G Protein-Coupled Receptors and the Rise of Type 2 Diabetes in Children
Abstract
1. Type 2 Diabetes Prevalence on the Rise in Youth
2. Learning from Yeast History
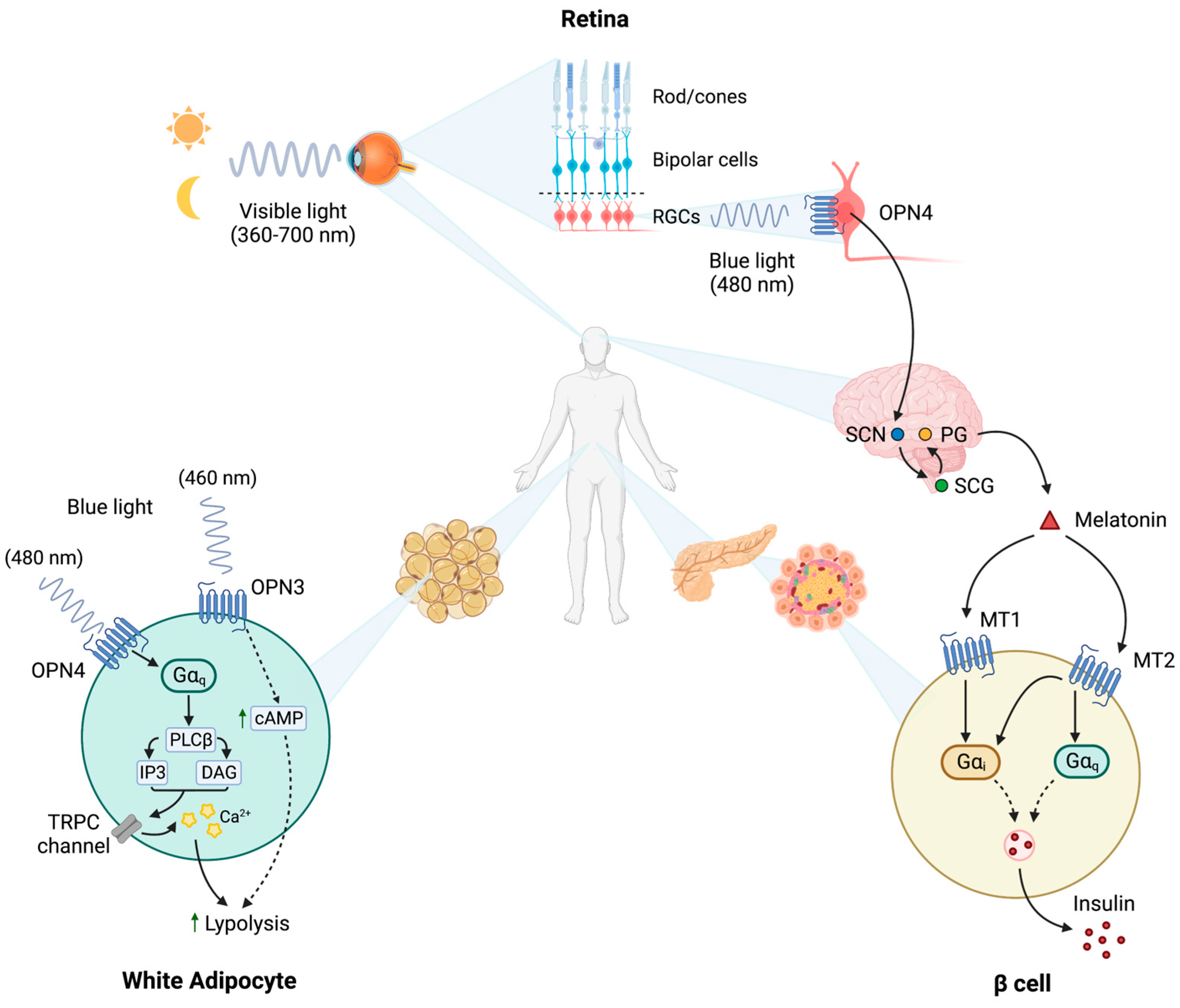
3. Type 2 Diabetes and Circadian Rhythm
4. Food Intake and Type 2 Diabetes: Future Pharmacological Strategies for Children
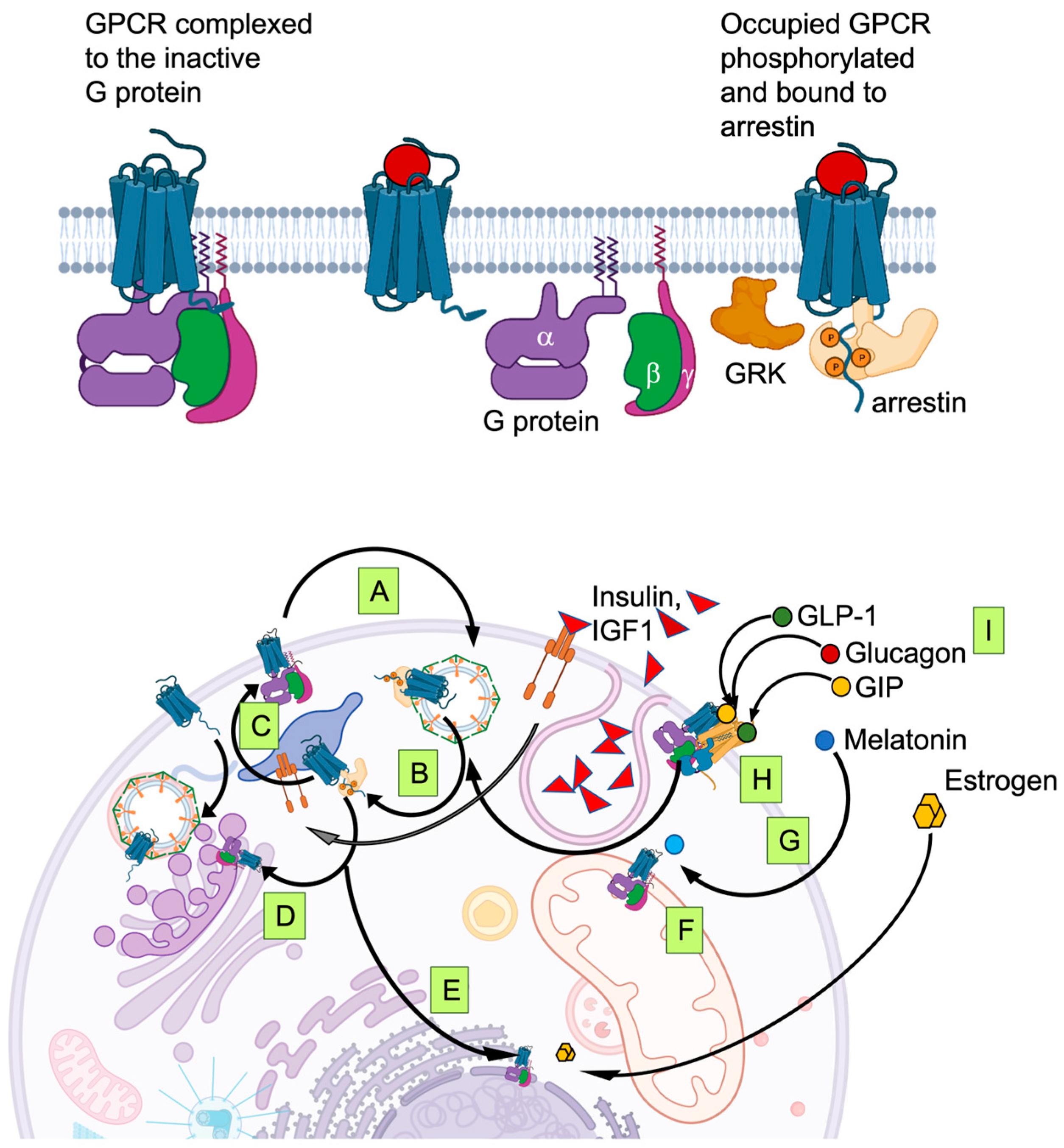
5. Signaling Integration on the Cell Surface: Biased Agonism, Multi-Agonists and Dimerism
6. Signaling Integration inside the Cell: GPCR Phosphorylation, Trafficking and Membrane Permeable Agonists
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2022, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.T.; Stafford, J.M.; Saydah, S.; D’agostino, R.B.; Dolan, L.M.; Lawrence, J.M.; Marcovina, S.; Mayer-Davis, E.J.; Pihoker, C.; Rewers, A.; et al. Treatment of Diabetes Mellitus in Children and Adolescents. Diabetes Care 2021, 44, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, G.; Boyle, J.P.; Thompson, T.J.; Case, D.; Dabelea, D.; Hamman, R.F.; Lawrence, J.M.; Liese, A.D.; Liu, L.L.; Mayer-Davis, E.J.; et al. Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged. Diabetes Care 2012, 35, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, M.; Long, Z.; Ning, H.; Li, J.; Cao, Y.; Liao, Y.; Liu, G.; Wang, F.; Pan, A. Global Burden of Type 2 Diabetes in Adolescents and Young Adults, 1990–2019: Systematic Analysis of the Global Burden of Disease Study 2019. BMJ 2022, 379, e072385. [Google Scholar] [CrossRef] [PubMed]
- Szypowska, A.; Skórka, A. The Risk Factors of Ketoacidosis in Children with Newly Diagnosed Type 1 Diabetes Mellitus. Pediatr. Diabetes 2011, 12, 302–306. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Thompson, M.; Ercole, A.; Walter, F.M. Variation between Countries in the Frequency of Diabetic Ketoacidosis at First Presentation of Type 1 Diabetes in Children: A Systematic Review. Diabetologia 2012, 55, 2878–2894. [Google Scholar] [CrossRef]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diabetes Rep. 2016, 16, 64. [Google Scholar] [CrossRef]
- Zhang, F.; Sjöholm, Å.; Zhang, Q. Growth Hormone Signaling in Pancreatic β-Cells—Calcium Handling Regulated by Growth Hormone. Mol. Cell. Endocrinol. 2009, 297, 50–57. [Google Scholar] [CrossRef]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Ripoll, C.; Fuentes, E.; Quesada, I.; Ropero, A.B. Role of Estrogen Receptors Alpha, Beta and GPER1/GPR30 in Pancreatic Beta-Cells. Front. Biosci. 2011, 16, 251–260. [Google Scholar] [CrossRef]
- Pi, M.; Wu, Y.; Quarles, L.D. GPRC6A Mediates Responses to Osteocalcin in β-Cells in Vitro and Pancreas in Vivo. J. Bone Miner. Res. 2011, 26, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Spears, E.; Serafimidis, I.; Powers, A.C.; Gavalas, A. Debates in Pancreatic Beta Cell Biology: Proliferation Versus Progenitor Differentiation and Transdifferentiation in Restoring β Cell Mass. Front. Endocrinol. 2021, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Castell, A.L.; Goubault, C.; Ethier, M.; Fergusson, G.; Tremblay, C.; Baltz, M.; Dal Soglio, D.; Ghislain, J.; Poitout, V. β Cell Mass Expansion during Puberty Involves Serotonin Signaling and Determines Glucose Homeostasis in Adulthood. JCI Insight 2022, 7, e160854. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Hang, Y.; Shostak, A.; Poffenberger, G.; Hart, N.; Prasad, N.; Phillips, N.; Levy, S.E.; Greiner, D.L.; Shultz, L.D.; et al. Age-Dependent Human β Cell Proliferation Induced by Glucagon-like Peptide 1 and Calcineurin Signaling. J. Clin. Investig. 2017, 127, 3835–3844. [Google Scholar] [CrossRef]
- Kulkarni, R.N.; Mizrachi, E.B.; Ocana, A.G.; Stewart, A.F. Human β-Cell Proliferation and Intracellular Signaling: Driving in the Dark without a Road Map. Diabetes 2012, 61, 2205–2213. [Google Scholar] [CrossRef]
- Iafusco, D.; Franceschi, R.; Maguolo, A.; Guercio Nuzio, S.; Crinò, A.; Delvecchio, M.; Iughetti, L.; Maffeis, C.; Calcaterra, V.; Manco, M. From Metabolic Syndrome to Type 2 Diabetes in Youth. Children 2023, 10, 516. [Google Scholar] [CrossRef]
- Kufe, C.N.; Micklesfield, L.K.; Masemola, M.; Chikowore, T.; Kengne, A.P.; Karpe, F.; Norris, S.A.; Crowther, N.J.; Olsson, T.; Goedecke, J.H. Increased Risk for Type 2 Diabetes in Relation to Adiposity in Middle-Aged Black South African Men Compared to Women. Eur. J. Endocrinol. 2022, 186, 523–533. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender Difference in Oxidative Stress: A New Look at the Mechanisms for Cardiovascular Diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Bishai, R.; Geller, D.; Shehadeh, N.; Al-Abdulrazzaq, D.; Vazquez, E.M.; Karoly, E.; Troja, T.; Doehring, O.; Carter, D.; et al. Once-Weekly Exenatide in Youth With Type 2 Diabetes. Diabetes Care 2022, 45, 1833–1840. [Google Scholar] [CrossRef]
- Bagepally, B.S.; Chaikledkaew, U.; Gurav, Y.K.; Anothaisintawee, T.; Youngkong, S.; Chaiyakunapruk, N.; McEvoy, M.; Attia, J.; Thakkinstian, A. Glucagon-like Peptide 1 Agonists for Treatment of Patients with Type 2 Diabetes Who Fail Metformin Monotherapy: Systematic Review and Meta-Analysis of Economic Evaluation Studies. BMJ Open Diabetes Res. Care 2020, 8, e001020. [Google Scholar] [CrossRef]
- Kelly, A.S.; Bensignor, M.O.; Hsia, D.S.; Shoemaker, A.H.; Shih, W.; Peterson, C.; Varghese, S.T. Phentermine/Topiramate for the Treatment of Adolescent Obesity. NEJM Evid. 2022, 1, EVIDoa2200014. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Scheel, D.W.; Macias, H.; Miyatsuka, T.; Kimc, H.; Hoang, P.; Ku, G.M.; Honig, G.; Liou, A.; Tang, Y.; et al. Gαi/o-Coupled Receptor Signaling Restricts Pancreatic β-Cell Expansion. Proc. Natl. Acad. Sci. USA 2015, 112, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Castorani, V.; Polidori, N.; Giannini, C.; Blasetti, A.; Chiarelli, F. Insulin Resistance and Type 2 Diabetes in Children. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 217. [Google Scholar] [CrossRef]
- Rendina, D.; Campanozzi, A.; De Filippo, G. Methodological Approach to the Assessment of the Obesogenic Environment in Children and Adolescents: A Review of the Literature. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 561–571. [Google Scholar] [CrossRef]
- Malina, C.; Larsson, C.; Nielsen, J. Yeast Mitochondria: An Overview of Mitochondrial Biology and the Potential of Mitochondrial Systems Biology. FEMS Yeast Res. 2018, 18, 40. [Google Scholar] [CrossRef]
- Thevelein, J.M.; Voordeckers, K. Functioning and Evolutionary Significance of Nutrient Transceptors. Mol. Biol. Evol. 2009, 26, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Stoffers, D.A. The Development of Beta-Cell Mass: Recent Progress and Potential Role of GLP-1. Horm. Metab. Res. 2004, 36, 811–821. [Google Scholar] [CrossRef]
- Delobel, M.; Dalle, S. G-Protein–Coupled Receptors Controlling Pancreatic β-Cell Functional Mass for the Treatment of Type 2 Diabetes. Curr. Opin. Endocr. Metab. Res. 2021, 16, 113–118. [Google Scholar] [CrossRef]
- Lane, J.M.; Qian, J.; Mignot, E.; Redline, S.; Scheer, F.A.J.L.; Saxena, R. Genetics of Circadian Rhythms and Sleep in Human Health and Disease. Nat. Rev. Genet. 2022, 24, 4–20. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; Stenvers, D.J.; Jansen, R.D.; Foppen, E.; Fliers, E.; Kalsbeek, A. Light at Night Acutely Impairs Glucose Tolerance in a Time-, Intensity- and Wavelength-Dependent Manner in Rats. Diabetologia 2017, 60, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Pévet, P. Melatonin. Dialogues Clin. Neurosci. 2002, 4, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lu, Q.; Guo, Q.; Teng, M.; Gong, Q.; Li, X.; Du, Y.; Liu, Z.; Tao, Y. Structural Basis of the Ligand Binding and Signaling Mechanism of Melatonin Receptors. Nat. Commun. 2022, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Tosini, G.; Owino, S.; Guillaume, J.L.; Jockers, R. Understanding Melatonin Receptor Pharmacology: Latest Insights from Mouse Models, and Their Relevance to Human Disease. BioEssays 2014, 36, 778–787. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, H.; Ahmad, N.; Mishra, P.; Tiwari, A. The Role of Melatonin in Diabetes: Therapeutic Implications. Arch. Endocrinol. Metab. 2015, 59, 391–399. [Google Scholar] [CrossRef]
- Stumpf, I.; Mühlbauer, E.; Peschke, E. Involvement of the CGMP Pathway in Mediating the Insulin-Inhibitory Effect of Melatonin in Pancreatic β-Cells. J. Pineal Res. 2008, 45, 318–327. [Google Scholar] [CrossRef]
- Garaulet, M.; Qian, J.; Florez, J.C.; Arendt, J.; Saxena, R.; Scheer, F.A.J.L. Melatonin Effects on Glucose Metabolism: Time To Unlock the Controversy. Trends Endocrinol. Metab. 2020, 31, 192–204. [Google Scholar] [CrossRef]
- Masters, M.; Pandi-Perumal, S.R.; Seixas, A.; Girardin, J.-L.; McFarlane, S.I. Melatonin, the Hormone of Darkness: From Sleep Promotion to Ebola Treatment. Brain Disord. Ther. 2014, 4, 1000151. [Google Scholar] [CrossRef]
- Tauman, R.; Zisapel, N.; Laudon, M.; Nehama, H.; Sivan, Y. Melatonin Production in Infants: Association with Perinatal Factors and Development. Pediatr. Neurol. 2002, 26, 379–382. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, Human Aging, and Age-Related Diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Skrzelowski, M.; Brookhaus, A.; Shea, L.A.; Berlau, D.J. Melatonin Use in Pediatrics: Evaluating the Discrepancy in Evidence Based on Country and Regulations Regarding Production. J. Pediatr. Pharmacol. Ther. JPPT 2021, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Pourhanifeh, M.H.; Hosseinzadeh, A.; Dehdashtian, E.; Hemati, K.; Mehrzadi, S. Melatonin: New Insights on Its Therapeutic Properties in Diabetic Complications. Diabetol. Metab. Syndr. 2020, 12, 30. [Google Scholar] [CrossRef]
- Onaolapo, O.J.; Onaolapo, A.Y.; Onaolapo, A.Y.; Neuroscience, B. Melatonin, Adolescence, and the Brain: An Insight into the Period-Specific Influences of a Multifunctional Signaling Molecule. Birth Defects Res. 2017, 109, 1659–1671. [Google Scholar] [CrossRef]
- Li, X.; Zhu, S.; Qi, F. Blue Light Pollution Causes Retinal Damage and Degeneration by Inducing Ferroptosis. J. Photochem. Photobiol. B 2023, 238, 112617. [Google Scholar] [CrossRef] [PubMed]
- Ekechukwu, O.N.; Christian, M. Metabolic Responses of Light and Taste Receptors—Unexpected Actions of GPCRs in Adipocytes. Rev. Endocr. Metab. Disord. 2022, 23, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.-Q.; Mao, C.; Xiao, P.; Zhao, R.-J.; Jiang, Y.; Yang, Z.; An, W.-T.; Shen, D.-D.; Yang, F.; Zhang, H.; et al. Structures of the Glucocorticoid-Bound Adhesion Receptor GPR97-G o Complex. Nature 2021, 589, 620–626. [Google Scholar] [CrossRef]
- Joseph, J.J.; Golden, S.H. Cortisol Dysregulation: The Bidirectional Link between Stress, Depression, and Type 2 Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef]
- Sohn, S.; Rees, P.; Wildridge, B.; Kalk, N.J.; Carter, B. Prevalence of Problematic Smartphone Usage and Associated Mental Health Outcomes amongst Children and Young People: A Systematic Review, Meta-Analysis and GRADE of the Evidence. BMC Psychiatry 2019, 19, 356. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, Stress, and Diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Ucar, C.; Senol, D.; Cevirgen, F.; Ozbag, D.; Altay, Z.; Yildiz, S. Mahmut Cay, 1 Effect of Increase in Cortisol Level Due to Stress in Healthy Young Individuals on Dynamic and Static Balance Scores. North. Clin. Istanb. 2018, 5, 295–301. [Google Scholar] [CrossRef]
- Bucci, M.; Marques, S.S.; Oh, D.; Harris, N.B. Toxic Stress in Children and Adolescents. Adv. Pediatr. 2016, 63, 403–428. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. Adv. Exp. Med. Biol. 2017, 960, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Szosland, D. Shift Work and Metabolic Syndrome, Diabetes Mellitus and Ischaemic Heart Disease. Int. J. Occup. Med. Environ. Health 2010, 23, 287–291. [Google Scholar] [CrossRef]
- Kimple, M.E.; Neuman, J.C.; Linnemann, A.K.; Casey, P.J. Inhibitory G Proteins and Their Receptors: Emerging Therapeutic Targets for Obesity and Diabetes. Exp. Mol. Med. 2014, 46, e102. [Google Scholar] [CrossRef]
- Atanes, P.; Persaud, S.J. GPCR Targets in Type 2 Diabetes. In GPCRs Structure, Function, and Drug Discovery; Academic Press: Cambridge, MA, USA, 2019; pp. 367–391. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Lenard, N.R.; Shin, A.C. Food Reward, Hyperphagia, and Obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1266–1277. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D.; Baler, R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. In Brain Imaging in Behavioral Neuroscience; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2011; Volume 11, pp. 1–24. [Google Scholar] [CrossRef]
- Stice, E.; Spoor, S.; Ng, J.; Zald, D.H. Relation of Obesity to Consummatory and Anticipatory Food Reward. Physiol. Behav. 2009, 97, 551. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, J.J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Groot, P.F.C.; Ruhé, H.G.; Barkhof, F.; Sloan, J.H.; Diamant, M.; Ijzerman, R.G. Brain Reward-System Activation in Response to Anticipation and Consumption of Palatable Food Is Altered by Glucagon-like Peptide-1 Receptor Activation in Humans. Diabetes Obes. Metab. 2015, 17, 878–886. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Drent, M.L.; Barkhof, F.; Diamant, M.; Ijzerman, R.G. Emotional Eating Is Associated with Increased Brain Responses to Food-Cues and Reduced Sensitivity to GLP-1 Receptor Activation. Obesity 2015, 23, 2075–2082. [Google Scholar] [CrossRef]
- Dockray, G.J. Enteroendocrine Cell Signalling via the Vagus Nerve. Curr. Opin. Pharmacol. 2013, 13, 954–958. [Google Scholar] [CrossRef]
- Nakagawa, A.; Satake, H.; Nakabayashi, H.; Nishizawa, M.; Furuya, K.; Nakano, S.; Kigoshi, T.; Nakayama, K.; Uchida, K. Receptor Gene Expression of Glucagon-like Peptide-1, but Not Glucose-Dependent Insulinotropic Polypeptide, in Rat Nodose Ganglion Cells. Auton. Neurosci. 2004, 110, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Vahl, T.P.; Tauchi, M.; Durler, T.S.; Elfers, E.E.; Fernandes, T.M.; Bitner, R.D.; Ellis, K.S.; Woods, S.C.; Seeley, R.J.; Herman, J.P.; et al. Glucagon-Like Peptide-1 (GLP-1) Receptors Expressed on Nerve Terminals in the Portal Vein Mediate the Effects of Endogenous GLP-1 on Glucose Tolerance in Rats. Endocrinology 2007, 148, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J. The Incretin System in Healthy Humans: The Role of GIP and GLP-1. Metabolism 2019, 96, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gromada, J. Role of Incretin Hormones in the Regulation of Insulin Secretion in Diabetic and Nondiabetic Humans. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E199–E206. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Oya, M.; Kitaguchi, T.; Pais, R.; Reimann, F.; Gribble, F.; Tsuboi, T. The G Protein-Coupled Receptor Family C Group 6 Subtype A (GPRC6A) Receptor Is Involved in Amino Acid-Induced Glucagon-like Peptide-1 Secretion from GLUTag Cells. J. Biol. Chem. 2013, 288, 4513. [Google Scholar] [CrossRef]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. GPRC6A: Jack of All Metabolism (or Master of None). Mol. Metab. 2016, 6, 185–193. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Prud’Homme, D.; Blanchet, R.; Giroux, I. Nutritional Modulation of Endogenous Glucagon-like Peptide-1 Secretion: A Review. Nutr. Metab. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 Is Expressed in Enteroendocrine Cells and Mediates Free Fatty Acid Stimulation of Incretin Secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Hauge, M.; Vestmar, M.A.; Husted, A.S.; Ekberg, J.P.; Wright, M.J.; Di Salvo, J.; Weinglass, A.B.; Engelstoft, M.S.; Madsen, A.N.; Lückmann, M.; et al. GPR40 (FFAR1)—Combined Gs and Gq Signaling in Vitro Is Associated with Robust Incretin Secretagogue Action Ex Vivo and in Vivo. Mol. Metab. 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Tazoe, H.; Otomo, Y.; Karaki, S.I.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of Short-Chain Fatty Acid Receptor GPR41 in the Human Colon. Biomed. Res. 2009, 30, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Secor, J.D.; Fligor, S.C.; Tsikis, S.T.; Yu, L.J.; Puder, M. Free Fatty Acid Receptors as Mediators and Therapeutic Targets in Liver Disease. Front. Physiol. 2021, 12, 438. [Google Scholar] [CrossRef]
- Burant, C.F. Activation of GPR40 as a Therapeutic Target for the Treatment of Type 2 Diabetes. Diabetes Care 2013, 36, S175–S179. [Google Scholar] [CrossRef]
- Goldspink, D.A.; Lu, V.B.; Billing, L.J.; Larraufie, P.; Tolhurst, G.; Gribble, F.M.; Reimann, F. Mechanistic Insights into the Detection of Free Fatty and Bile Acids by Ileal Glucagon-like Peptide-1 Secreting Cells. Mol. Metab. 2018, 7, 90–101. [Google Scholar] [CrossRef]
- Mancini, A.D.; Poitout, V. The Fatty Acid Receptor FFA1/GPR40 a Decade Later: How Much Do We Know? Trends Endocrinol. Metab. 2013, 24, 398–407. [Google Scholar] [CrossRef]
- Marcinak, J.; Cao, C.; Lee, D.; Ye, Z. Fasiglifam for Glycaemic Control in People with Type 2 Diabetes: A Phase III, Placebo-Controlled Study. Diabetes Obes. Metab. 2017, 19, 1714–1721. [Google Scholar] [CrossRef]
- Lückmann, M.; Shenol, A.; Nissen, T.A.D.; Petersen, J.E.; Kouvchinov, D.; Schwartz, T.W.; Frimurer, T.M. Optimization of First-in-Class Dual-Acting FFAR1/FFAR4 Allosteric Modulators with Novel Mode of Action. ACS Med. Chem. Lett. 2022, 13, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Tamborlane, W.V.; Barrientos-Pérez, M.; Fainberg, U.; Frimer-Larsen, H.; Hafez, M.; Hale, P.M.; Jalaludin, M.Y.; Kovarenko, M.; Libman, I.; Lynch, J.L.; et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Estrada, A.; Nieto-Rodríguez, C.; León-Figueroa, D.A.; Moreno-Ramos, E.; Cabanillas-Ramirez, C.; Barboza, J.J. Efficacy of Liraglutide in Obesity in Children and Adolescents: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Children 2023, 10, 208. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Okorokov, P.L.; Bezlepkina, O.B. Сoвременные Стратегии Лечения Ожирения у Детей. Probl. Endocrinol. 2023, 68, 131. [Google Scholar] [CrossRef]
- Stinson, S.E.; Jonsson, A.E.; Lund, M.A.V.; Frithioff-Bøjsøe, C.; Aas Holm, L.; Pedersen, O.; Ängquist, L.; Sørensen, T.I.A.; Holst, J.J.; Christiansen, M.; et al. Fasting Plasma GLP-1 Is Associated with Overweight/Obesity and Cardiometabolic Risk Factors in Children and Adolescents. J. Clin. Endocrinol. Metab. 2021, 106, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Bensignor, M.O.; Wolf, J.M.; Rudser, K.D.; Kelly, A.S.; Arslanian, S. Glucagon-like Peptide-1 Receptor Agonist Prescribing Patterns in Adolescents with Type 2 Diabetes. Diabetes Obes. Metab. 2022, 24, 1380. [Google Scholar] [CrossRef] [PubMed]
- Bensignor, M.O.; Kelly, A.S.; Arslanian, S. Anti-Obesity Pharmacotherapy for Treatment of Pediatric Type 2 Diabetes: Review of the Literature and Lessons Learned from Adults. Front. Endocrinol. 2022, 13, 2745. [Google Scholar] [CrossRef] [PubMed]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Carullo, G.; Mazzotta, S.; Vega-Holm, M.; Iglesias-Guerra, F.; Manuel Vega-Pérez, J.; Aiello, F.; Brizzi, A. GPR120/FFAR4 Pharmacology: Focus on Agonists in Type 2 Diabetes Mellitus Drug Discovery. J. Med. Chem. 2021, 64, 4332. [Google Scholar] [CrossRef]
- Du, Y.Q.; Sha, X.Y.; Cheng, J.; Wang, J.; Lin, J.Y.; An, W.T.; Pan, W.; Zhang, L.J.; Tao, X.N.; Xu, Y.F.; et al. Endogenous Lipid-GPR120 Signaling Modulates Pancreatic Islet Homeostasis to Different Extents. Diabetes 2022, 71, 1454–1471. [Google Scholar] [CrossRef]
- Karamitri, A.; Plouffe, B.; Bonnefond, A.; Chen, M.; Gallion, J.; Guillaume, J.L.; Hegron, A.; Boissel, M.; Canouil, M.; Langenberg, C.; et al. Type 2 Diabetes–Associated Variants of the MT2 Melatonin Receptor Affect Distinct Modes of Signaling. Sci. Signal. 2018, 11, eaan6622. [Google Scholar] [CrossRef] [PubMed]
- Ast, J.; Nasteska, D.; Fine, N.H.F.; Nieves, D.J.; Koszegi, Z.; Lanoiselée, Y.; Cuozzo, F.; Viloria, K.; Bacon, A.; Luu, N.T.; et al. Revealing the Tissue-Level Complexity of Endogenous Glucagon-like Peptide-1 Receptor Expression and Signaling. Nat. Commun. 2023, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Jones, B. The Therapeutic Potential of GLP-1 Receptor Biased Agonism. Br. J. Pharmacol. 2022, 179, 492–510. [Google Scholar] [CrossRef] [PubMed]
- El Eid, L.; Reynolds, C.A.; Tomas, A.; Ben, J. Biased Agonism and Polymorphic Variation at the GLP-1 Receptor: Implications for the Development of Personalised Therapeutics. Pharmacol. Res. 2022, 184, 106411. [Google Scholar] [CrossRef]
- Clemmensen, C.; Finan, B.; Müller, T.D.; Dimarchi, R.D.; Tschöp, M.H.; Hofmann, S.M. Emerging Hormonal-Based Combination Pharmacotherapies for the Treatment of Metabolic Diseases. Nat. Rev. Endocrinol. 2018, 15, 90–104. [Google Scholar] [CrossRef]
- Heise, T.; DeVries, J.H.; Urva, S.; Li, J.; Pratt, E.J.; Thomas, M.K.; Mather, K.J.; Karanikas, C.A.; Dunn, J.; Haupt, A.; et al. Tirzepatide Reduces Appetite, Energy Intake, and Fat Mass in People with Type 2 Diabetes. Diabetes Care 2023, 46, 998–1004. [Google Scholar] [CrossRef]
- Novikoff, A.; O’Brien, S.L.; Bernecker, M.; Grandl, G.; Kleinert, M.; Knerr, P.J.; Stemmer, K.; Klingenspor, M.; Zeigerer, A.; DiMarchi, R.; et al. Spatiotemporal GLP-1 and GIP Receptor Signaling and Trafficking/Recycling Dynamics Induced by Selected Receptor Mono- and Dual-Agonists. Mol. Metab. 2021, 49, 101181. [Google Scholar] [CrossRef]
- Tse, L.H.; Wong, Y.H. Modeling the Heterodimer Interfaces of Melatonin Receptors. Front. Cell. Neurosci. 2021, 15, 725296. [Google Scholar] [CrossRef]
- Al-Zaid, B.; Chacko, S.; Ezeamuzie, C.I.; Bünemann, M.; Krasel, C.; Karimian, T.; Lanzerstorfer, P.; Al-Sabah, S. Differential Effects of Glucose-Dependent Insulinotropic Polypeptide Receptor/Glucagon-like Peptide-1 Receptor Heteromerization on Cell Signaling When Expressed in HEK-293 Cells. Pharmacol. Res. Perspect. 2022, 10, e01013. [Google Scholar] [CrossRef]
- Shen, A.; Nieves-Cintron, M.; Deng, Y.; Shi, Q.; Chowdhury, D.; Qi, J.; Hell, J.W.; Navedo, M.F.; Xiang, Y.K. Functionally Distinct and Selectively Phosphorylated GPCR Subpopulations Co-Exist in a Single Cell. Nat. Commun. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, F.; Zhang, D.; Liu, Z.; Lin, A.; Liu, C.; Xiao, P.; Yu, X.; Sun, J.P. Phosphorylation of G Protein-Coupled Receptors: From the Barcode Hypothesis to the Flute Model. Mol. Pharmacol. 2017, 92, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Innamorati, G.; Le Gouill, C.; Balamotis, M.; Birnbaumer, M. The Long and the Short Cycle. J. Biol. Chem. 2001, 276, 13096–13103. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Carli, M.; Petragnano, F.; Colaianni, F.; Aloisi, G.; Maggio, R.; Scarselli, M.; Rossi, M. GPCRs in Intracellular Compartments: New Targets for Drug Discovery. Biomolecules 2022, 12, 1343. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Chen, X.; Li, X.; Ying, G.; Jin, L.; Ma, Q.; Li, G.; Shi, Y.; Zhang, G.; et al. Differential Requirements of Arrestin-3 and Clathrin for Ligand-Dependent and -Independent Internalization of Human G Protein-Coupled Receptor 40. Cell. Signal. 2014, 26, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Samvelyan, S.; Chiarelli, F. An Update of the Consensus Statement on Insulin Resistance in Children 2010. Front. Endocrinol. 2022, 13, 1061524. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Innamorati, G.; Sadeghi, H.M.; Tran, N.T.; Birnbaumer, M. A Serine Cluster Prevents Recycling of the V2 Vasopressin Receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 2222–2226. [Google Scholar] [CrossRef]
- Alharbi, A.G.; Tobin, A.B.; Milligan, G. How Arrestins and GRKs Regulate the Function of Long Chain Fatty Acid Receptors. Int. J. Mol. Sci. 2022, 23, 12237. [Google Scholar] [CrossRef]
- Villegas-Comonfort, S.; Guzmán-Silva, A.; Romero-Ávila, M.T.; Takei, Y.; Tsujimoto, G.; Hirasawa, A.; García-Sáinz, J.A. Receptor Tyrosine Kinase Activation Induces Free Fatty Acid 4 Receptor Phosphorylation, β-Arrestin Interaction, and Internalization. Eur. J. Pharmacol. 2019, 855, 267–275. [Google Scholar] [CrossRef]
- Crudden, C.; Song, D.; Cismas, S.; Trocmé, E.; Pasca, S.; Calin, G.A.; Girnita, A.; Girnita, L. Below the Surface: IGF-1R Therapeutic Targeting and Its Endocytic Journey. Cells 2019, 8, 1223. [Google Scholar] [CrossRef]
- Ansarullah; Jain, C.; Far, F.F.; Homberg, S.; Wißmiller, K.; von Hahn, F.G.; Raducanu, A.; Schirge, S.; Sterr, M.; Bilekova, S.; et al. Inceptor Counteracts Insulin Signalling in β-Cells to Control Glycaemia. Nature 2021, 590, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Frithioff-Bøjsøe, C.; Lund, M.A.V.; Kloppenborg, J.T.; Nielsen, T.T.H.; Fonvig, C.E.; Lausten-Thomsen, U.; Hedley, P.L.; Hansen, T.; Pedersen, O.B.; Christiansen, M.; et al. Glucose Metabolism in Children and Adolescents: Population-Based Reference Values and Comparisons to Children and Adolescents Enrolled in Obesity Treatment. Pediatr. Diabetes 2019, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Lee, S. Appetite-Related Peptides in Childhood and Adolescence: Role of Ghrelin, PYY, and GLP-1. Appl. Physiol. Nutr. Metab. 2015, 40, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, C.; Schug, J.; Canaday, P.S.; Russ, H.A.; Tarlow, B.D.; Grompe, M.T.; Horton, T.; Hebrok, M.; Streeter, P.R.; Kaestner, K.H.; et al. Human Islets Contain Four Distinct Subtypes of β Cells. Nat. Commun. 2016, 7, 11756. [Google Scholar] [CrossRef]
| GPCR | Ligand | Effects | Research Phase | Reference |
|---|---|---|---|---|
| GLP1R | GLP1 | Insulin secretion and glucose uptake increase, appetite reduction, gastric emptying inhibition | Commercialized drugs (liraglutide, exenatide, semaglutide) | [21] |
| MT1 and MT2 | Melatonin | Circadian rhythm regulation, appetite modulation | Pre-clinical | [43] |
| OPN3 and OPN4 | Light | Diet-induced obesity and insulin resistance protection | Pre-clinical | [46] |
| Cortisol G protein-coupled receptor | Cortisol | Insulin resistance and glucose intolerance after chronic stimulation | Pre-clinical | [52] |
| GPRC6A | Testosterone, Ca2+, zinc, magnesium, osteocalcin | β cell proliferation and insulin sensitivity increase, obesity and metabolic syndrome protection | Pre-clinical | [71] |
| FFARs | Short-, medium- and long-chain free fatty acids | GLP1, GIP and PYY release stimulation | Pre-clinical | [78] |
| GIPR-GLP1R | GIP, GLP1 | Incretinic effect stimulation | Phase III clinical trials (Tirzepatide) | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallatana, A.; Cremonesi, L.; Trombetta, M.; Fracasso, G.; Nocini, R.; Giacomello, L.; Innamorati, G. G Protein-Coupled Receptors and the Rise of Type 2 Diabetes in Children. Biomedicines 2023, 11, 1576. https://doi.org/10.3390/biomedicines11061576
Dallatana A, Cremonesi L, Trombetta M, Fracasso G, Nocini R, Giacomello L, Innamorati G. G Protein-Coupled Receptors and the Rise of Type 2 Diabetes in Children. Biomedicines. 2023; 11(6):1576. https://doi.org/10.3390/biomedicines11061576
Chicago/Turabian StyleDallatana, Alessia, Linda Cremonesi, Maddalena Trombetta, Giulio Fracasso, Riccardo Nocini, Luca Giacomello, and Giulio Innamorati. 2023. "G Protein-Coupled Receptors and the Rise of Type 2 Diabetes in Children" Biomedicines 11, no. 6: 1576. https://doi.org/10.3390/biomedicines11061576
APA StyleDallatana, A., Cremonesi, L., Trombetta, M., Fracasso, G., Nocini, R., Giacomello, L., & Innamorati, G. (2023). G Protein-Coupled Receptors and the Rise of Type 2 Diabetes in Children. Biomedicines, 11(6), 1576. https://doi.org/10.3390/biomedicines11061576









