem, H. Use of Gliadel (BCNU) Wafer in the Surgical Treatment of Malignant Glioma: A 10-Year Institutional Experience. Ann. Surg. Oncol. 2008, 15, 2887–2893. [Google Scholar] [CrossRef]
- McGovern, P.C.; Lautenbach, E.; Brennan, P.J.; Lustig, R.A.; Fishman, N.O. Risk Factors for Postcraniotomy Surgical Site Infection after 1,3-Bis (2-Chloroethyl)-1-Nitrosourea (Gliadel) Wafer Placement. Clin. Infect. Dis. 2003, 36, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.-K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205. [Google Scholar] [CrossRef]
- Flüh, C.; Chitadze, G.; Adamski, V.; Hattermann, K.; Synowitz, M.; Kabelitz, D.; Held-Feindt, J. NKG2D ligands in glioma stem-like cells: Expression in situ and in vitro. Histochem. Cell Biol. 2018, 149, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Hauck, M.; Dittmann, J.; Zeller-Plumhoff, B.; Madurawala, R.; Hellmold, D.; Kubelt, C.; Synowitz, M.; Held-Feindt, J.; Adelung, R.; Wulfinghoff, S.; et al. Fabrication and Modelling of a Reservoir-Based Drug Delivery System for Customizable Release. Pharmaceutics 2022, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Rasch, F.; Schmitt, C.; Saure, L.M.; Meyer, R.; Adamski, V.; Dengiz, D.; Scherließ, R.; Lucius, R.; Synowitz, M.; Mishra, Y.K. Macroscopic Silicone Microchannel Matrix for Tailored Drug Release and Localized Glioblastoma Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3388–3397. [Google Scholar] [CrossRef]
- Cohrs, G.; Drucks, B.; Synowitz, M.; Held-Feindt, J.; Knerlich-Lukoschus, F. Expression Patterns of Hypoxia-Inducible Factors, Proinflammatory, and Neuroprotective Cytokines in Neuroepithelial Tissues of Lumbar Spinal Lipomas–A Pilot Study. World Neurosurg. 2020, 141, e633–e644. [Google Scholar] [CrossRef]
- Westphal, M.; Ram, Z.; Riddle, V.; Hilt, D.; Bortey, E.; On behalf of the Executive Committee of the Gliadel® Study Group. Gliadel® wafer in initial surgery for malignant glioma: Long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006, 148, 269–275. [Google Scholar] [CrossRef]
- Corroyer-Dulmont, A.; Pérès, E.A.; Petit, E.; Guillamo, J.-S.; Varoqueaux, N.; Roussel, S.; Toutain, J.; Divoux, D.; MacKenzie, E.T.; Delemare, J.; et al. Detection of glioblastoma response to temozolomide combined with bevacizumab based on µMRI and µPET imaging reveals [18F]-fluoro-l-thymidine as an early and robust predictive marker for treatment efficacy. Neuro-Oncology 2013, 15, 41–56. [Google Scholar] [CrossRef]
- Bampoe, J.; Glen, J.; Mackenzie, I.; Porter, P.; Bernstein, M. Effect of implant dose/volume and surgical resection on survival in a rat glioma brachytherapy model: Implications for brain tumor therapy. Neurosurgery 1997, 41, 1374–1383. [Google Scholar] [CrossRef]
- Grobben, B.; De Deyn, P.; Slegers, H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002, 310, 257–270. [Google Scholar] [CrossRef]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- San-Galli, F.; Vrignaud, P.; Robert, J.; Coindre, J.M.; Cohadon, F. Assessment of the experimental model of transplanted C6 glioblastoma in wistar rats. J. Neurooncol. 1989, 7, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Huszthy, P.C.; Daphu, I.; Niclou, S.P.; Stieber, D.; Nigro, J.M.; Sakariassen, P.Ø.; Miletic, H.; Thorsen, F.; Bjerkvig, R. In vivo models of primary brain tumors: Pitfalls and perspectives. Neuro-Oncology 2012, 14, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Günther, H.S.; Schmidt, N.O.; Phillips, H.S.; Kemming, D.; Kharbanda, S.; Soriano, R.; Modrusan, Z.; Meissner, H.; Westphal, M.; Lamszus, K. Glioblastoma-derived stem cell-enriched cultures form distinct subgroups according to molecular and phenotypic criteria. Oncogene 2008, 27, 2897–2909. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Candolfi, M.; Curtin, J.F.; Nichols, W.S.; Muhammad, A.G.; King, G.D.; Pluhar, G.E.; McNiel, E.A.; Ohlfest, J.R.; Freese, A.B.; Moore, P.F. Intracranial glioblastoma models in preclinical neuro-oncology: Neuropathological characterization and tumor progression. J. Neurooncol. 2007, 85, 133–148. [Google Scholar] [CrossRef]
- Gómez-Oliva, R.; Domínguez-García, S.; Carrascal, L.; Abalos-Martínez, J.; Pardillo-Díaz, R.; Verástegui, C.; Castro, C.; Nunez-Abades, P.; Geribaldi-Doldán, N. Evolution of Experimental Models in the Study of Glioblastoma: Toward Finding Efficient Treatments. Front. Oncol. 2021, 10, 614295. [Google Scholar] [CrossRef]
- Colston, M.J.; Fieldsteel, A.H.; Dawson, P.J. Growth and regression of human tumor cell lines in congenitally athymic (rnu/rnu) rats. J. Natl. Cancer Inst. 1981, 66, 843–848. [Google Scholar]
- Maruo, K.; Ueyama, Y.; Kuwahara, Y.; Hioki, K.; Saito, M.; Nomura, T.; Tamaoki, N. Human tumour xenografts in athymic rats and their age dependence. Br. J. Cancer 1982, 45, 786–789. [Google Scholar] [CrossRef]
- Lohr, J.; Ratliff, T.; Huppertz, A.; Ge, Y.; Dictus, C.; Ahmadi, R.; Grau, S.; Hiraoka, N.; Eckstein, V.; Ecker, R.C. Effector T-Cell Infiltration Positively Impacts Survival of Glioblastoma Patients and Is Impaired by Tumor-Derived TGF-β. Clin. Cancer Res. 2011, 17, 4296–4308. [Google Scholar] [CrossRef]
- Gieryng, A.; Pszczolkowska, D.; Bocian, K.; Dabrowski, M.; Rajan, W.D.; Kloss, M.; Mieczkowski, J.; Kaminska, B. Immune microenvironment of experimental rat C6 gliomas resembles human glioblastomas. Sci. Rep. 2017, 7, 17556. [Google Scholar] [CrossRef] [PubMed]
- Knauth, M.; Egelhof, T.; Roth, S.U.; Wirtz, C.R.; Sartor, K. Monocrystalline iron oxide nanoparticles: Possible solution to the problem of surgically induced intracranial contrast enhancement in intraoperative MR imaging. AJNR Am. J. Neuroradiol. 2001, 22, 99–102. [Google Scholar] [PubMed]
- Moonshi, S.S.; Bejot, R.; Atcha, Z.; Vijayaragavan, V.; Bhakoo, K.K.; Goggi, J.L. A comparison of PET imaging agents for the assessment of therapy efficacy in a rodent model of glioma. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 397–407. [Google Scholar] [PubMed]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Combination Therapy of Surgical Tumor Resection with Implantation of a Hydrogel Containing Camptothecin-Loaded Poly(lactic-co-glycolic acid) Microspheres in a C6 Rat Glioma Model. Biol. Pharm. Bull. 2012, 35, 545–550. [Google Scholar] [CrossRef]
- Denbo, J.W.; Williams, R.F.; Orr, W.S.; Sims, T.L.; Ng, C.Y.; Zhou, J.; Spence, Y.; Morton, C.L.; Nathwani, A.C.; Duntsch, C.; et al. Continuous local delivery of interferon-β stabilizes tumor vasculature in an orthotopic glioblastoma xenograft resection model. Surgery 2011, 150, 497–504. [Google Scholar] [CrossRef]
- Emerich, D.F.; Winn, S.R.; Bartus, R.T. Injection of chemotherapeutic microspheres and glioma. IV: Eradicating tumors in rats. Cell Transplant. 2002, 11, 47–54. [Google Scholar] [CrossRef]
- Bianco, J.; Bastiancich, C.; Joudiou, N.; Gallez, B.; des Rieux, A.; Danhier, F. Novel model of orthotopic U-87 MG glioblastoma resection in athymic nude mice. J. Neurosci. Method. 2017, 284, 96–102. [Google Scholar] [CrossRef]
- Bastiancich, C.; Lemaire, L.; Bianco, J.; Franconi, F.; Danhier, F.; Préat, V.; Bastiat, G.; Lagarce, F. Evaluation of lauroyl-gemcitabine-loaded hydrogel efficacy in glioblastoma rat models. Nanomedicine 2018, 13, 1999–2013. [Google Scholar] [CrossRef]
- Song, X.; Shu, X.-H.; Wu, M.-L.; Zheng, X.; Jia, B.; Kong, Q.-Y.; Liu, J.; Li, H. Postoperative resveratrol administration improves prognosis of rat orthotopic glioblastomas. BMC Cancer 2018, 18, 871. [Google Scholar] [CrossRef]
- Geisler, S.; Stegmayr, C.; Niemitz, N.; Lohmann, P.; Rapp, M.; Stoffels, G.; Willuweit, A.; Galldiks, N.; Filss, C.; Sabel, M.C. Treatment-Related Uptake of O-(2-18F-Fluoroethyl)-l-Tyrosine and l-[Methyl-3H]-Methionine After Tumor Resection in Rat Glioma Models. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 1373–1379. [Google Scholar] [CrossRef]
- Tamargo, R.J.; Epstein, J.I.; Reinhard, C.S.; Chasin, M.; Brem, H. Brain biocompatibility of a biodegradable, controlled-release polymer in rats. J. Biomed. Mater. Res. 1989, 23, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Leiss, L.; Yang, N.; Rygih, C.B.; Mitra, S.S.; Cheshier, S.H.; Weissman, I.L.; Huang, B.; Miletic, H.; Bjerkvig, R.; et al. Surgical debulking promotes recruitment of macrophages and triggers glioblastoma phagocytosis in combination with CD47 blocking immunotherapy. Oncotarget 2017, 8, 12145–12157. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.J.; Jarzabek, M.A.; Dicker, P.; O’Brien, D.F.; Callanan, J.J.; Byrne, A.T.; Prehn, J.H.M. Validation of an imageable surgical resection animal model of Glioblastoma (GBM). J. Neurosci. Methods 2014, 233, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Majós, C.; Cos, M.; Castañer, S.; Gil, M.; Plans, G.; Lucas, A.; Bruna, J.; Aguilera, C. Early post-operative magnetic resonance imaging in glioblastoma: Correlation among radiological findings and overall survival in 60 patients. Eur. Radiol. 2016, 26, 1048–1055. [Google Scholar] [CrossRef]
- Ferrari, A.; Peters, J.; Anikeeva, M.; Pravdivtsev, A.; Ellermann, F.; Them, K.; Will, O.; Peschke, E.; Yoshihara, H.; Jansen, O.; et al. Performance and reproducibility of 13C and 15N hyperpolarization using a cryogen-free DNP polarizer. Sci. Rep. 2022, 12, 11694. [Google Scholar] [CrossRef]







| Cell Line | Cell Count (PBS) | Cell Count (Ringer’s Solution) | Difference in Cell Count PBS/Ringer’s Solution [n-Fold] |
|---|---|---|---|
| C6 | 59,300 ± 1900 | 55,850 ± 3650 | 1.07 ± 0.08 |
| U87MG | 38,800 ± 2600 | 41,150 ± 1350 | 0.94 ± 0.07 |
| Patient-derived GSCs | 93,800 ± 1600 | 96,750 ± 1750 | 0.97 ± 0.02 * |
| Cell Line | Cell Count 26sG Hamilton Needle (Outer Diameter 0.474 mm, Inner Diameter 0.127 mm) | Cell Count 26G Hamilton Needle (Outer Diameter 0.464 mm, Inner Diameter 0.26 mm) | Difference in Cell Count 26sG/26G Hamilton Needle [n-Fold] |
|---|---|---|---|
| C6 | 61,117 ± 6447 | 92,175 ± 4007 | 1.52 ± 0.17 *** |
| U87MG | 39,975 ± 2750 | 83,367 ± 3691 | 2.09 ± 0.17 *** |
| Patient-derived GSCs | 45,467 ± 13,943 | 95,417 ± 6947 | 2.32 ± 0.73 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubelt, C.; Hellmold, D.; Peschke, E.; Hauck, M.; Will, O.; Schütt, F.; Lucius, R.; Adelung, R.; Scherließ, R.; Hövener, J.-B.; et al. Establishment of a Rodent Glioblastoma Partial Resection Model for Chemotherapy by Local Drug Carriers—Sharing Experience. Biomedicines 2023, 11, 1518. https://doi.org/10.3390/biomedicines11061518
Kubelt C, Hellmold D, Peschke E, Hauck M, Will O, Schütt F, Lucius R, Adelung R, Scherließ R, Hövener J-B, et al. Establishment of a Rodent Glioblastoma Partial Resection Model for Chemotherapy by Local Drug Carriers—Sharing Experience. Biomedicines. 2023; 11(6):1518. https://doi.org/10.3390/biomedicines11061518
Chicago/Turabian StyleKubelt, Carolin, Dana Hellmold, Eva Peschke, Margarethe Hauck, Olga Will, Fabian Schütt, Ralph Lucius, Rainer Adelung, Regina Scherließ, Jan-Bernd Hövener, and et al. 2023. "Establishment of a Rodent Glioblastoma Partial Resection Model for Chemotherapy by Local Drug Carriers—Sharing Experience" Biomedicines 11, no. 6: 1518. https://doi.org/10.3390/biomedicines11061518





