Physiological Activity of Trace Element Germanium including Anticancer Properties
Abstract
:1. Introduction
2. Historical Digression and Toxicity of Germanium Compounds
3. Organic Germanium Compounds
3.1. Germanium Sesquioxides
3.2. Germatranes, Germocanes
3.3. Other Germanium Compounds
4. Inorganic and Coordination Germanium Compounds
5. A Possible Mechanism of Anticancer Action of Germanium Compounds
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukevics, E.; Ignatovich, L. Biological activity of organogermanium compounds. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Rappoport, Z., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, pp. 1653–1683. [Google Scholar] [CrossRef]
- World Health Organization. WHO Drug Information 2014. WHO Drug Inf. 2014, 28, 125–210. [Google Scholar]
- Kadomtseva, A.V.; Mochalov, G.M.; Kuzina, O.V. Biologically Active Coordination Compounds of Germanium. Synthesis and Physicochemical Properties. Russ. J. Org. Chem. 2021, 57, 879–888. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Teng, G.-Q.; Zhou, H.; Dong, C.-L. Germanium Reduces Inflammatory Damage in Mammary Glands During Lipopolysaccharide-Induced Mastitis in Mice. Biol. Trace Elem. Res. 2020, 198, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in effect of germanium or germanium compounds on animals—A review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Parish, W.W.; Andrus, G.M.; Simpson, J.S.A.; Field, C.J. Germane Facts About Germanium Sesquioxide: I. Chemistry and Anticancer Properties. J. Altern. Complement. Med. 2004, 10, 337–344. [Google Scholar] [CrossRef]
- Lukevics, E.; Gar, T.K.; Ignatovich, L.M.; Mironov, V.F. Biological Activity of Germanium Compounds; Zinatne: Riga, Latvia, 1990; p. 191. (In Russian) [Google Scholar]
- Rosenberg, E. Germanium-Containing Compounds, Current Knowledge and Applications. In Encyclopedia of Metalloproteins; Kretsinger, R., Uversky, V., Permyakov, E., Eds.; Springer: New York, NY, USA, 2013; pp. 847–855. [Google Scholar] [CrossRef]
- Ali, M.M. Germanium l-Cysteine Alpha-Tocopherol Complex as Stimulator to Antioxidant Defense System. In Encyclopedia of Metalloproteins; Kretsinger, R., Uversky, V., Permyakov, E., Eds.; Springer: New York, NY, USA, 2013; pp. 836–841. [Google Scholar] [CrossRef]
- Marczynski, B. Carcinogenesis as the result of the deficiency of some essential trace elements. Med. Hypotheses 1988, 26, 239–249. [Google Scholar] [CrossRef]
- Al-Zamely, O.M.Y. New approach for evaluation of Co, Ni, Mo, V, and Ge levels in serum of no Hodgkin lymphoma patients. Int. J. Biotechnol. Biochem. 2011, 7, 39–60. [Google Scholar]
- Kamil, Z.H.; Ewadh, M.J. Determination of serum trace elements and haematological parameters in lymphoma patients receiving chemotherapy. J. Babylon Univ. Pure Appl. Sci. 2016, 24, 2489–2500. [Google Scholar]
- Cheng, X.; Zhou, Y.-C.; Zhou, B.; Huang, Y.-C.; Wang, G.-Z.; Zhou, G.-B. Systematic analysis of concentrations of 52 elements in tumor and counterpart normal tissues of patients with non-small cell lung cancer. Cancer Med. 2019, 8, 7720–7727. [Google Scholar] [CrossRef]
- Schroeder, H.A.; Balassa, J.J. Abnormal trace metals in man: Germanium. J. Chronic Dis. 1967, 20, 211–224. [Google Scholar] [CrossRef]
- Chen, T.J.; Lin, C.H. Germanium: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, NJ, USA, 2011; pp. 927–933. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Niedzielski, P.; Budka, A.; Gąsecka, M.; Kalač, P.; Jasińska, A.; Budzyńska, S.; Kozak, L.; Mleczek, M. Comparison of multielemental composition of Polish and Chinese mushrooms (Ganoderma spp.). Eur. Food Res. Technol. 2017, 243, 1555–1566. [Google Scholar] [CrossRef]
- Wiche, O.; Székely, B.; Moschner, C.; Heilmeier, H. Germanium in the soil-plant system—A review. Environ. Sci. Pollut. Res. 2018, 25, 31938–31956. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Kim, Y.-S.; Jeong, C.-S.; Kim, S.-J.; Zhong, J.-J.; Paek, K.-Y. Production of Ginsenosides from Adventitious Root Cultures of Panax ginseng. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 625–651. [Google Scholar] [CrossRef]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Shin, D.Y.; Lee, J.H. Immune activation of Bio-Germanium in a randomized, double-blind, placebo-controlled clinical trial with 130 human subjects: Therapeutic opportunities from new insights. PLoS ONE 2020, 15, e0240358. [Google Scholar] [CrossRef]
- McMahon, M.; Regan, F.; Hughes, H. The determination of total germanium in real food samples including Chinese herbal remedies using graphite furnace atomic absorption spectroscopy. Food Chem. 2006, 97, 411–417. [Google Scholar] [CrossRef]
- Ohri, L.K.; Vicari, S.M.; Malone, P.M. Germanium Use and Associated Adverse Effects: A Review. J. Pharm. Technol. 1993, 9, 237–241. [Google Scholar] [CrossRef]
- Yajing, Z.; Xuan, G.; Sueichin, H.; Lihua, W. Determination of Germanium in Lucid Ganoderma and Ginseng by GFASS. Chin. J. Mod. Appl. Pharm. 1993, 10, 11–12. [Google Scholar]
- Avula, B.; Wang, Y.-H.; Smillie, T.J.; Duzgoren-Aydin, N.S.; Khan, I.A. Quantitative Determination of Multiple Elements in Botanicals and Dietary Supplements Using ICP-MS. J. Agric. Food Chem. 2010, 58, 8887–8894. [Google Scholar] [CrossRef]
- Rosenberg, E. Germanium: Environmental occurrence, importance and speciation. Rev. Environ. Sci. Biotechnol. 2009, 8, 29–57. [Google Scholar] [CrossRef]
- Yang, L.-L.; Zhang, D.-Q. Direct determination of germanium in botanical samples by graphite furnace atomic absorption spectrometry with palladium–zirconium as chemical modifier. Talanta 2002, 56, 1123–1129. [Google Scholar] [CrossRef]
- Chen, X.-C.; Zhu, Y.-G.; Zhu, L.-A.; Huang, C.; Chen, Y.; Chen, L.-M.; Fang, F.; Zhou, Y.-C.; Zhao, C.-H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Ahuja, A.; Kim, J.H.; Kim, J.-H.; Yi, Y.-S.; Cho, J.Y. Functional role of ginseng-derived compounds in cancer. J. Ginseng Res. 2018, 42, 248–254. [Google Scholar] [CrossRef]
- Najafi, T.F.; Bahri, N.; Tohidinik, H.R.; Feyz, S.; Bloki, F.; Savarkar, S.; Jahanfar, S. Treatment of cancer-related fatigue with ginseng: A systematic review and meta-analysis. J. Herb. Med. 2021, 28, 100440. [Google Scholar] [CrossRef]
- Sadeghian, M.; Rahmani, S.; Zendehdel, M.; Hosseini, S.A.; Zare Javid, A. Ginseng and Cancer-Related Fatigue: A Systematic Review of Clinical Trials. Nutr. Cancer 2021, 73, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Baatar, D.; Hwang, S.G. Anticancer Activities of Ginsenosides, the Main Active Components of Ginseng. Evid.-Based Complement. Altern. Med. 2021, 2021, 8858006. [Google Scholar] [CrossRef] [PubMed]
- Awais, M.; Aizaz, A.; Nazneen, A.; Bhatti, Q.u.A.; Akhtar, M.; Wadood, A.; Rehman, M.A.U. A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments. Prosthesis 2022, 4, 263–316. [Google Scholar] [CrossRef]
- Popov, A.V.; Menchikov, L.G. The Warburg Effect Is a Guide to Multipurpose Cancer Therapy Including Trace Element Delivery. In Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalised Treatment; Coelho, J., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 4, pp. 255–270. [Google Scholar] [CrossRef]
- Menchikov, L.G.; Shestov, A.A.; Popov, A.V. Warburg effect revisited: Embodiment of classical biochemistry and organic chemistry. Current state and prospects. Biochemistry 2023, 88 (Suppl. 1), S1–S20. [Google Scholar] [CrossRef]
- Unakar, N.J.; Tsui, J.; Johnson, M. Effect of pretreatment of germanium-132 on Na(+)-K(+)-ATPase and galactose cataracts. Curr. Eye Res. 1997, 16, 832–837. [Google Scholar] [CrossRef]
- Ishiwara, F. The influence of different types of metals on mouse carcinoma (Uber die Beeinflussung von Mausecarcinom durch verschiedene Metallarten). Ber. Gesamte Physiol. Exp. Pharmakol. 1928, 49, 615. [Google Scholar]
- Menchikov, L.G.; Ignatenko, M.A. Biological Activity of Organogermanium Compounds (A Review). Pharm. Chem. J. 2012, 46, 635–638. [Google Scholar] [CrossRef]
- Thayer, J.S. Germapharmaca: Some recent studies on biologically active organogermanium compounds. Appl. Organomet. Chem. 1987, 1, 227–234. [Google Scholar] [CrossRef]
- Patai, S. (Ed.) The Chemistry of Organic Germanium, Tin and Lead Compounds; John Wiley & Sons, Ltd.: Chichester, UK, 1995; Volume 1. [Google Scholar]
- Rappoport, Z. (Ed.) The Chemistry of Organic Germanium, Tin and Lead Compounds; John Wiley & Sons, Ltd.: Chichester, UK, 2002; Volume 2. [Google Scholar]
- Chase, T.A.; Cupp, M.J.; Tracy, T.S. Germanium. In Dietary Supplements: Toxicology and Clinical Pharmacology; Cupp, M.J., Tracy, T.S., Eds.; Humana Press: Totowa, NJ, USA, 2003; pp. 197–207. [Google Scholar] [CrossRef]
- Satge, J. Some Applications of Germanium and its Derivatives. Main Group Met. Chem. 2004, 27, 301–308. [Google Scholar] [CrossRef]
- Lukevics, E.; Ignatovich, L. Biological Activity of Organogermanium Compounds. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents. The Use of Metals in Medicine; Gielen, M., Tiekink, E.R.T., Eds.; J. Wiley: Chichester, UK, 2005; Volume 15, pp. 279–295. [Google Scholar] [CrossRef]
- Narokha, V.; Nizhenkovska, I.; Kuznetsova, O. Potential of germanium-based compounds in coronavirus infection. Acta Pharm. 2022, 72, 245–258. [Google Scholar] [CrossRef]
- Lee, V.Y. (Ed.) Organogermanium Compounds: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Singh, R.V.; Gupta, P.; Chaudhary, P.; Deshmukh, C.N. Coordination compounds of germanium(IV) formed with soft and hard donor atoms: A look into the past and present work. Main Group Met. Chem. 2005, 28, 93–118. [Google Scholar] [CrossRef]
- Seifullina, I.I.; Martsinko, E.E. Coordination Compounds of Germanium (IV) with Anions of Citric, Tartaric and Xylaric Acids; Odessa Mechnikov National University: Odessa, Ukraine, 2015. (In Russian) [Google Scholar]
- Kaplan, B.J.; Andrus, G.M.; Parish, W.W. Germane facts about germanium sesquioxide: II. Scientific error and misrepresentation. J. Altern. Complement. Med. 2004, 10, 345–348. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide. Biol. Trace Elem. Res. 1991, 29, 267–280. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity in Humans by the Ultratrace Element Germanium. Ren. Fail. 1991, 13, 1–4. [Google Scholar] [CrossRef]
- Tao, S.-H.; Bolger, P.M. Hazard Assessment of Germanium Supplements. Regul. Toxicol. Pharmacol. 1997, 25, 211–219. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, C.; Zhao, D. Successful management of germanium poisoning-induced multiple organ dysfunctions by combined blood purification therapy. Curr. Med. Res. Opin. 2020, 36, 687–691. [Google Scholar] [CrossRef]
- Nakamura, T.; Shimada, Y.; Takeda, T.; Sato, K.; Akiba, M.; Fukaya, H. Organogermanium compound, Ge-132, forms complexes with adrenaline, ATP and other physiological cis-diol compounds. Future Med. Chem. 2015, 7, 1233–1246. [Google Scholar] [CrossRef]
- Kim, E.; Hwang, S.-U.; Yoon, J.D.; Jeung, E.-B.; Lee, E.; Kim, D.Y.; Hyun, S.-H. Carboxyethylgermanium sesquioxide (Ge-132) treatment during in vitro culture protects fertilized porcine embryos against oxidative stress induced apoptosis. J. Reprod. Dev. 2017, 63, 581–590. [Google Scholar] [CrossRef]
- Wada, T.; Hanyu, T.; Nozaki, K.; Kataoka, K.; Kawatani, T.; Asahi, T.; Sawamura, N. Antioxidant Activity of Ge-132, a Synthetic Organic Germanium, on Cultured Mammalian Cells. Biol. Pharm. Bull. 2018, 41, 749–753. [Google Scholar] [CrossRef]
- Gerber, G.B.; Leonard, A. Mutagenicity, carcinogenicity and teratogenicity of germanium compounds. Mutat. Res. Rev. Mutat. Res. 1997, 387, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.S.; Faroon, O.M.; Maples-Reynolds, N.; Fowler, B.A. Germanium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 799–816. [Google Scholar] [CrossRef]
- Keith, L.S.; Maples-Reynolds, N. Germanium. In Handbook on the Toxicology of Metals. Volume II: Specific Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: London, UK; San Diego, CA, USA, 2022; pp. 289–316. [Google Scholar] [CrossRef]
- Reddeman, R.A.; Glávits, R.; Endres, J.R.; Murbach, T.S.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Germanium Sesquioxide (Organic Germanium). J. Toxicol. 2020, 2020, 6275625. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.H.; Macintosh, D.; Allen, J.; McCarthy, J. Germanium, Tin, and Copper. In Patty’s Toxicology; Bingham, E., Cohrssen, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 355–380. [Google Scholar] [CrossRef]
- Banasik, M. Germanium. In Hamilton & Hardy’s Industrial Toxicology; Harbison, R.D., Bourgeois, M.M., Johnson, G.T., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 119–122. [Google Scholar] [CrossRef]
- Matsusaka, T.; Fujii, M.; Nakano, T.; Terai, T.; Kurata, A.; Imaizumi, M.; Abe, H. Germanium-induced nephropathy: Report of two cases and review of the literature. Clin. Nephrol. 1988, 30, 341–345. [Google Scholar]
- Sanai, T.; Okuda, S.; Onoyama, K.; Oochi, N.; Oh, Y.; Kobayashi, K.; Shimamatsu, K.; Fujimi, S.; Fujishima, M. Germanium Dioxide-Induced Nephropathy: A New Type of Renal Disease. Nephron 1990, 54, 53–60. [Google Scholar] [CrossRef]
- Takeuchi, A.; Yoshizawa, N.; Oshima, S.; Kubota, T.; Oshikawa, Y.; Akashi, Y.; Oda, T.; Niwa, H.; Imazeki, N.; Seno, A.; et al. Nephrotoxicity of germanium compounds: Report of a case and review of the literature. Nephron 1992, 60, 436–442. [Google Scholar] [CrossRef]
- Kolesnikov, S.P. Research in Chemistry of Trihalogermans and Germanium Analogues of Dihalocarbenes; N.D. Zelinsky Institute of Organic Chemistry, Academy of Sciencies of USSR: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Kolesnikov, S.P.; Nefedov, O.M. The reaction of trichlorogermane with ketones (in Connection with the Paper by T.K.Gar and V.F.Mironov). J. Gen. Chem. USSR Engl. Transl. 1967, 37, 701. [Google Scholar]
- Nefedov, O.M.; Kolesnikov, S.P.; Perlmutter, B.L. Reactions of Trichlorogermane with Ketones and Alcohols. Angew. Chem. Int. Ed. 1967, 6, 628–629. [Google Scholar] [CrossRef]
- Tomilov, Y.V.; Menchikov, L.G.; Shapiro, E.A.; Gvozdev, V.D.; Shavrin, K.N.; Volchkov, N.V.; Lipkind, M.B.; Egorov, M.P.; Boganov, S.E.; Khabashesku, V.N.; et al. Carbenes, related intermediates, and small-sized cycles: Contribution from Professor Nefedov’s laboratory. Mendeleev Commun. 2021, 31, 750–768. [Google Scholar] [CrossRef]
- Mironov, V.F.; Berliner, E.M.; Gar, T.K. Reactions of trichlorogermane with acrylic acid and its derivatives. J. Gen. Chem. USSR Engl. Transl. 1967, 37, 911–912. [Google Scholar]
- Mironov, V.F.; Berliner, E.M.; Gar, T.K.; Rybakov, E.A. Reactions of Trichlorogermane with Unsaturated Carboxylic Acids. J. Gen. Chem. USSR Engl. Transl. 1968, 38, 2218. [Google Scholar]
- Zhang, C.L.; Li, T.H.; Niu, S.H.; FuWang, R.; Fu, Z.L.; Guo, F.Q.; Yang, M. Synthesis and Evaluation of Novel Organogermanium Sesquioxides As Antitumor Agents. Bioinorg. Chem. Appl. 2009, 2009, 908625. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shimada, Y.; Sato, K. Bioorganic and Medicinal Organogermanium Chemistry. In Organogermanium Compounds: Theory, Experiment, and Applications; Lee, V.Y., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; pp. 839–865. [Google Scholar] [CrossRef]
- Lahans, T. Integrating Conventional and Chinese Medicine in Cancer Care: A Clinical Guide; Elsevier Health Sciences: Philadelphia, PA, USA, 2007; p. 405. [Google Scholar]
- Mainwaring, M.G.; Poor, C.; Zander, D.S.; Harman, E. Complete remission of pulmonary spindle cell carcinoma after treatment with oral germanium sesquioxide. Chest 2000, 117, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Nefedov, O.M.; Kolesnikov, S.P. Trichlorogermane etherates. Bull. Acad. Sci. USSR Div. Chem. Sci. 1963, 12, 1910. [Google Scholar] [CrossRef]
- Shangguan, G.Q.; Zhang, S.G.; Ni, J.Z. Synthesis, structures and antitumor activities of beta-phenol ester propyl germanium sesquioxides. Chin. Chem. Lett. 1995, 6, 945–946. [Google Scholar]
- Doi, Y.; Imai, N.; Suguro, M.; Numano, T.; Furukawa, F. No carcinogenicity of poly-trans-[(2-carboxyethyl) germasesquioxane](Ge-132): 26-week feeding study using rasH2 mice. Fundam. Toxicol. Sci. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Iwadate, K.; Yamaguchi, Y.; Sasaki, M.; Nakatani, M.; Doi, Y.; Imai, N.; Tamano, S.; Nishihori, Y. Carcinogenicity study of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) in F344 rats. Fundam. Toxicol. Sci. 2018, 5, 127–140. [Google Scholar] [CrossRef]
- Ogwapit, S.M. Analysis of Ge-132 and development of a simple oral anticancer formulation. Biosci. Horiz. 2011, 4, 128–139. [Google Scholar] [CrossRef]
- Vinodhini, J.; Kumar, D.S.R.S.; Sudha, S. Cytotoxic Effect of Carboxyethylgermanium Sesquioxide (Ge-132) on MCF-7 Human Breast Cancer Cell Line. Int. J. Curr. Sci. Res. IJCSR 2011, 2, 178–181. [Google Scholar]
- Masuda, T.; Noda, M.; Kogawa, T.; Kitagawa, D.; Hayashi, N.; Jomori, T.; Nakanishi, Y.; Nakayama, K.I.; Ohno, S.; Mimori, K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020, 111, 924–931. [Google Scholar] [CrossRef]
- Masuda, T.; Tsuruda, Y.; Matsumoto, Y.; Uchida, H.; Nakayama, K.I.; Mimori, K. Drug repositioning in cancer: The current situation in Japan. Cancer Sci. 2020, 111, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Vinodhini, J.; Sudha, S. Effect of bis-carboxy ethyl germanium sesquoxide on N-nitroso-N-methylurea-induced rat mammary carcinoma. Asian J. Pharm. Clin. Res. 2013, 6, 242–244. [Google Scholar]
- Ayodele, O.T. Anticancer activities of organogermanium compounds. Int. J. Progress. Sci. Technol. 2016, 3, 23–25. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Tokuji, Y.; Nakamura, T. Nuclear magnetic resonance studies of the interactions between the organic germanium compound Ge-132 and saccharides. Carbohydr. Res. 2015, 407, 10–15. [Google Scholar] [CrossRef]
- Nakamura, T.; Takeda, T.; Tokuji, Y. The Oral Intake of Organic Germanium, Ge-132, Elevates α-Tocopherol Levels in the Plasma and Modulates Hepatic Gene Expression Profiles to Promote Immune Activation in Mice. Int. J. Vitam. Nutr. Res. 2014, 84, 0183–0195. [Google Scholar] [CrossRef]
- Guo, F.; Xu, D.; Lin, Y.; Wang, G.; Wang, F.; Gao, Q.; Wei, Q.; Lei, S. Chemokine CCL2 contributes to BBB disruption via the p38 MAPK signaling pathway following acute intracerebral hemorrhage. FASEB J. 2020, 34, 1872–1884. [Google Scholar] [CrossRef]
- He, S.; Liu, R.; Li, B.; Huang, L.; Fan, W.; Tembachako, C.R.; Zheng, X.; Xiong, X.; Miyata, M.; Xu, B.; et al. Propagermanium, a CCR2 inhibitor, attenuates cerebral ischemia/reperfusion injury through inhibiting inflammatory response induced by microglia. Neurochem. Int. 2019, 125, 99–110. [Google Scholar] [CrossRef]
- Mizuno, N.; Nishibori, E.; Oka, M.; Jomori, T.; Takata, M.; Kumasaka, T. Structural Basis for Polymer Packing and Solvation Properties of the Organogermanium Crystalline Polymer Propagermanium and Its Derivatives. J. Pharm. Sci. 2015, 104, 2482–2488. [Google Scholar] [CrossRef]
- Baidya, S.; Nishimoto, Y.; Sato, S.; Shimada, Y.; Sakurai, N.; Nonaka, H.; Noguchi, K.; Kido, M.; Tadano, S.; Ishikawa, K.; et al. Dual Effect of Organogermanium Compound THGP on RIG-I-Mediated Viral Sensing and Viral Replication during Influenza a Virus Infection. Viruses 2021, 13, 1674. [Google Scholar] [CrossRef]
- Alimbarova, L.M.; Ambrosov, I.V.; Matelo, S.K.; Barinsky, I.F. Antiviral activity of the organic germanium complex with aciclovir against herpes simplex virus (Herpesviridae: Alphaherpesvirinae: Simplexvirus: Human alphaherpesvirus 1/2) in the in vitro and in vivo systems. Probl. Virol. 2021, 66, 368–382. [Google Scholar] [CrossRef]
- Mulder, P.; van den Hoek, A.M.; Kleemann, R. The CCR2 Inhibitor Propagermanium Attenuates Diet-Induced Insulin Resistance, Adipose Tissue Inflammation and Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0169740. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Li, M.; Kim, E.H.; Park, J.S.; Lee, J.C.; Ham, S.W. Antioxidant and Radical Scavenging Activities of Ascorbic Acid Derivatives Conjugated with Organogermanium. Bull. Korean Chem. Soc. 2010, 31, 3513–3514. [Google Scholar] [CrossRef]
- Tezuka, T.; Higashino, A.; Akiba, M.; Nakamura, T. Organogermanium (Ge-132) Suppresses Activities of Stress Enzymes Responsible for Active Oxygen Species in Monkey Liver Preparation. Adv. Enzym. Res. 2017, 5, 13. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, Y.; Kim, D.Y.; Lee, E.; Hyun, S.-H. Antioxidative effect of carboxyethylgermanium sesquioxide (Ge-132) on IVM of porcine oocytes and subsequent embryonic development after parthenogenetic activation and IVF. Theriogenology 2015, 84, 226–236. [Google Scholar] [CrossRef]
- Matsumoto, H.; Iwafuji, H.; Yamane, J.; Takeuchi, R.; Utsunomiya, T.; Fujii, A. Restorative effect of organic germanium compound (Ge-132) on dermal injury. Wound Med. 2016, 15, 6–10. [Google Scholar] [CrossRef]
- Takeda, T.; Doiyama, S.; Azumi, J.; Shimada, Y.; Tokuji, Y.; Yamaguchi, H.; Nagata, K.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium suppresses cell death due to oxidative stress in normal human dermal fibroblasts. Sci. Rep. 2019, 9, 13637. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.-A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S.D. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. [Google Scholar] [CrossRef] [PubMed]
- Azumi, J.; Takeda, T.; Shimada, Y.; Zhuang, T.; Tokuji, Y.; Sakamoto, N.; Aso, H.; Nakamura, T. Organogermanium THGP Induces Differentiation into M1 Macrophages and Suppresses the Proliferation of Melanoma Cells Via Phagocytosis. Int. J. Mol. Sci. 2023, 24, 1885. [Google Scholar] [CrossRef]
- Sekiguchi, F.; Koike, N.; Shimada, Y.; Sugimoto, K.; Masuda, H.; Nakamura, T.; Yamaguchi, H.; Tanabe, G.; Marumoto, S.; Kasanami, Y.; et al. A hydrolysate of poly-trans-[(2-carboxyethyl)germasesquioxane] (Ge-132) suppresses Cav3.2-dependent pain by sequestering exogenous and endogenous sulfide. Redox Biol. 2023, 59, 102579. [Google Scholar] [CrossRef]
- Azumi, J.; Shimada, Y.; Takeda, T.; Aso, H.; Nakamura, T. The Organogermanium Compound 3-(Trihydroxygermyl) Propanoic Acid (THGP) Suppresses Inflammasome Activation Via Complexation with ATP. Int. J. Mol. Sci. 2022, 23, 13364. [Google Scholar] [CrossRef]
- Shimada, Y.; Sato, K.; Takeda, T.; Tokuji, Y. The Organogermanium Compound Ge-132 Interacts with Nucleic Acid Components and Inhibits the Catalysis of Adenosine Substrate by Adenosine Deaminase. Biol. Trace Elem. Res. 2018, 181, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Zhao, Y.; Sui, B.; Shangguan, G. Studies on the interaction of novel organogermanium sesquioxides with DNA. Chem. Res. Chin. Univ. 2015, 31, 31–37. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Sui, B.; Shangguan, G. Synthesis and cytotoxicity of novel organogermanium sesquioxides. J. Jining Med. Univ. 2017, 40, 14. [Google Scholar] [CrossRef]
- Yao, S.; Jiang, J.; Yang, P.; Cai, J.-Y. Synthesis, characterization and antioxidant activity of a novel organogermanium sesquioxide with resveratrol. Bull. Korean Chem. Soc. 2012, 33, 1121–1122. [Google Scholar] [CrossRef]
- Chernyshev, E.A.; Knyazev, S.P.; Kirin, V.N.; Vasilev, I.M.; Alekseev, N.V. Structural features of silatranes and germatranes. Russ. J. Gen. Chem. 2004, 74, 58–65. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Sundius, T.R.; Vrazhnov, D.V.; Kochina, T.A.; Voronkov, M.G. Bonding in germatranyl cation and germatranes. J. Organomet. Chem. 2007, 692, 5697–5700. [Google Scholar] [CrossRef]
- Zabalov, M.; Karlov, S.; Zaitseva, G.; Lemenovskii, D. The molecular and electronic structure features of silatranes, germatranes, and their carbon analogs. Russ. Chem. Bull. 2006, 55, 464–476. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Korlyukov, A.A.; Samokhin, G.S.; Vrazhnov, D.V.; Kochina, T.A. Germatranes and their quasi and hypo analogs with highly electronegative substituent at the Ge atom. Russ. Chem. Bull. 2012, 61, 992–998. [Google Scholar] [CrossRef]
- Karlov, S.S.; Zaitseva, G.S. Germatranes and their Analogs. Synthesis, Structure, and Reactivity. (Review). Chem. Heterocycl. Compd. 2001, 37, 1325–1357. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Xiao, B. Germatranes and carbagermatranes: (hetero)aryl and alkyl coupling partners in Pd-catalyzed cross-coupling reactions. Chem. Commun. 2021, 57, 11764–11775. [Google Scholar] [CrossRef]
- Lukevics, E.; Germane, S.; Zidermane, A.; Dauvarte, A.; Kravchenko, I.M.; Trusule, M.; Mironov, V.F.; Gar, T.K.; Khromova, N.Y. Synthesis, neurotropic and antitumor activity of some germatranes, germasesquioxanes and their organotin analogs. Pharm. Chem. J. 1984, 18, 89–94. [Google Scholar] [CrossRef]
- Kakimoto, N.; Sato, K.; Takada, T.; Akiba, M. Organogermanium compounds: Synthesis, structure, and properties of masked ethoxycarbonylgermanium sesquioxide (GE-132) and related compounds with one triethanolamine component. Heterocycles 1987, 26, 347–353. [Google Scholar] [CrossRef]
- Song, X.; Yang, Z.; Su, G.; Xieqinglan. Synthesis, characterization and anticancer activity of some bis(germylpropionato-di-n-butyltin) oxides. Phosphorus Sulfur Silicon Relat. Elem. 1999, 150–151, 367–374. [Google Scholar] [CrossRef]
- Ye, L.; Ou, X.; Peng, X.; Luo, Y. Synthesis of 3-(2, 8, 9-trioxa-5-aza-1-germatricyclo [3.3.3.0] Undecane-1- yl)-3-(4-hydroxyl-3-methoxyphenyl)-propionic Acid and its Inhibitory Effect on the Cervical Tumor U14 in vitro and in vivo. Med. Chem. 2012, 8, 595–598. [Google Scholar] [CrossRef]
- Zhigacheva, I.V.; Baryshok, V.P.; Rasulov, M.M.; Storozhenko, P.A. 2-(Germatran-1-yloxy)ethylamine as an inhibitor of the total activity of mononuclear alkaline phospholipase A2. Russ. Chem. Bull. 2021, 70, 444–448. [Google Scholar] [CrossRef]
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetic characteristics of mitochondria under stress and action of organogermanium compounds. J. Nat. Sci. Sustain. Technol. 2015, 9, 439. [Google Scholar]
- Binyukov, V.I.; Mil, E.M.; Zhigacheva, I.V.; Generozova, I.P.; Rasulov, M.M. Morphological and bioenergetical characteristics of mitochondria. In Chemical and Biochemical Physics: A Systematic Approach to Experiments, Evaluation, and Modeling; Schiraldi, D., Zaikov, G.E., Eds.; Apple Academic Press: Oakville, ON, Canada, 2016; pp. 259–276. [Google Scholar]
- Zhigacheva, I.V.; Burlakova, E.B. Plant Growth and Development Regulators and their Effect on the Functional State of Mitochondria. In Chemistry and Technology of Plant Substances; Kutchin, A.V., Shishkina, L.N., Weisfeld, L.I., Eds.; Apple Academic Press: New York, NY, USA, 2017; pp. 243–278. [Google Scholar] [CrossRef]
- Shigarova, A.M.; Grabelnych, O.I.; Baryshok, V.P.; Borovskii, G.B. Impossible mechanisms of germatranol influence on the thermal stability of wheat germs. Appl. Biochem. Microbiol. 2016, 52, 429–434. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Lezov, D.V.; Kondratenko, Y.A.; Kochina, T.A. Interaction of simple amino acids (glycine, α-alanine, β-alanine and L-valine) with germatranol hydrate. J. Mol. Struct. 2022, 1253, 132245. [Google Scholar] [CrossRef]
- Baukov, Y.I.; Tandura, S.N. Hypervalent Compounds of Organic Germanium, Tin and Lead Derivatives. In The Chemistry of Organic Germanium, Tin and Lead Compounds; Rappoport, Z., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002; Volume 2, pp. 963–1239. [Google Scholar] [CrossRef]
- Selina, A.; Karlov, S.; Zaitseva, G. Metallocanes of group 14 elements. 1. Derivatives of silicon and germanium. (Review). Chem. Heterocycl. Compd. 2006, 42, 1518–1556. [Google Scholar] [CrossRef]
- Lermontova, E.K.; Churakov, A.V.; Quan, M.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S. Synthesis, structure, and reactivity of germanium-containing derivatives of substituted diethanolamines. Russ. J. Inorg. Chem. 2009, 54, 211–218. [Google Scholar] [CrossRef]
- de Velasco, D.O.-G.; Sánchez-Jiménez, R.; Hernández-Ortega, S.; Toscano, R.A.; García-Montalvo, V. Study on the transannular bond formation and hypercoordination in tin and germanium spirometallocanes. Polyhedron 2010, 29, 2435–2439. [Google Scholar] [CrossRef]
- Shainyan, B.A. Structural and Conformational Aspects in the Chemistry of Heterocycles. Molecules 2020, 25, 3461. [Google Scholar] [CrossRef] [PubMed]
- Lukevics, E.; Ignatovich, L. Comparative study of the biological activity of organosilicon and organogermanium compounds. Appl. Organomet. Chem. 1992, 6, 113–126. [Google Scholar] [CrossRef]
- Dawara, L.; Singh, D.; Singh, R.V. Antimicrobial and pesticidal activity of some organogermanium (IV) complexes synthesized under microwave irradiation. Main Group Met. Chem. 2011, 34, 69–75. [Google Scholar] [CrossRef]
- Dawara, L.; Fahmi, N.; Singh, R.V. Synthesis, characterization, antimicrobial, pesticidal and DNA cleavage activity of germanium (IV) derivatives of 3-(2-methyl-2, 3-dihydro-benzthiazo-2-yl)-chromen-2-one and N′-[1-2-oxo-2H-chrome-3yl-ethylidene]-hydrazinecarbodithionic acid benzyl ester ligands. Main Group Met. Chem. 2011, 34, 139–146. [Google Scholar] [CrossRef]
- Fahmi, N.; Khedar, R.; Singh, R.V. Green synthesis of new Ge(IV) complexes with bio-potent ligands and their antimicrobial, DNA cleavage, and antioxidant activities. Russ. J. Gen. Chem. 2016, 86, 958–964. [Google Scholar] [CrossRef]
- Singh, N.; Watts, S.; Joshi, S.C.; Singh, R.V. Pesticidal and antifertility activities of triorganogermanium(IV) complexes synthesized using a green chemical approach. Appl. Organomet. Chem. 2013, 27, 269–276. [Google Scholar] [CrossRef]
- Mahawar, P.; Wasson, M.K.; Sharma, M.K.; Jha, C.K.; Mukherjee, G.; Vivekanandan, P.; Nagendran, S. A Prelude to Biogermylene Chemistry. Angew. Chem. Int. Ed. 2020, 59, 21377–21381. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Seo, J.-A.; Lim, K.-M.; Ham, S.W. A novel organogermanium protected atopic dermatitis induced by oxazolone. Bioorg. Med. Chem. Lett. 2010, 20, 4032–4034. [Google Scholar] [CrossRef]
- Lim, D.H.; Li, M.; Kim, E.-h.; Ham, S.W. Synthesis of Novel Organogermanium Derivative Conjugated with Vitamin C and Study of its Antioxidant Effects. Bull. Korean Chem. Soc. 2010, 31, 1839–1840. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, S.; Cai, H.-H.; Yang, P.-H.; Cai, J. Synthesis and synergetic effects of chrysin–organogermanium (IV) complex as potential anti-oxidant. Bioorg. Med. Chem. Lett. 2013, 23, 5727–5732. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, H.; Pi, J.; Jiang, J.-H.; Liu, L.; Bai, H.-H.; Yang, P.-H.; Cai, J.-Y. Anti-tumor activity evaluation of novel chrysin–organogermanium(IV) complex in MCF-7 cells. Bioorg. Med. Chem. Lett. 2013, 23, 5544–5551. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gong, L.; Jin, H.; Pi, J.; Bai, H.; Wang, H.; Cai, H.; Yang, P.; Cai, J. Chrysin–organogermanium (IV) complex induced Colo205 cell apoptosis-associated mitochondrial function and anti-angiogenesis. Scanning 2015, 37, 246–257. [Google Scholar] [CrossRef]
- Lu, P.; Yao, S.; Cai, J.; Yang, P.-h. Synthesis and synergetic anti-tumor activity evaluation of dihydroartemisinin-organogermanium(IV) compound. Bioorg. Med. Chem. Lett. 2014, 24, 5294–5297. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 1. Hydrogermylation of conjugated steroid enones. Russ. Chem. Bull. 1998, 47, 180–182. [Google Scholar] [CrossRef]
- Karpenko, R.G.; Kolesnikov, S.P. Germylated steroids. 2. Synthesis of steroid germatranes. Russ. Chem. Bull. 1999, 48, 1185–1186. [Google Scholar] [CrossRef]
- Karpenko, R.G.; Krylova, I.V.; Kamernitskii, A.V. Germylated steroids. 3. Synthesis of trialkylgermylated steroids. Russ. Chem. Bull. 2011, 60, 2100–2102. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Biological Activities of Organometalloid (As, At, B, Ge, Si, Se, Te) Steroids. J. Appl. Pharm. Sci. Vol 2017, 7, 184–202. [Google Scholar] [CrossRef]
- Ignatovich, L.; Starkova, O.; Romanovs, V.; Sleiksha, I.; Shestakova, I.; Popelis, J.; Lukevics, E. Novel trialkylsilyl(germyl)-substituted thienyl- and furylbenzimidazoles and their N-substituted derivatives—Synthesis, structure and cytotoxic activity. Comptes Rendus Chim. 2013, 16, 621–627. [Google Scholar] [CrossRef]
- Ignatovich, L.; Spura, J.; Muravenko, V.; Belyakov, S.; Popelis, J.; Shestakova, I.; Domrachova, I.; Gulbe, A.; Rudevica, Z.; Leonchiks, A. Synthesis, structure and biological activity of new 6,6-dimethyl-2-oxo-4-{2-[5-organylsilyl(germyl)]furan(thiophen)-2-yl}vinyl-5,6-dihydro-2H-pyran-3-carbonitriles. Appl. Organomet. Chem. 2015, 29, 756–763. [Google Scholar] [CrossRef]
- Fedoruk, R.S.; Kovalchuk, I.I.; Mezentseva, L.M.; Tesarivska, U.I.; Pylypets, A.Z.; Kaplunenko, V.H. Germanium compounds and their role in animal body. Anim. Biol. 2022, 24, 51. [Google Scholar] [CrossRef]
- Seifullina, I.I. New page in coordination chemistry of germanium. Odesa Natl. Univ. Herald. Chem. 2003, 8, 8–25. (In Russian) [Google Scholar]
- Korlyukov, A.A. Coordination compounds of tetravalent silicon, germanium and tin: The structure, chemical bonding and intermolecular interactions in them. Russ. Chem. Rev. 2015, 84, 422. [Google Scholar] [CrossRef]
- Grishanov, D.A.; Churakov, A.V.; Medvedev, A.G.; Mikhaylov, A.A.; Lev, O.; Prikhodchenko, P.V. Crystalline Ammonium Peroxogermanate as a Waste-Free, Fully Recyclable Versatile Precursor for Germanium Compounds. Inorg. Chem. 2019, 58, 1905–1911. [Google Scholar] [CrossRef]
- Fedoruk, R.S.; Khrabko, M.I.; Dolaychuk, O.P. Effect of Germanium Citrate on Immunophysiological Activity of the Rats’ Organism. Int. J. Physiol. Pathophysiol. 2018, 9, 17–26. [Google Scholar] [CrossRef]
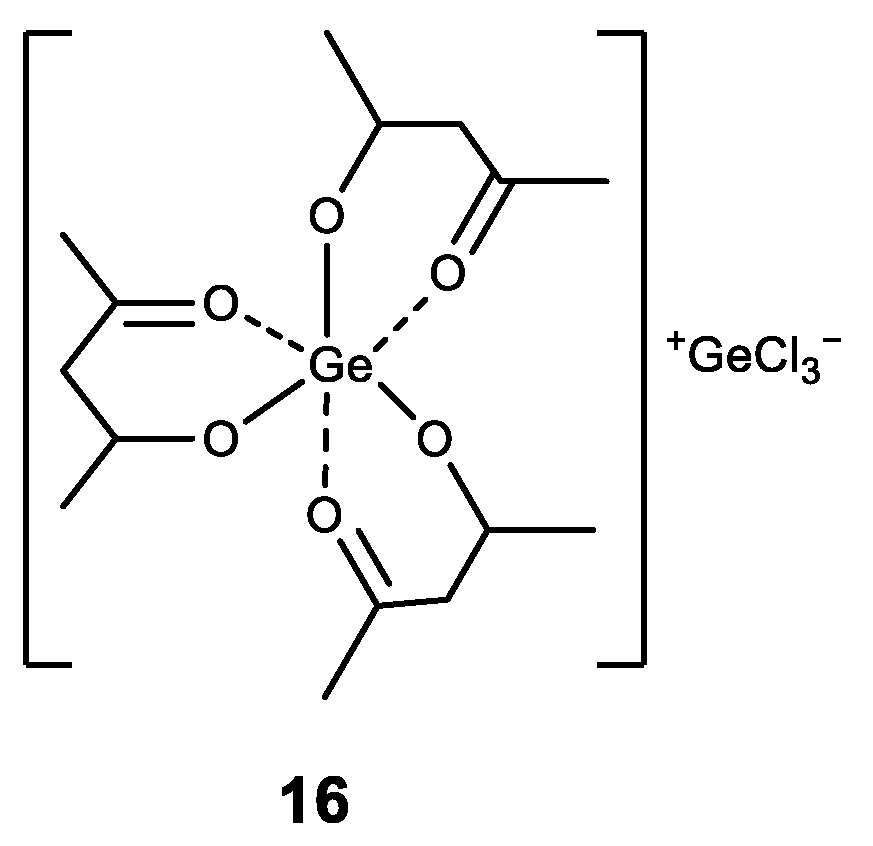
- Mertens, R.T.; Parkin, S.; Awuah, S.G. Exploring six-coordinate germanium(IV)-diketonate complexes as anticancer agents. Inorg. Chim. Acta 2020, 503, 119375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-P.; Xie, W.-L.; Cai, H.-H.; Cai, J.-Y.; Yang, P.-H. Hydroxyl radical scavenging mechanism of human erythrocytes by quercetin–germanium (IV) complex. Eur. J. Pharm. Sci. 2012, 47, 28–34. [Google Scholar] [CrossRef]
- Zhai, G.; Zhu, W.; Duan, Y.; Qu, W.; Yan, Z. Synthesis, characterization and antitumor activity of the germanium-quercetin complex. Main Group Met. Chem. 2012, 35, 103–109. [Google Scholar] [CrossRef]
- Pi, J.; Zeng, J.; Luo, J.-J.; Yang, P.-H.; Cai, J.-Y. Synthesis and biological evaluation of Germanium(IV)–polyphenol complexes as potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2902–2908. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mana, S. Pharmacological evaluation germanium (IV)-hesperidin complex for hepatocellular carcinoma on rats. World J. Pharm. Res. 2021, 10, 1795–1805. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Warburg, O.H. The Prime Cause and Prevention of Cancer (with Two Prefaces on Prevention); National Cancer Institute: Bethesda, MD, USA, 1967.
- Warburg, O. Otto Warburg on the Prime Cause & Prevention of Cancer: Respiration of Oxygen in Normal Body Cells vs. Fermentation of Sugar in Cancer Cells, 2nd ed.; Konrad Triltsch: Würzburg, Germany, 1969. [Google Scholar]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Chakraborti, S.; Ray, B.K.; Roychoudhury, S. (Eds.) Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Singapore, 2022; p. LVI, 2675. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Sotgia, F.; Martinez-Outschoorn, U.E.; Lisanti, M.P. Mitochondrial oxidative stress drives tumor progression and metastasis: Should we use antioxidants as a key component of cancer treatment and prevention? BMC Med. 2011, 9, 62. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Betül, Ç.; Ali Cengiz, Ç. Antioxidant and Oxidative Stress. In Antioxidants; Viduranga, W., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 6. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Schwarz, R.; Giese, H. Beiträge zur Chemie des Germaniums, III. Mitteil.: Sulfo-und Pergermanate. Ber. Der Dtsch. Chem. Ges. A B Ser. 1930, 63, 778–782. [Google Scholar] [CrossRef]
- Schwarz, R.; Heinrich, F. Zur Kenntnis der peroxydischen Verbindungen. Z. Für Anorg. Und Allg. Chem. 1935, 223, 387–392. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, L.; Deng, Y.; Zhang, C.; Zhang, Y.; Xiong, S.; Ding, C.; Zhao, J.; Liao, C.; Gong, D. A review of public and environmental consequences of organic germanium. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1384–1409. [Google Scholar] [CrossRef]
- Filella, M. Comment on “A review of public and environmental consequences of organic germanium” by Zheng and co-workers. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1279–1287. [Google Scholar] [CrossRef]
- Zheng, J.; Deng, Y. Response to comment on “A review of public and environmental consequences of organic germanium”. Crit. Rev. Environ. Sci. Technol. 2022, 53, 1478–1488. [Google Scholar] [CrossRef]



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menchikov, L.G.; Popov, A.V. Physiological Activity of Trace Element Germanium including Anticancer Properties. Biomedicines 2023, 11, 1535. https://doi.org/10.3390/biomedicines11061535
Menchikov LG, Popov AV. Physiological Activity of Trace Element Germanium including Anticancer Properties. Biomedicines. 2023; 11(6):1535. https://doi.org/10.3390/biomedicines11061535
Chicago/Turabian StyleMenchikov, Leonid G., and Anatoliy V. Popov. 2023. "Physiological Activity of Trace Element Germanium including Anticancer Properties" Biomedicines 11, no. 6: 1535. https://doi.org/10.3390/biomedicines11061535
APA StyleMenchikov, L. G., & Popov, A. V. (2023). Physiological Activity of Trace Element Germanium including Anticancer Properties. Biomedicines, 11(6), 1535. https://doi.org/10.3390/biomedicines11061535






