Therapeutic Approaches in Pancreatic Cancer: Recent Updates
Abstract
:1. Introduction
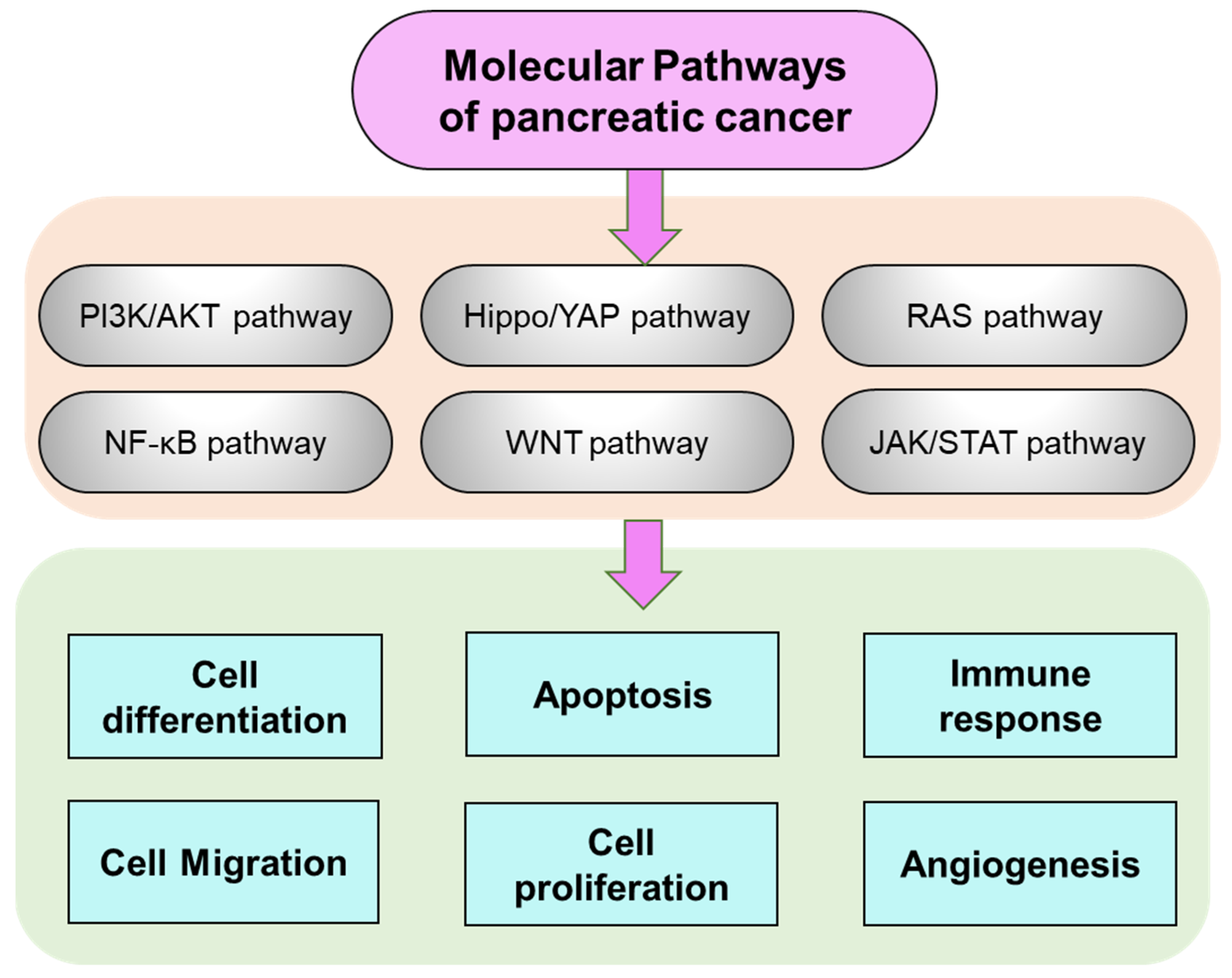
2. Pancreatic Cancer Molecular Manifestation and Pathways Regulation
3. Therapeutic Strategies
3.1. Non-Coding RNAs
3.2. Cyclin-Dependent Kinases
3.3. Oncolytic Virus
3.4. Nanomedicine
3.5. Adjuvants, Immunological Targets and Peptide
3.6. Natural Bioactive and Organic Compounds
3.7. Microbiome
3.8. Clustered Regularly Interspaced Short Palindromic Repeats, and Associated Protein 9 (CRISPR/Cas9)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. C.A. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mugaanyi, J.; Lu, C.; Huang, J.; Lu, C. Undifferentiated pancreatic carcinomas, clinical features and therapeutic options: What we know. Cancers 2022, 14, 6102. [Google Scholar] [CrossRef] [PubMed]
- Abou Khouzam, R.; Lehn, J.-M.; Mayr, H.; Clavien, P.-A.; Wallace, M.B.; Ducreux, M.; Limani, P.; Chouaib, S. Hypoxia, a targetable culprit to counter pancreatic cancer resistance to therapy. Cancers 2023, 15, 1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.-Q.; Wang, Z.-F.; Wu, X.-L.; Zhou, C.-H.; Yan, J.-Y.; Hu, B.-Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef]
- Le Cosquer, G.; Maulat, C.; Bournet, B.; Cordelier, P.; Buscail, E.; Buscail, L. Pancreatic cancer in chronic pancreatitis: Pathogenesis and diagnostic approach. Cancers 2023, 15, 761. [Google Scholar] [CrossRef]
- Cintoni, M.; Grassi, F.; Palombaro, M.; Rinninella, E.; Pulcini, G.; Di Donato, A.; Salvatore, L.; Quero, G.; Tortora, G.; Alfieri, S.; et al. Nutritional interventions during chemotherapy for pancreatic cancer: A systematic review of prospective studies. Nutrients 2023, 15, 727. [Google Scholar] [CrossRef]
- Lan, X.; Robin, G.; Kasnik, J.; Wong, G.; Abdel-Rahman, O. Challenges in diagnosis and treatment of pancreatic exocrine insufficiency among patients with pancreatic ductal adenocarcinoma. Cancers 2023, 15, 1331. [Google Scholar] [CrossRef]
- Gajewska-Naryniecka, A.; Szwedowicz, U.; Łapińska, Z.; Rudno-Rudzińska, J.; Kielan, W.; Kulbacka, J. Irreversible electroporation in pancreatic cancer—An evolving experimental and clinical method. Int. J. Mol. Sci. 2023, 24, 4381. [Google Scholar] [CrossRef]
- Gautam, S.K.; Khan, P.; Natarajan, G.; Atri, P.; Aithal, A.; Ganti, A.K.; Batra, S.K.; Nasser, M.W.; Jain, M. Mucins as potential biomarkers for early detection of cancer. Cancers 2023, 15, 1640. [Google Scholar] [CrossRef]
- Ge, W.; Yue, M.; Wang, Y.; Wang, Y.; Xue, S.; Shentu, D.; Mao, T.; Zhang, X.; Xu, H.; Li, S.; et al. A novel molecular signature of cancer-associated fibroblasts predicts prognosis and immunotherapy response in pancreatic cancer. Int. J. Mol. Sci. 2023, 24, 156. [Google Scholar] [CrossRef]
- de Santibañes, M.; Pekolj, J.; Sanchez Claria, R.; de Santibañes, E.; Mazza, O.M. Technical implications for surgical resection in locally advanced pancreatic cancer. Cancers 2023, 15, 1509. [Google Scholar] [CrossRef] [PubMed]
- Raufi, A.G.; May, M.S.; Hadfield, M.J.; Seyhan, A.A.; El-Deiry, W.S. Advances in liquid biopsy technology and implications for pancreatic cancer. Int. J. Mol. Sci. 2023, 24, 4238. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Galdiero, R.; Maggialetti, N.; Silvestro, L.; De Bellis, M.; Di Girolamo, E.; Grazzini, G.; Chiti, G.; et al. Risk assessment and pancreatic cancer: Diagnostic management and artificial intelligence. Cancers 2023, 15, 351. [Google Scholar] [CrossRef] [PubMed]
- Korn, R.L.; Burkett, A.; Geschwind, J.; Zygadlo, D.; Brodie, T.; Cridebring, D.; Von Hoff, D.D.; Demeure, M.J. Can imaging using radiomics and fat fraction analysis detect early tissue changes on historical CT scans in the regions of the pancreas gland that subsequently develop adenocarcinoma? Diagnostics 2023, 13, 941. [Google Scholar] [CrossRef]
- Padinharayil, H.; Rai, V.; George, A. Mitochondrial metabolism in pancreatic ductal adenocarcinoma: From mechanism-based perspectives to therapy. Cancers 2023, 15, 1070. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, B.; Ramai, D.; Galentino, M.; Martino, B.; Facciorusso, A. New perspectives on endoscopic management of liver and pancreatic cancer. Cancers 2023, 15, 1549. [Google Scholar] [CrossRef] [PubMed]
- Shetu, S.A.; James, N.; Rivera, G.; Bandyopadhyay, D. Molecular research in pancreatic cancer: Small molecule inhibitors, their mechanistic pathways and beyond. Curr. Issues Mol. Biol. 2023, 45, 1914–1949. [Google Scholar] [CrossRef]
- Lin, T.; Reddy, A.; Hill, C.; Sehgal, S.; He, J.; Zheng, L.; Herman, J.; Meyer, J.; Narang, A. The timing of surgery following stereotactic body radiation therapy impacts local control for borderline resectable or locally advanced pancreatic cancer. Cancers 2023, 15, 1252. [Google Scholar] [CrossRef]
- Fávaro, W.J.; dos Santos, M.M.; Pereira, M.M.; Garcia, P.V.; Durán, N. Effects of P-MAPA immunotherapy associated with gemcitabine on chemically-induced pancreatic cancer in animal model: New therapeutic perspectives. Biointerface Res. Appl. Chem. 2022, 12, 7540–7555. [Google Scholar]
- Steinhart, Z.; Pavlovic, Z.; Chandrashekhar, M.; Hart, T.; Wang, X.; Zhang, X.; Robitaille, M.; Brown, K.R.; Jaksani, S.; Overmeer, R.; et al. Genome-wide CRISPR screens reveal a Wnt-FZD5 signaling circuit as a druggable vulnerability of RNF43-mutant pancreatic tumors. Nat. Med. 2017, 23, 60–68. [Google Scholar] [CrossRef]
- Liu, J.; Mroczek, M.; Mach, A.; Stępień, M.; Aplas, A.; Pronobis-Szczylik, B.; Bukowski, S.; Mielczarek, M.; Gajewska, E.; Topolski, P.; et al. Genetics, genomics and emerging molecular therapies of pancreatic cancer. Cancers 2023, 15, 779. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Hua, J.; Liu, J.; Zhang, B.; Wang, W.; Yu, X.; Xu, J. Mesenchymal stromal cell-based targeted therapy pancreatic cancer: Progress and challenges. Int. J. Mol. Sci. 2023, 24, 3559. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.L.; Schad, F. Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy? Cancers 2023, 15, 1116. [Google Scholar] [CrossRef] [PubMed]
- Akl, L.; El-Hafeez, A.A.; Ibrahim, T.M.; Salem, R.; Marzouk, H.M.M.; El-Domany, R.A.; Ghosh, P.; Eldehna, W.M.; Abou-Seri, S.M. Identification of novel piperazine-tethered phthalazines as selective CDK1 inhibitors endowed with in vitro anticancer activity toward the pancreatic cancer. Eur. J. Med. Chem. 2022, 243, 114704. [Google Scholar] [CrossRef]
- Hung, J.; Perez, S.M.; Dasa, S.S.K.; Hall, S.P.; Heckert, D.B.; Murphy, B.P.; Crawford, H.C.; Kelly, K.A.; Brinton, L.T. A bitter taste receptor as a novel molecular target on cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Pharmaceuticals 2023, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, X.; Chen, Z.; Jiang, W.; Dong, S.; He, R.; Zhou, W. Nomograms for predicting the risk and prognosis of liver metastases in pancreatic cancer: A population-based analysis. J. Pers. Med. 2023, 13, 409. [Google Scholar] [CrossRef]
- Nakaoka, K.; Ohno, E.; Kawabe, N.; Kuzuya, T.; Funasaka, K.; Nakagawa, Y.; Nagasaka, M.; Ishikawa, T.; Watanabe, A.; Tochio, T.; et al. Current status of the diagnosis of early-stage pancreatic ductal adenocarcinoma. Diagnostics 2023, 13, 215. [Google Scholar] [CrossRef]
- Xu, Q.; Zhou, Y.; Zhang, H.; Li, H.; Qin, H.; Wang, H. Bibliometric analysis of hotspots and frontiers of immunotherapy in pancreatic cancer. Healthcare 2023, 11, 304. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Lu, Y.-F.; Xu, J.-X.; Du, Y.-Z.; Yu, R.-S. Recent advances in well-designed therapeutic nanosystems for the pancreatic ductal adenocarcinoma treatment dilemma. Molecules 2023, 28, 1506. [Google Scholar] [CrossRef]
- Thakur, P.; Thakur, V.; Kumar, P.; Patel, S.K.S. Emergence of novel omicron hybrid variants: BA(x), XE, XD, XF more than just alphabets. Int. J. Surg. 2022, 104, 106727. [Google Scholar] [CrossRef]
- Thakur, V.; Bhola, S.; Thakur, P.; Patel, S.K.S.; Kulshrestha, S.; Ratho, R.K.; Kumar, P. Waves and variants of SARS-CoV-2: Understanding the causes and effect of the COVID-19 catastrophe. Infection 2022, 50, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.A. Signaling pathways in pancreatic cancer. Cancer J. 2001, 7, 274–286. [Google Scholar] [PubMed]
- Iovanna, J.; Mallmann, M.C.; Goncalves, A.; Turrini, O.; Dagorn, J.-C. Current knowledge on pancreatic cancer. Front. Oncol. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sachdev, E.; Robbins, L.A.; Lin, E.; Hendifar, A.E.; Mita, M.M. Statins and pancreatic cancer. Oncol. Lett. 2017, 71, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Liao, Q.; Zhao, Y. Pancreatic cancer, gut microbiota, and therapeutic efficacy. J. Cancer 2020, 11, 2749–2758. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Hogendorf, P. The role of microRNA in pancreatic cancer. Biomedicines 2021, 9, 1322. [Google Scholar] [CrossRef]
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic cancer: A review of current treatment and novel therapies. J. Invest. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Stott, M.C.; Oldfield, L.; Hale, J.; Costello, E.; Halloran, C.M. Recent advances in understanding pancreatic cancer. Fac. Rev. 2022, 11, 9. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Q. Histone modifications represent a key epigenetic feature of epithelial-to-mesenchyme transition in pancreatic cancer. Int. J. Mol. Sci. 2023, 24, 4820. [Google Scholar] [CrossRef]
- Sherman, M.H.; Beatty, G.L. Tumor microenvironment in pancreatic cancer pathogenesis and therapeutic resistance. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 123–148. [Google Scholar] [CrossRef]
- Falcomatà, C.; Bärthel, S.; Schneider, G.; Rad, R.; Schmidt-Supprian, M.; Saur, D. Context-specific determinants of the immunosuppressive tumor microenvironment in pancreatic cancer. Cancer Discov. 2023, OF1–OF20. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef] [PubMed]
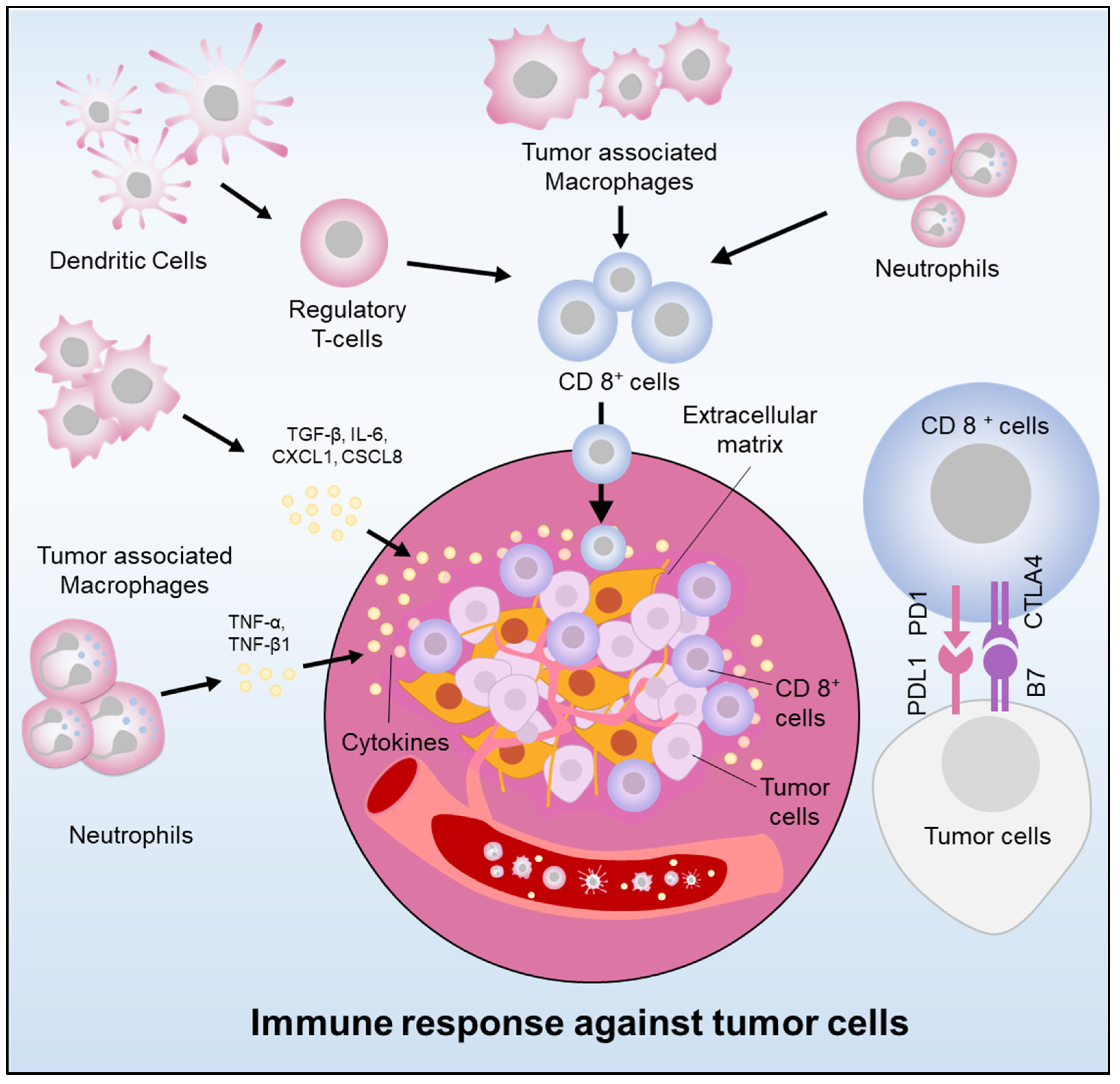
- Peng, C.; Li, Z.; Yu, X. The role of pancreatic infiltrating innate immune cells in acute pancreatitis. Int. J. Med. Sci. 2021, 18, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef]
- Ng, S.S.W.; Dawson, L.A. Inflammatory cytokines and radiotherapy in pancreatic ductal adenocarcinoma. Biomedicines 2022, 10, 3215. [Google Scholar] [CrossRef]
- Zhang, Y.; Lazarus, J.; Steele, N.G.; Yan, W.; Lee, H.-J.; Nwosu, Z.C.; Halbrook, C.J.; Menjivar, R.E.; Kemp, S.B.; Sirihorachai, V.R.; et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 2020, 10, 422–439. [Google Scholar] [CrossRef]
- Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Murakami, T.; Hiroshima, Y.; Matsuyama, R.; Homma, Y.; Hoffman, R.M.; Endo, I. Role of the tumor microenvironment in pancreatic cancer. Ann. Gastroenterol. Surg. 2019, 3, 130–137. [Google Scholar] [CrossRef]
- Sideras, K.; Braat, H.; Kwekkeboom, J.; Van Eijck, C.H.; Peppelenbosch, M.P.; Sleijfer, S.; Bruno, M. Role of the Immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat. Rev. 2014, 40, 513–522. [Google Scholar] [CrossRef]
- Ansari, D.; Ohlsson, H.; Althini, C.; Bauden, M.; Zhou, Q.; Hu, D.; Andersson, R. The hippo signaling pathway in pancreatic cancer. Anticancer. Res. 2019, 39, 3317–3321. [Google Scholar] [CrossRef]
- Korc, M.; Preis, M. Signaling pathways in pancreatic cancer. Crit. Rev. Eukaryot. Gene Expr. 2011, 2, 115–129. [Google Scholar]
- Ahmed, S.; Bradshaw, A.-D.; Gera, S.; Dewan, M.Z.; Xu, R. The TGF-β/Smad4 signaling pathway in pancreatic carcinogenesis and its clinical significance. J. Clin. Med. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Quatannens, D.; Verhoeven, Y.; Van Dam, P.; Lardon, F.; Prenen, H.; Roeyen, G.; Peeters, M.; Smits, E.L.J.; Van Audenaerde, J. Targeting hedgehog signaling in pancreatic ductal adenocarcinoma. Pharmacol. Ther. 2022, 236, 108107. [Google Scholar] [CrossRef]
- Mortazavi, M.; Moosavi, F.; Martini, M.; Giovannetti, E.; Firuzi, O. Prospects of targeting PI3K/AKT/MTOR pathway in pancreatic cancer. Crit. Rev. Oncol. Hematol. 2022, 176, 103749. [Google Scholar] [CrossRef] [PubMed]
- Orth, M.; Metzger, P.; Gerum, S.; Mayerle, J.; Schneider, G.; Belka, C.; Schnurr, M.; Lauber, K. Pancreatic ductal adenocarcinoma: Biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat. Oncol. 2019, 14, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhou, X.-Y.; Zhou, C.-L.; Liu, J.; Yong, T.; Fan, Y.; Wang, C. Insulin receptor tyrosine kinase substrate (IRTKS) promotes the tumorigenesis of pancreatic cancer via PI3K/AKT signaling. Hum. Cell 2022, 35, 1885–1899. [Google Scholar] [CrossRef]
- Mirzaei, S.; Saghari, S.; Bassiri, F.; Raesi, R.; Zarrabi, A.; Hushmandi, K.; Sethi, G.; Tergaonkar, V. NF-κB as a regulator of cancer metastasis and therapy response: A focus on epithelial–mesenchymal transition. J. Cell. Physiol. 2022, 237, 2770–2795. [Google Scholar] [CrossRef]
- Wu, J.; Ding, J.; Yang, J.; Guo, X.; Zheng, Y. MicroRNA roles in the nuclear factor kappa B signaling pathway in cancer. Front. Immunol. 2018, 9, 546. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Nayyab, S.; Martinelli, C.; Berardi, R.; Katifelis, H.; Gazouli, M.; Cho, W.C. Regulation of hippo, TGFβ/SMAD, Wnt/β-Catenin, JAK/STAT, and NOTCH by Long Non-Coding RNAs in Pancreatic Cancer. Front. Oncol. 2021, 11, 657965. [Google Scholar] [CrossRef]
- Javadinia, S.A.; Shahidsales, S.; Fanipakdel, A.; Joudi-Mashhad, M.; Mehramiz, M.; Talebian, S.; Maftouh, M.; Mardani, R.; Hassanian, S.M.; Khazaei, M. Therapeutic potential of targeting the Wnt/Β-catenin pathway in the treatment of pancreatic cancer. J. Cell. Biochem. 2019, 120, 6833–6840. [Google Scholar] [CrossRef]
- Aguilera, K.Y.; Dawson, D.W. WNT ligand dependencies in pancreatic cancer. Front. Cell Dev. Biol. 2021, 9, 671022. [Google Scholar] [CrossRef] [PubMed]
- Altan, Z.; Sahin, Y. miR-203 suppresses pancreatic cancer cell proliferation and migration by modulating DUSP5 expression. Mol. Cell Probe. 2022, 66, 101866. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Eldehna, W.M.; Ali, M.M.; Ella, D.A.A.E. 1-Piperazinylphthalazines as potential VEGFR-2 inhibitors and anticancer agents: Synthesis and in vitro biological evaluation. Eur. J. Med. Chem. 2016, 107, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Araki, H.; Tazawa, H.; Kanaya, N.; Kajiwara, Y.; Yamada, M.; Hashimoto, M.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Umeda, Y.; et al. Oncolytic virus-mediated p53 overexpression promotes immunogenic cell death and efficacy of PD-1 blockade in pancreatic cancer. Mol. Ther-Oncolytics 2022, 27, 3–13. [Google Scholar] [CrossRef]
- Sugito, N.; Heishima, K.; Akao, Y. Chemically modified MIR143-3p exhibited anticancer effects by impairing the KRAS network in colorectal cancer cells. Mol. Ther.-Nucl. Acids 2021, 6, 249. [Google Scholar]
- Shim, M.K.; Yang, S.; Park, J.; Yoon, J.S.; Moon, Y.; Shim, N.; Moon, Y.; Shim, N.; Jo, M.; Choi, Y.; et al. Preclinical development of carrier-free prodrug nanoparticles for enhanced antitumor therapeutic potential with less toxicity. J. Nanotechnol. 2022, 20, 436. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.; Predoi, D.; Burtea, C.; Dinischiotu, A. New insights into the biological response triggered by dextran-coated maghemite nanoparticles in pancreatic cancer cells and their potential for theranostic applications. Int. J. Mol. Sci. 2023, 24, 3307. [Google Scholar] [CrossRef]
- Alhussan, A.; Jackson, N.; Eaton, S.; Santos, N.D.; Barta, I.; Zaifman, J.; Chen, S.; Tam, Y.Y.C.; Krishnan, S.; Chithrani, D.B. Lipid-nanoparticle-mediated delivery of docetaxel prodrug for exploiting full potential of gold nanoparticles in the treatment of pancreatic cancer. Cancers 2022, 14, 6137. [Google Scholar] [CrossRef]
- Brero, F.; Calzolari, P.; Albino, M.; Antoccia, A.; Arosio, P.; Berardinelli, F.; Bettega, D.; Ciocca, M.; Facoetti, A.; Gallo, S.; et al. Proton therapy, magnetic nanoparticles and hyperthermia as combined treatment for pancreatic BxPC3 tumor cells. Nanomaterials 2023, 13, 791. [Google Scholar] [CrossRef]
- Mosiane, K.S.; Nweke, E.E.; Balogun, M.; Fru, P.N. Polyethyleneglycol-betulinic acid (PEG-BA) polymer-drug conjugate induces apoptosis and antioxidation in a biological model of pancreatic cancer. Polymers 2023, 15, 448. [Google Scholar] [CrossRef]
- Kobayashi, K.; Einama, T.; Takihata, Y.; Yonamine, N.; Fujinuma, I.; Tsunenari, T.; Kouzu, K.; Nakazawa, A.; Iwasaki, T.; Ueno, H.; et al. Therapeutic efficacy of dose-reduced adjuvant chemotherapy with S-1 in patients with pancreatic cancer: A retrospective study. BMC Cancer 2022, 22, 1028. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hayashi, H.; Uemura, N.; Ogata, Y.; Zhao, L.; Sato, H.; Shiraishi, Y.; Kuroki, H.; Kitamura, F.; Kaida, T.; et al. Thrombospondin-1 overexpression stimulates loss of Smad4 and accelerates malignant behavior via TGF-β signal activation in pancreatic ductal adenocarcinoma. Transl. Oncol. 2022, 26, 101533. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Deploying biomolecules as anti-COVID-19 agents. Indian J. Microbiol. 2020, 60, 263–268. [Google Scholar] [CrossRef]
- Guo, W.; Ding, Y.; Pu, C.; Wang, Z.; Deng, W.; Jin, X. Curcumin inhibits pancreatic cancer cell proliferation by regulating beclin1 expression and inhibiting the hypoxia-inducible factor-1α-mediated glycolytic pathway. J. Gastrointest. Oncol. 2022, 13, 3254–3262. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Xie, C.; Zhu, J.; Meng, Y.; Chen, Y.; Li, Y.; Jiang, Y.; Yang, X.; Wang, S.; et al. Sonic hedgehog and Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer stem cells. Anticancer. Drugs 2018, 29, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Du, Y.; Ben, Q.; Huang, L.; He, X.; Gong, Y.; Gao, J.; Wu, H.; Man, X.; Jin, J.; et al. Bulk pancreatic cancer cells can convert into cancer stem cells(CSCs) in vitro and 2 compounds can target these CSCs. Cell Cycle 2015, 15, 403–412. [Google Scholar] [CrossRef]
- Akbari, A.; Sedaghat, M.; Heshmati, J.; Tabaeian, S.P.; Dehghani, S.; Pizarro, A.B.; Rostami, Z.; Agah, S. Molecular mechanisms underlying curcumin-mediated microRNA regulation in carcinogenesis; focused on gastrointestinal cancers. Biomed. Pharmacother. 2021, 141, 111849. [Google Scholar] [CrossRef]
- Li, L.; Aggarwal, B.B.; Shishodia, S.; Abbruzzese, J.; Kurzrock, R. Nuclear Factor-KappaB and IkappaB Kinase Are Constitutively Active in Human Pancreatic Cells, and Their down-Regulation by Curcumin (Diferuloylmethane) Is Associated with the Suppression of Proliferation and the Induction of Apoptosis. Cancer 2004, 101, 2351–2362. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead Box O1 and inhibition of the PI3K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef]
- Yoshida, K.; Toden, S.; Ravindranathan, P.; Han, H.; Goel, A. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the LncRNA PVT1 expression. Carcinogenesis 2017, 38, 1036–1046. [Google Scholar] [CrossRef]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jia, X.; Gao, Y.; Zhang, Q. Chaetospirolactone reverses the apoptotic resistance towards TRAIL in pancreatic cancer. Biochem. Biophys. Res. Commun. 2018, 495, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Ha, J.; Kim, J.; Cho, Y.; Ahn, J.; Cheon, C.; Kim, S.-H.; Ko, S.-G.; Kim, B. Natural Products for pancreatic cancer treatment: From traditional medicine to modern drug discovery. Nutrients 2021, 13, 3801. [Google Scholar] [CrossRef]
- Luan, Y.-P.; Li, Q.-F.; Wu, S.-G.; Mao, D.-C.; Deng, Y.-Y.; Chen, R.-W. Tsoong induces apoptosis and inhibits proliferation, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol. Med. Rep. 2018, 17, 3527–3536. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Lee, K.; Kim, M.K.; Lee, K.M.; Shin, Y.C.; Cho, S.-G.; Ko, S.-G. DSGOST inhibits tumor growth by blocking VEGF/VEGFR2-activated angiogenesis. Oncotarget 2016, 7, 21775–21785. [Google Scholar] [CrossRef]
- Koul, M.; Meena, S.; Kumar, A.; Sharma, P.R.; Singamaneni, V.; Riyaz-Ul-Hassan, S.; Hamid, A.; Chaubey, A.; Prabhakar, A.; Gupta, P.; et al. Secondary metabolites from endophytic fungus Penicillium pinophilum induce ROS-mediated apoptosis through mitochondrial pathway in pancreatic cancer cells. Planta Med. 2016, 82, 344–355. [Google Scholar] [CrossRef]
- Torphy, R.J.; Fujiwara, Y.; Schulick, R.D. Pancreatic cancer treatment: Better, but a long way to go. Surg. Today 2020, 50, 1117–1125. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Xiao, R.; Ma, J.; Tang, Y.; Chen, W.; Zhang, R.; Jiang, L.; Chen, H.; Shen, B.; et al. Synthesis and anti-tumor activity of nitrogen-containing derivatives of the natural product diphyllin. Eur. J. Med. Chem. 2022, 243, 114708. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Y.; Gao, J.; Zhong, K.; Wei, H.; Li, K.; Tang, M.; Zhao, X.; Liu, X.; Nie, C.; et al. Diosgenin Exhibits tumor suppressive function via down-regulation of EZH2 in pancreatic cancer cells. Cell Cycle 2019, 18, 1745–1758. [Google Scholar] [CrossRef]
- Semwal, P.; Painuli, S.; Abu-Izneid, T.; Rauf, A.; Sharma, A.; Daştan, S.D.; Kumar, M.; Alshehri, M.M.; Taheri, Y.; Das, R.; et al. Diosgenin: An updated pharmacological review and therapeutic perspectives. Oxid. Med. Cell Longev. 2022, 2022, 1035441. [Google Scholar] [CrossRef]
- Wang, W.; Luo, J.; Liang, Y.; Li, X. Echinacoside suppresses pancreatic adenocarcinoma cell growth by inducing apoptosis via the mitogen-activated protein kinase pathway. Mol. Med. Rep. 2016, 13, 2613–2618. [Google Scholar] [CrossRef]
- Long, J.; Liu, Z.; Hui, L. Anti-Tumor Effect and Mechanistic Study of Elemene on Pancreatic Carcinoma. BMC Complement. Altern. Med. 2019, 19, 133. [Google Scholar] [CrossRef]
- Hou, S.; Li, Z.; Chen, X.; Wang, W.; Duan, T.; Scampavia, L.; Yuan, Y.; Spicer, T.P.; Chen, X.; Xie, T. Elemene Sensitizes pancreatic cancer cells to bortezomib by enhancing proteasome inhibition via molecular patch mechanism. Sig. Transduct. Target Ther. 2023, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Wang, J.; Li, X.; Lu, W.; Yang, J.; Hu, Y.; Huang, P.; Wen, S. New mild and simple approach to isothiocyanates: A class of potent anticancer agents. Molecules 2017, 22, 773. [Google Scholar] [CrossRef]
- De Dosso, S.; Siebenhüner, A.R.; Winder, T.; Meisel, A.; Fritsch, R.; Astaras, C.; Szturz, P.; Borner, M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102180. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, H.; Mizushina, Y.; Yoshida, K.; Ejima, Y.; Mukumoto, N.; Wang, T.; Inubushi, S.; Nakayama, M.; Wakahara, Y.; Sasaki, R. MGDG extracted from spinach enhances the cytotoxicity of radiation in pancreatic cancer cells. Radiat. Oncol. 2016, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Zhou, Y.; Jiang, H.; Pan, S.; Sun, B. Piperlongumine suppresses growth and sensitizes pancreatic tumors to gemcitabine in a xenograft mouse model by modulating the NF-kappa B pathway. Cancer Prev. Res. (Phila) 2016, 9, 234–244. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Zhang, W.; Zhang, H.; Miao, Z.; Zhuang, C. Radiosensitization of human pancreatic cancer by piperlongumine analogues. Chin. Chem. Lett. 2021, 32, 1197–1201. [Google Scholar] [CrossRef]
- Karki, K.; Hedrick, E.; Kasiappan, R.; Jin, U.-H.; Safe, S. Piperlongumine induces reactive oxygen species (ROS)-dependent downregulation of specificity protein transcription factors. Cancer Prev. Res. (Phila) 2017, 10, 467–477. [Google Scholar] [CrossRef]
- Cheng, S.; Castillo, V.; Sliva, D. CDC20 associated with cancer metastasis and novel mushroom-derived CDC20 inhibitors with antimetastatic activity. Int. J. Oncol. 2019, 54, 2250–2256. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.; Lee, Y.G.; Kang, H.J.; Lee, S.-H.; Park, S.Y.; Min, J.-K.; Lee, S.-R.; Chung, S.J. Identification of sennoside A as a novel inhibitor of the slingshot (SSH) family proteins related to cancer metastasis. Pharmacol. Res. 2017, 119, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Fu, Y.; Han, Q.; Ma, Y.; Ji, H.; Wei, X.; Chen, Y.; Sun, Y.; Gao, Y.; Wu, H. Transcriptome analysis of the inhibitory effect of sennoside a on the metastasis of hepatocellular carcinoma cells. Front. Pharmacol. 2021, 11, 566099. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, M.K.; Lee, K.; Lee, K.M.; Choi, Y.K.; Shin, Y.C.; Cho, S.-G.; Ko, S.-G. SH003 Represses tumor angiogenesis by blocking VEGF binding to VEGFR2. Oncotarget 2016, 7, 32969–32979. [Google Scholar] [CrossRef]
- He, X.; Wang, N.; Zhang, Y.; Huang, X.; Wang, Y. The therapeutic potential of natural products for treating pancreatic cancer. Front. Pharmacol. 2022, 13, 1051952. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhang, X.; Zhang, H.; Shang, H.; Bao, J.; Wang, H.; Li, Z. Sugiol (127horbar;Hydroxyabieta-8,11,13-Trien-7-One) targets human pancreatic carcinoma cells (Mia-PaCa2) by inducing apoptosis, G2/M cell cycle arrest, ROS production and inhibition of cancer cell migration. J BUON. 2018, 23, 205–210. [Google Scholar] [PubMed]
- Bajpai, V.K.; Sonwal, S.; Hwang, S.-K.; Shukla, S.; Khan, I.; Dey, D.K.; Chen, L.; Simal-Gandara, J.; Xiao, J.; Huh, Y.S.; et al. Sugiol, a diterpenoid: Therapeutic Actions and molecular pathways involved. Pharmacol. Res. 2021, 163, 105313. [Google Scholar] [CrossRef]
- Pei, Z.; Fu, W.; Wang, G. A natural product toosendanin inhibits epithelial-mesenchymal transition and tumor growth in pancreatic cancer via deactivating Akt/MTOR signaling. Biochem. Biophys. Res. Commun. 2017, 493, 455–460. [Google Scholar] [CrossRef]
- Chen, P.; Wang, M.; Wang, C. Qingyihuaji formula reverses gemcitabine resistant human pancreatic cancer through regulate LncRNA AB209630/MiR-373/EphB2-NANOG signals. Biosci. Rep. 2019, 39, BSR20190610. [Google Scholar] [CrossRef]
- LeJeune, T.M.; Tsui, H.Y.; Parsons, L.B.; Miller, G.E.; Whitted, C.; Lynch, K.E.; Ramsauer, R.E.; Patel, J.U.; Wyatt, J.E.; Street, D.S.; et al. Mechanism of action of two flavone isomers targeting cancer cells with varying cell differentiation status. PLoS ONE 2015, 10, e0142928. [Google Scholar] [CrossRef]
- Poormolaie, N.; Mohammadi, M.; Mir, A.; Asadi, M.; Kararoudi, A.N.; Vahedian, V.; Maroufi, N.F.; Rashidi, M. Xanthomicrol: Effective therapy for cancer treatment. Toxicol. Rep. 2023, 10, 436–440. [Google Scholar] [CrossRef]
- Batooie, N.; Khodaei, M.M.; Bahrami, K.; Miraghaee, S.S.; Hosseinzadeh, N.; Sajadimajd, S. One-pot synthesis of new benzo[4,5]imidazo[2,1-b]pyrimido[4,5-d][1,3] thiazine-2,4(3H)-dione and benzo[4,5]imidazo[2,1-b][1,3]thiazin-4-one derivatives as new anti-cancer components. J. Mol. Struct. 2023, 1271, 134037. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Cho, B.K.; Wood, T.K.; Lee, J.-K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 62–90. [Google Scholar] [CrossRef] [PubMed]
- Doocey, C.M.; Finn, K.; Murphy, C.; Guinane, C.M. The impact of the human microbiome in tumorigenesis, cancer progression, and biotherapeutic development. BMC Microbiol. 2022, 22, 53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Patel, S.K.S.; Kharga, K.; Kumar, R.; Kumar, P.; Pandohee, J.; Kulshresha, S.; Harjai, K.; Chhibber, S. Molecular mechanisms and applications of N-acyl homoserine lactone mediated quorum sensing in bacteria. Molecules 2022, 27, 7584. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Patel, S.K.S.; Cho, B.K.; Wood, T.K.; Lee, J.-K. Emerging applications of bacteria as antitumor agents. Semin. Cancer Biol. 2022, 86, 1014–1025. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; You, L.; Yang, J.; Feng, M.; Qiu, J.; Zhao, F.; Liu, Y.; Cao, Z.; Zheng, L. Role of the Microbiome in occurrence, development and treatment of pancreatic cancer. Mol. Cancer 2019, 18, 173. [Google Scholar] [CrossRef]
- Miyabayashi, K.; Ijichi, H.; Fujishiro, M. The role of the microbiome in pancreatic cancer. Cancers 2022, 14, 4479. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, D. The huge clinical potential of microbiota in the treatment of pancreatic cancer: The next frontier. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188733. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Dong, S.; Xu, H.; Zhou, W. Association of the microbiota and pancreatic cancer: Opportunities and limitations. Front. Immunol. 2022, 13, 844401. [Google Scholar] [CrossRef]
- Memba, R.; Duggan, S.N.; Chonchubhair, H.M.N.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.M. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatology 2017, 17, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-B.; Meng, J.; Zhang, Q.-X.; Kang, T.-T.; Lu, R.-R. Protective effect of surface layer proteins isolated from four Lactobacillus strains on hydrogen-peroxide-induced HT-29 cells oxidative stress. Int. J. Biol. Macromol. 2017, 102, 76–83. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Oldani, M.; Forcella, M.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Synergistic antioxidant effect of prebiotic ginseng berries extract and probiotic strains on healthy and tumoral colorectal cell lines. Int. J. Mol. Sci. 2023, 24, 373. [Google Scholar] [CrossRef]
- Manilla, V.; Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Endotoxemia and gastrointestinal cancers: Insight into the mechanisms underlying a dangerous relationship. Microorganisms 2023, 11, 267. [Google Scholar] [CrossRef]
- Udayasuryan, B.; Ahmad, R.N.; Nguyen, T.T.D.; Umaña, A.; Monét Roberts, L.; Sobol, P.; Jones, S.D.; Munson, J.M.; Slade, D.J.; Verbridge, S.S. Fusobacterium nucleatum induces proliferation and migration in pancreatic cancer cells through host autocrine and paracrine signaling. Sci. Signal. 2022, 15, eabn4948. [Google Scholar] [CrossRef]
- Nista, E.C.; Del Gaudio, A.; Del Vecchio, L.E.; Mezza, T.; Pignataro, G.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Pancreatic cancer resistance to treatment: The role of microbiota. Biomedicines 2023, 11, 157. [Google Scholar] [CrossRef]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Diet, gut microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 420–429. [Google Scholar] [CrossRef]
- Kharga, K.; Kumar, L.; Patel, S.K.S. Recent advances in monoclonal antibody-based approaches in the management of bacterial sepsis. Biomedicines 2023, 11, 765. [Google Scholar] [CrossRef]
- Hullar, M.A.J.; Burnett-Hartman, A.N.; Lampe, J.W. Gut microbes, diet, and cancer. Cancer Treat. Res. 2014, 159, 377–399. [Google Scholar]
- Jain, D.; Chaudhary, P.; Varshney, N.; Janmeda, P. Carcinogenic effects of N-nitroso compounds in the environment. Environ. Conserv. J. 2020, 21, 25–41. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, X.; Yin, M.; Gao, J.; Weng, Z.; Xu, C. The relationship between helicobacter pylori and pancreatic cancer: A meta-analysis. Transl. Cancer Res. 2022, 11, 2810. [Google Scholar] [CrossRef]
- Sexton, R.E.; Uddin, M.H.; Bannoura, S.; Khan, H.Y.; Mzannar, Y.; Li, Y.; Aboukameel, A.; Al-Hallak, M.N.; Al-Share, B.; Mohamed, A. Connecting the human microbiome and pancreatic cancer. Cancer Metastasis Rev. 2022, 41, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Daley, D. The Role of the microbiome in pancreatic oncogenesis. Int. Immunol. 2022, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, G.; Sakandar, H.A.; Kwok, L.-Y.; Sun, Z. Gut dysbiosis in pancreatic diseases: A causative factor and a novel therapeutic target. Front. Nutr. 2022, 9, 814269. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Osborn, M.J.; Belanto, J.J.; Tolar, J.; Voytas, D.F. Gene editing and its application for hematological diseases. Int. J. Hematol. 2016, 104, 18–28. [Google Scholar] [CrossRef]
- Watanabe, S.; Shimada, S.; Akiyama, Y.; Ishikawa, Y.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; Kudo, A.; et al. Loss of KDM6A characterizes a poor prognostic subtype of human pancreatic cancer and potentiates HDAC inhibitor lethality. Int. J. Cancer 2019, 145, 192–205. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Forte, G.; Dal Piaz, F.; Parente, L.; Petrella, A. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of formyl peptide receptor pathway. Sci. Rep. 2016, 6, 29660. [Google Scholar] [CrossRef]
- Barkeer, S.; Chugh, S.; Karmakar, S.; Kaushik, G.; Rauth, S.; Rachagani, S.; Batra, S.K.; Ponnusamy, M.P. Novel role of O-glycosyltransferases GALNT3 and B3GNT3 in the self-renewal of pancreatic cancer stem cells. BMC Cancer 2018, 18, 1157. [Google Scholar] [CrossRef] [PubMed]
- Pessolano, E.; Belvedere, R.; Bizzarro, V.; Franco, P.; Marco, I.D.; Porta, A.; Tosco, A.; Parente, L.; Perretti, M.; Petrella, A. Annexin A1 may induce pancreatic cancer progression as a key player of extracellular vesicles effects as evidenced in the in vitro MIA PaCa-2 model system. Int. J. Mol. Sci. 2018, 19, 3878. [Google Scholar] [CrossRef]
- Yuza, K.; Nakajima, M.; Nagahashi, M.; Tsuchida, J.; Hirose, Y.; Miura, K.; Tajima, Y.; Abe, M.; Sakimura, K.; Takabe, K.; et al. Different roles of sphingosine kinase 1 and 2 in pancreatic cancer progression. J. Surg. Res. 2018, 232, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.; Barkeer, S.; Rachagani, S.; Nimmakayala, R.K.; Perumal, N.; Pothuraju, R.; Atri, P.; Mahapatra, S.; Thapa, I.; Talmon, G.A.; et al. Disruption of C1galt1 gene promotes development and metastasis of pancreatic adenocarcinomas in mice. Gastroenterology 2018, 155, 1608–1624. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, H.; Xiao, T.; Cong, L.; Love, M.I.; Zhang, F.; Irizarry, R.A.; Liu, J.S.; Brown, M.; Liu, X.S. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 2014, 15, 554. [Google Scholar] [CrossRef]
- Hart, T.; Moffat, J. BAGEL: A computational framework for identifying essential genes from pooled library screens. BMC Bioinform. 2016, 17, 164. [Google Scholar] [CrossRef]
- Wang, B.; Krall, E.B.; Aguirre, A.J.; Kim, M.; Widlund, H.R.; Doshi, M.B.; Sicinska, E.; Sulahian, R.; Goodale, A.; Cowley, G.S.; et al. ATXN1L, CIC, and ETS transcription factors modulate sensitivity to MAPK pathway inhibition. Cell Rep. 2017, 18, 1543–1557. [Google Scholar] [CrossRef]
- Song, M. The CRISPR/Cas9 system: Their delivery, in vivo and ex vivo applications and clinical development by startups. Biotechnol. Prog. 2017, 33, 1035–1045. [Google Scholar] [CrossRef]
| Molecules/Biomolecules | Mechanism | Remarks/Application | Reference |
|---|---|---|---|
| miR-203 | DUSP5 downregulating expression | Proliferation, migration, and colony-forming potential of PANC-1 inhibition | [60] |
| Piperazine-tethered phthalazines | Selective CDK1 inhibitors | Potential activity against pancreatic cancer adenocarcinoma: MDA-PATC53 (IC50 = 0.51–0.88 μM), and PL45 (IC50 = 0.74–1.14 μM), and CDK1 inhibitory activity with IC50 of 36.8–44.5 nM | [24] |
| 1-Piperazinylphthalazines | VEGFR-2 inhibitors | IC50 of 0.30–0.40 μM for compounds 16 k and 21 d towards VEGFR-2 | [63] |
| Oncolytic adenovirus OBP-702 | p53 overexpression | Induces ICD and antitumor immune responses in PDAC with distinct p53 status | [64] |
| Chemically modified MIR143-3p | Suppressing the entire RAS network | MIR143#12 IC50 values of 63.25 and 4.74 nM at 72 and 96 h of incubation against Panc-1, respectively | [65] |
| Carrier-free prodrug nanoparticles (F68-FDOX) | Cytotoxicity against cancer cells | High drug loading above 50%, and showed a broad therapeutic spectrum against colon, breast, and pancreatic cancers | 66] |
| Dextran-coated maghemite nanoparticles | Alteration in heat shock proteins (HSPs) and p53 protein expression | Nanoparticles (56 μg/mL) exposure reduced 50% of PANC-1 cell viability after 72 h | [67] |
| Lipid nanoparticles (LNPDTX-P) on gold nanoparticles | Enhanced uptake of LNPDTX-P by pancreatic cancer cells, and exhibtes synergetic radiosensitization effects | 2-fold enhancement in nanoparticles uptake by LNPDTX-P-treated tumour cells compared to pure nanoparticles | [68] |
| Magnetic nanoparticles and hyperthermia | Increase DNA double strand breaks by radiosensitization effects and ROS production | The combined treatment of magnetic nanoparticles and hyperthermia enhanced cell death at 6 h in BxPC3 pancreatic tumor cells compared to irradiation or nanoparticles adminstration | [69] |
| Polyethyleneglycol-betulinic acid (PEG-BA) polymer-drug conjugate | Induces apoptosis mediated death of MIA-PaCa-2 by arresting sub-G1phase of cell cycl and involves in anti-inflammatory and antioxidant activities | PEG-BA (4 μM) treated cells showed over-expression of the proapoptotic genes TNF (23.7-fold) and CASPASE 3 (12,060-fold), and exhibits IC50 of 15.6 μM compared to BA-only of >100 μM for antioxidant potential | [70] |
| S-1 adjuvant chemotherapy | Total dose intensity-derived survival prediction | Maintenance of dose intensity >60% in S-1 adjuvant chemotherapy improves survival of pancreatic cancer patients | [71] |
| Protein aggregate magnesium-ammonium phospholinoleate-palmitoleate anhydride | Suppression of abnormal cell proliferation altering TLR4 signaling pathway | P-MAPA-based showed histopathological repair in 40% of rats, and P-MAPA/gemcitabine-associated treatment was effective in reducing neoplastic lesion progression, and enabling histopathological improvement in 80% of rats | [19] |
| LSKL peptide | Thrombospondin-1 (TSP-1) inhibitors | TSP-1 promotes Smad4 expression deficiency, and malignant potential by activation of TGF-β signal in PDAC | [72] |
| Curcumin | Sensitization pf cancer cells to gemcitabine by assuaging expressions of PRC2 subunit EZH2, and lncRNA PVT1 and overcoming drug-resisance | IC50 value 8 and 20 µM for curcumin against BxPC3 and Panc1 cells, respectively | [74,80,81] |
| Chaetospirolactone | Induction of apoptosis by upregulating apoptotic proteins such as c-caspases (3, 8, and 9), and downregulating EZH2 gene | 100 nM CSL treatment for 24 h reverses TRAIL resistance in PANC-1 cells via epigenetic regulation of DR4 | [80,83] |
| Cordifoliketones A | Apoptosis induction through upregulating Bad, Bax, and caspases (3, 8, and 9), and downregulating oncogenes (Bcl-2, and Bcl-xL) | Minimum IC50 of 4.18 μg/mL of cordifoliketones A is required for the maximum inhibition of cell growth in PANC-1 AsPC-1, BxPC-3 and PANC-1 after 48 h of treatment | [83,84] |
| Danggui-Sayuk-GaOsuyu-SaenggangTang (DSGOST) | Inhibition of migration, and tube formation by upregulating caspage-3 and downregulating p-IKKα/β, p-IκBα, p-NF-κB, p-AKT, p-VEGFR2, p-FAK, p-SRC, and MMP-9 | DSGOST at dose-100 µg/mL inhibited maximum cell growth after 2 h of tratement in PANC-1 | [83,85] |
| Dicatenarin | Induces apoptosis by inducing reactive oxygen species and increased induction of caspase-3 | IC50 values of 12 µg/mL against MIA PaCa-2 cell line | [86,87] |
| Diphyllin derivatives (amino derivative 15) | Cell cycle arrests at G0/G1 phase | 69-fold more potent than diphyllin with IC50 of 3 nM against pancreatic cancer CFPAC-1 cells | [88] |
| Diosgenin | Inhibition of tumor growth by upregulating tumor suppressor PTEN and downregulating oncogene EZH2, and vimentin using Patu8988 and Panc-1 cell lies | 75 μg/mL diosgenis for 72 h suppreses upto 70% growth of Patu8988 and Panc-1 cell lies | [89,90] |
| Echinacoside | Induction of apoptosis by upregulating ROS, Bax, p38 and downregulating MMP, JNK, and ERK1/2 in SW1990 cell lines | Maximum growth inhibition was observed at 72 h using 100 µM of echinacoside for SW1990 cell lines | [83,91] |
| Elemene | Cell proliferations inhibition, and cell cycle arrests by upregulating tumor suppressor gene p53 and down regulating oncogene Bcl-2 | 60 µg/mL od elemene induces more than 85% of cell death after 72 h of tratment in BxPC-3 and Panc-1 cell lines | [92,93] |
| Methyl4-(2-isothiocyanatoethyl)benzoate | Apoptosis induction by upregulating ROS and downregulating oncogene glutathione (GSH) | Maximum appoptosis at dose 10 µM methyl4-(2-isothiocyanatoethyl)benzoate after 72 h treatment in Panc1 and Capan2 cell lines | [94,95] |
| Monogalactosyl diacylglycerol (MGDG) | Apoptosis induction by upregulating cytochrome c, c-PARP, Bax, and c-caspase-3, and downregulating Bcl-2 | Maximum cell death was observed at IC50 of 25.6 in PANC-1, BxPC-3, MIAPaCa-2 and AsPC-1 cells for 72 h treatment | [83,96] |
| Piperlongumine | Apoptosis induction by upregulating procaspase-3, c-PARP, and downregulating Bcl-2, Bcl-xL, survivin, XIAP, miR-27a, and miR-17/miR-20a | Piperlongumine at dose 40 µmol/L inhibit maximum cell growth in PANC-1 after 72 h of treatment | [97,98,99] |
| Polyporenic acid | Inhibition of metastasis by down regulating of oncogene CDC20 | Polyporenic acid at dose 60 µM, for 24 h has shwon mximum inhibiot of cell growth in PANC-1 | [83,100] |
| Sennoside A | Cells invasion and migration inhibition through downregulating p-cofilin | Sennoside A at 10 µM dose produced maximimum cytotoxicity for 20 m in pancreatic cancer cell Panc-1 | [101,102] |
| SH003 | Angiogenesis inhibition by upregulating c-caspase-3, and downregulating p-VEGFR2, MMP-9, p-FAK, p-SRC, p-ERK, p-AKT, and p-STAT3 | SH003 at dose 20 µg/mL has shown maximimun cytotoxic effects for 62 h of treatment in PANC-1 | [103,104] |
| Sugiol | Apoptosis induction, cell cycle arrest, and increase of ROS production by upregulating Bax and downregulating Bcl-2, and MMP | At IC50 of 15 μM of suigiol treatment for 48 h, suppres maximum growth of Mia-PaCa2 pancreatic cancer cells | [105,106] |
| Toosendanin | Cells invasion and migration inhibition by upregulating E-cadherin and downregulating Vimentin, ZEB1, Snail, p-AKT, p-PRAS40, p-mTOR, and p-p70S6K | Toosendanin at concentration of 200 nM, decreased 7–8 fold in the migratory capacity of PANC-1 and AsP pancreatic cancer cells, after 5 days of treatment | [104,107] |
| Qingyihuaji | Cells invasion and migration inhibition by upregulating lncRNA AB209630 and downregulating miR-373, EphB2, and Nanog | Qingyihuaji at 40 µg/L demonstarted maximum inhibition of cell growth for 72 h in CFPAC-1 cell lines | [83,108] |
| 5,7-dihydroxy-3,6,8-trimethoxyflavone (flavone A) | Apoptosis induction and cell cycle arrests by upregulating p-ERK, p-c-JUN and downregulating pS6, p-Bad, Bcl-xL, and Bcl-2 | 40 μM flavone treatment for 9 h iduced maximum appoptosis in pancreatic cancer MIA Paca-2 cell lines. | [109,110] |
| 2,2-dimethyl-5-(4-nitrophenyl)-1,2,3,12a-tetrahydro4H-benzo[d]benzo[4,5]imidazo[2,1-b][1,3]thiazine-4-one | Cytotoxicity against cancer cells | IC50 values of 21.59 µM against Panc1 compared to etoposide (25.19 µM) | [111] |
| 1,3-dimethyl-5-(p-tolyl)-1,12a-dihydro-2H-benzo[4,5]imidazo[2,1-b]pyrimido[4,5-d][1,3]thiazine-2,4(3H)-dione | Cytotoxicity against cancer cells | IC50 values of 31.87 µM against Panc1 compared to etoposide (25.19 µM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, L.; Kumar, S.; Sandeep, K.; Patel, S.K.S. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines 2023, 11, 1611. https://doi.org/10.3390/biomedicines11061611
Kumar L, Kumar S, Sandeep K, Patel SKS. Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines. 2023; 11(6):1611. https://doi.org/10.3390/biomedicines11061611
Chicago/Turabian StyleKumar, Lokender, Sanjay Kumar, Kumar Sandeep, and Sanjay Kumar Singh Patel. 2023. "Therapeutic Approaches in Pancreatic Cancer: Recent Updates" Biomedicines 11, no. 6: 1611. https://doi.org/10.3390/biomedicines11061611
APA StyleKumar, L., Kumar, S., Sandeep, K., & Patel, S. K. S. (2023). Therapeutic Approaches in Pancreatic Cancer: Recent Updates. Biomedicines, 11(6), 1611. https://doi.org/10.3390/biomedicines11061611






