Microneedle-Assisted Topical Delivery of Idebenone-Loaded Bioadhesive Nanoparticles Protect against UV-Induced Skin Damage
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Manufacture of Hyperbranched Polyglycerol (HPG)
2.3. Synthesis of PLA-HPG and PLA-Cy5
2.3.1. PLA-HPG
2.3.2. PLA-Cy5
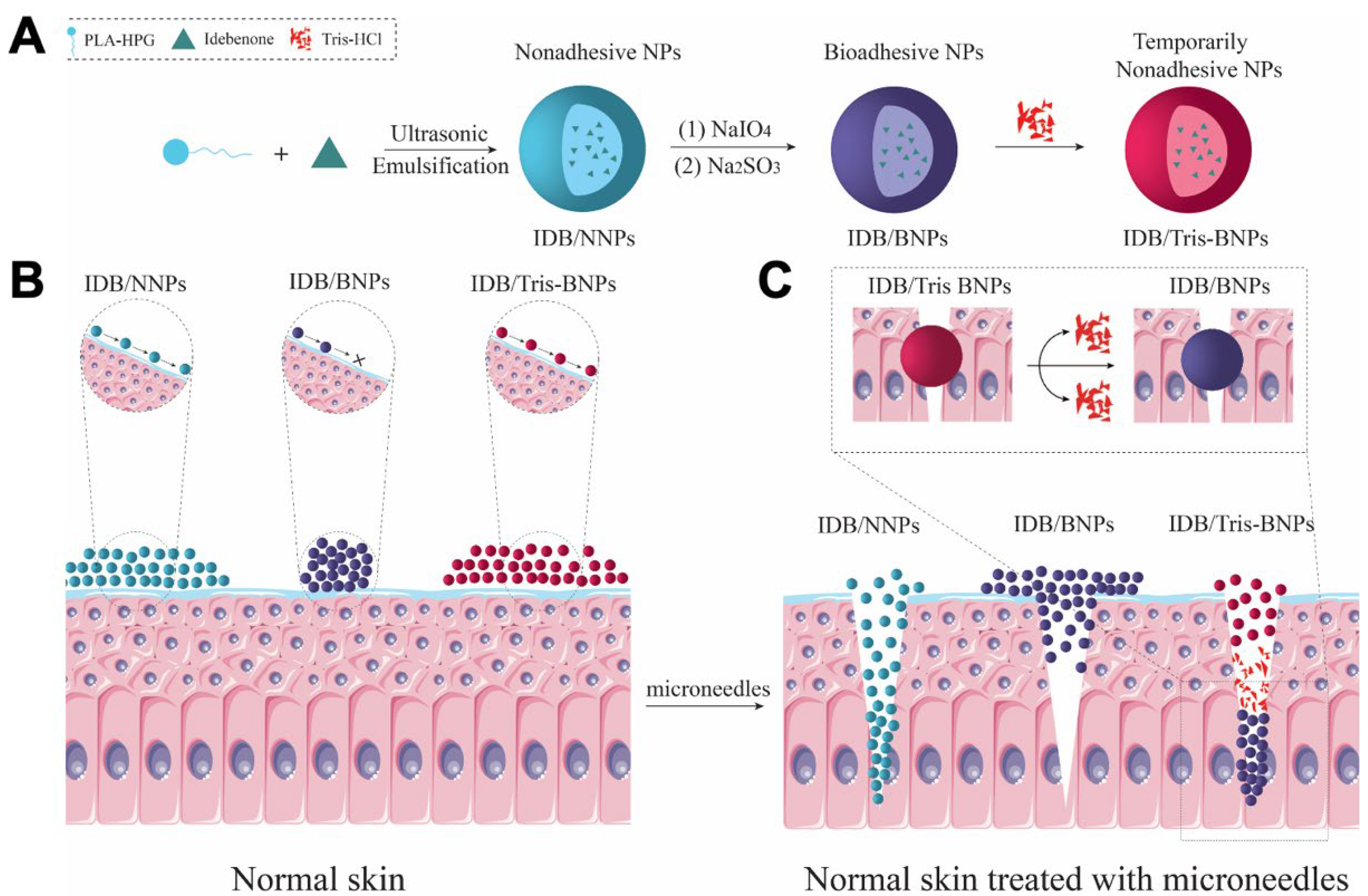
2.4. Preparation of NPs
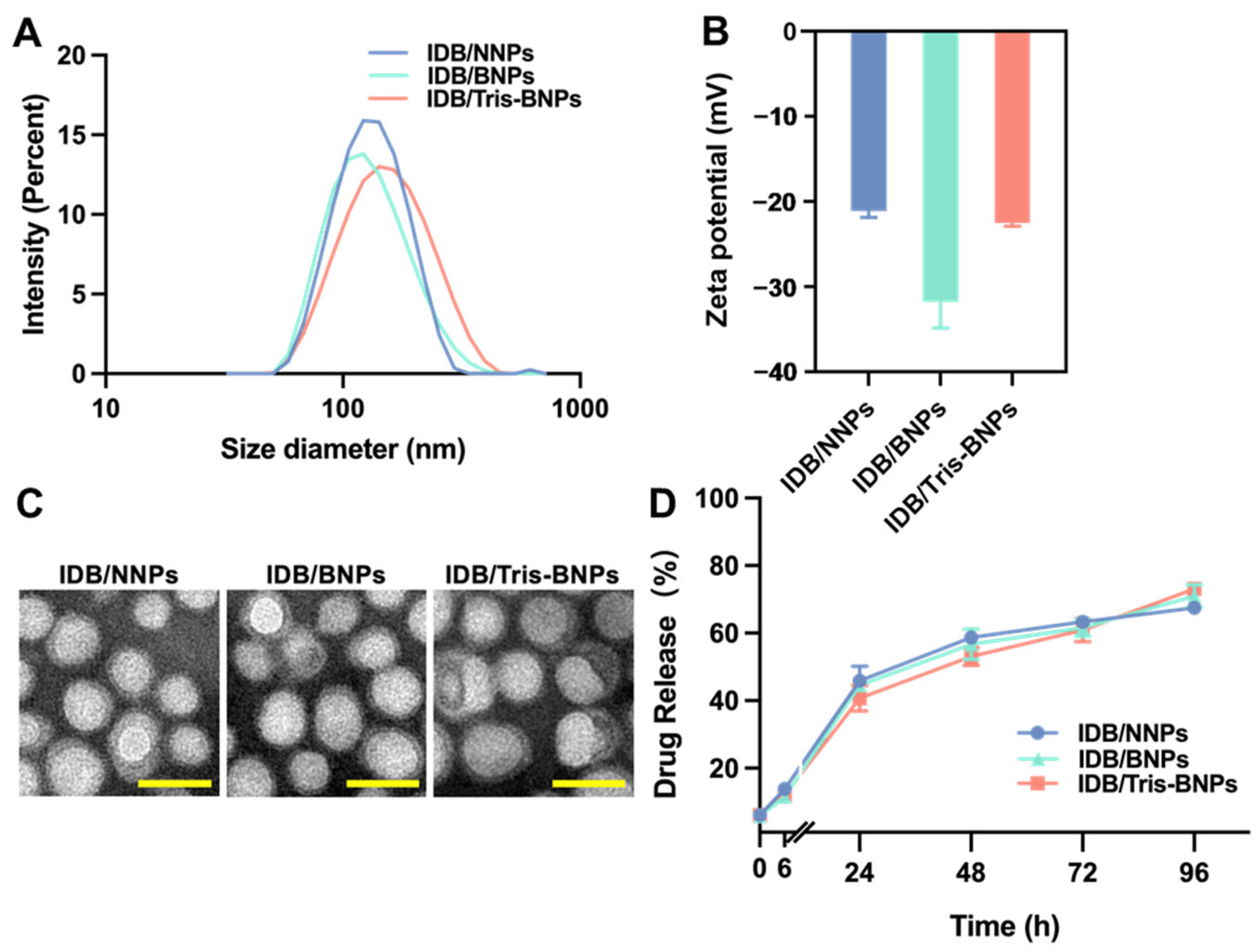
2.5. Characterization of Various NPs
2.6. In Vitro IDB Release Profile
2.7. In Vitro Cytotoxicity Evaluation of NPs
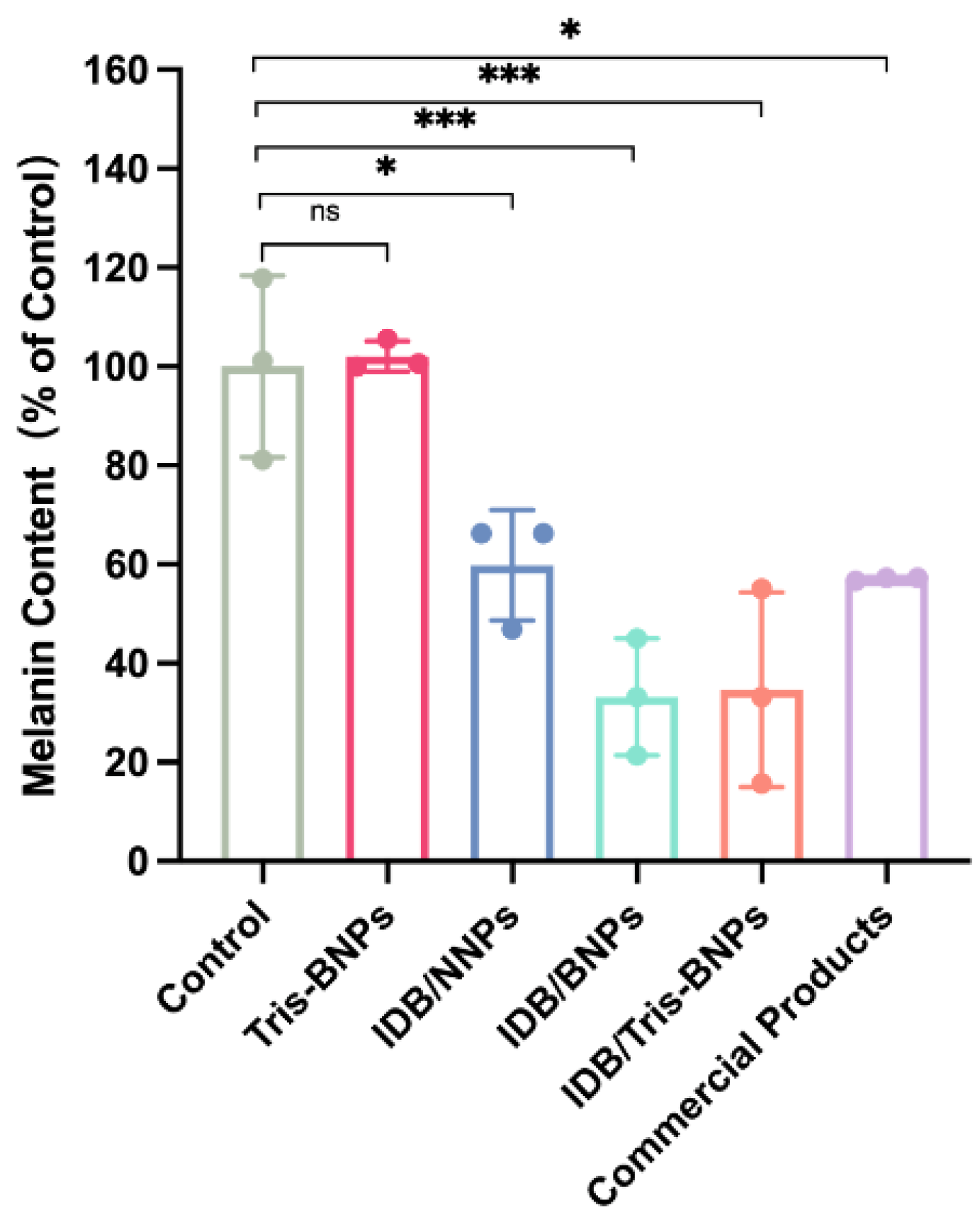
2.8. Melanogenesis Assessment of NPs
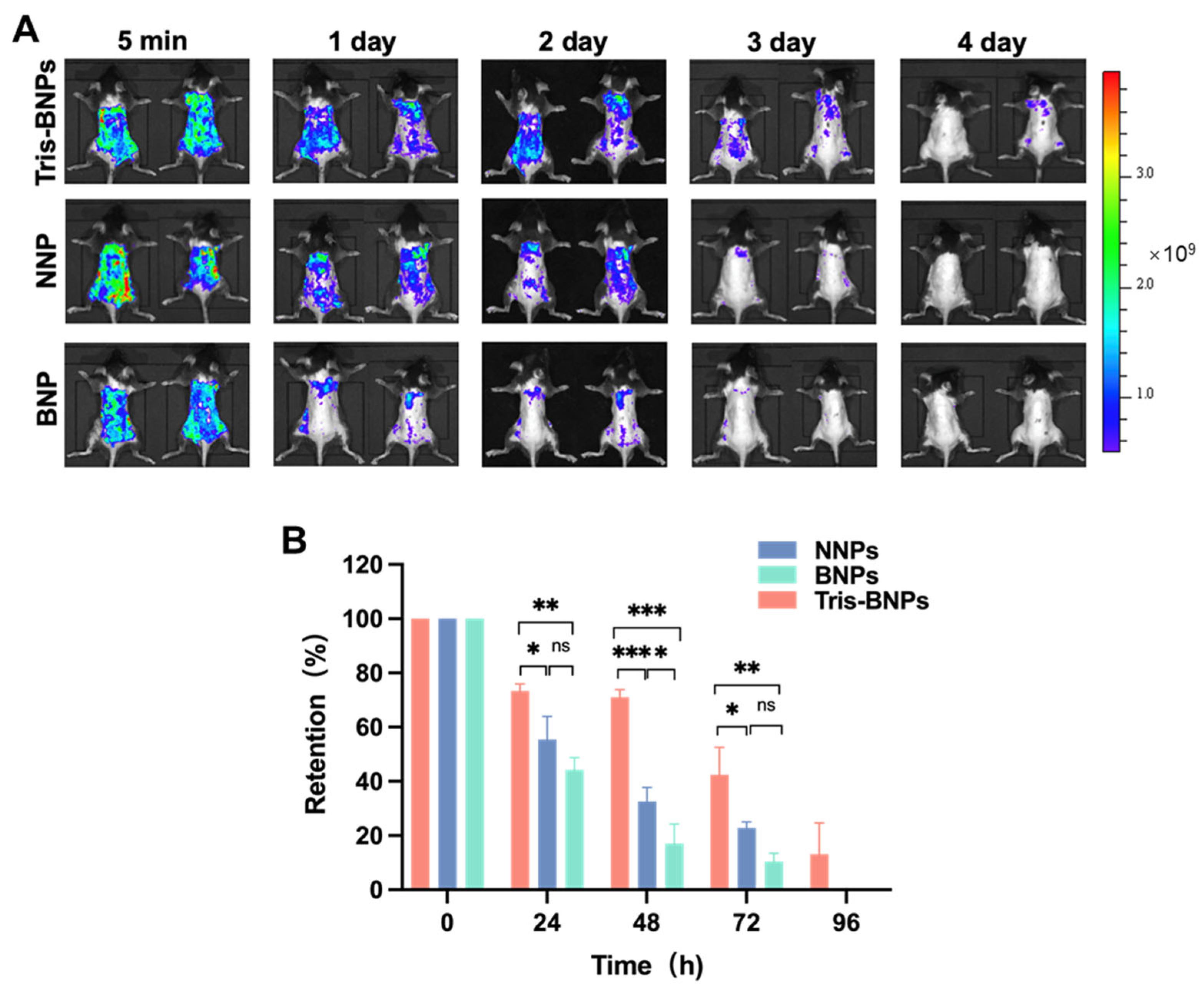
2.9. Retention and Penetration Analysis of Various NPs
2.10. Establishment of an Artificial Tanning Model
2.11. Skin-Protecting Activity of NPs
2.12. Histopathological Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of IDB/Tris-BNPs
3.2. In Vitro Cell Viability Evaluation
3.3. IDB/Tris-BNPs Reduced Melanin Synthesis after ɑ-MSH Stimulation of B16F10 Cells
3.4. Microneedle-Assisted Skin Penetration of NPs
3.5. Retention of Various NPs within Mice Skin following Pretreatment with Microneedles
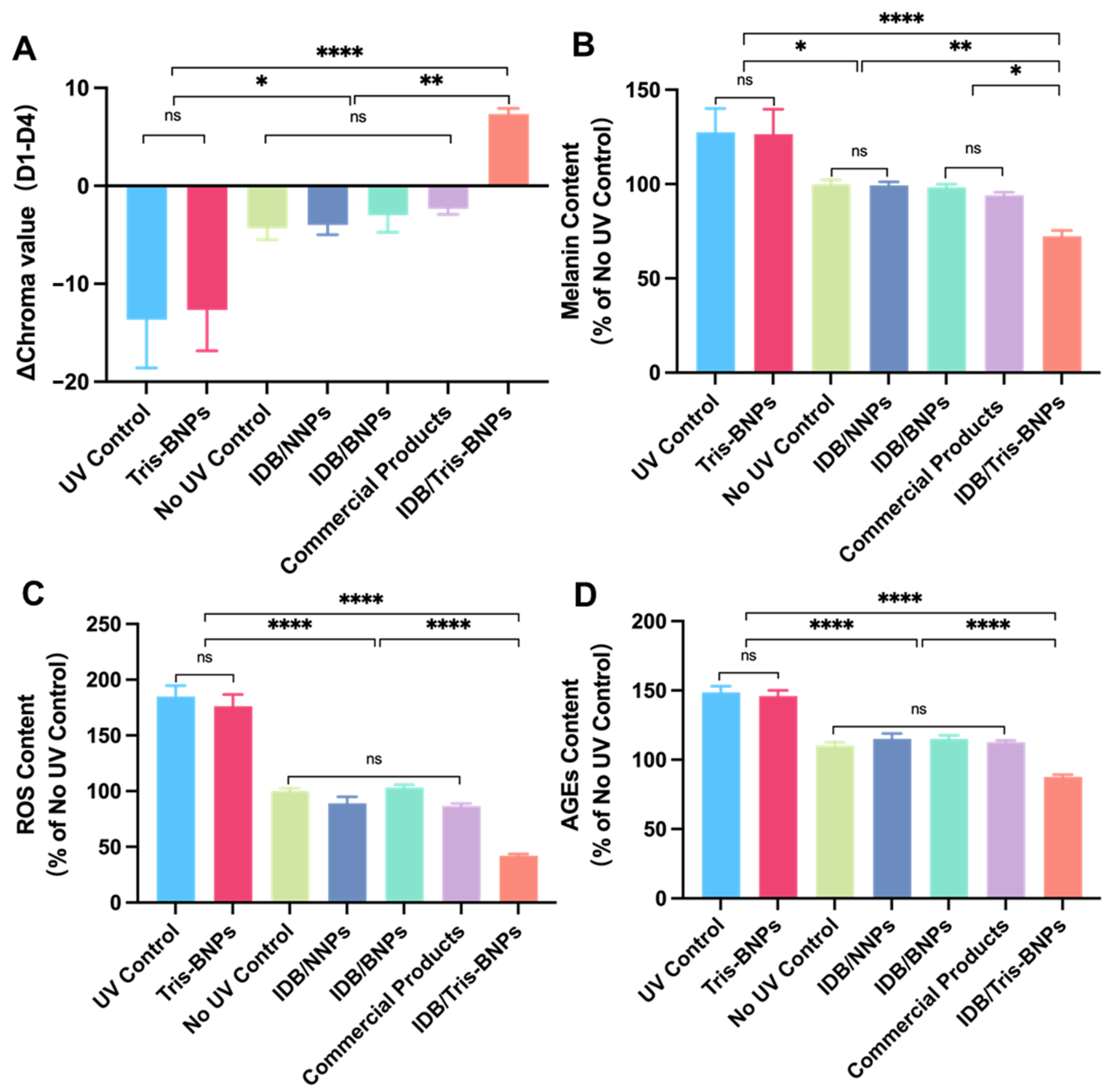
3.6. Skin Protective Effect of NPs Administered by Microneedles to C57BL/6 Mice Skin after UV Irradiation
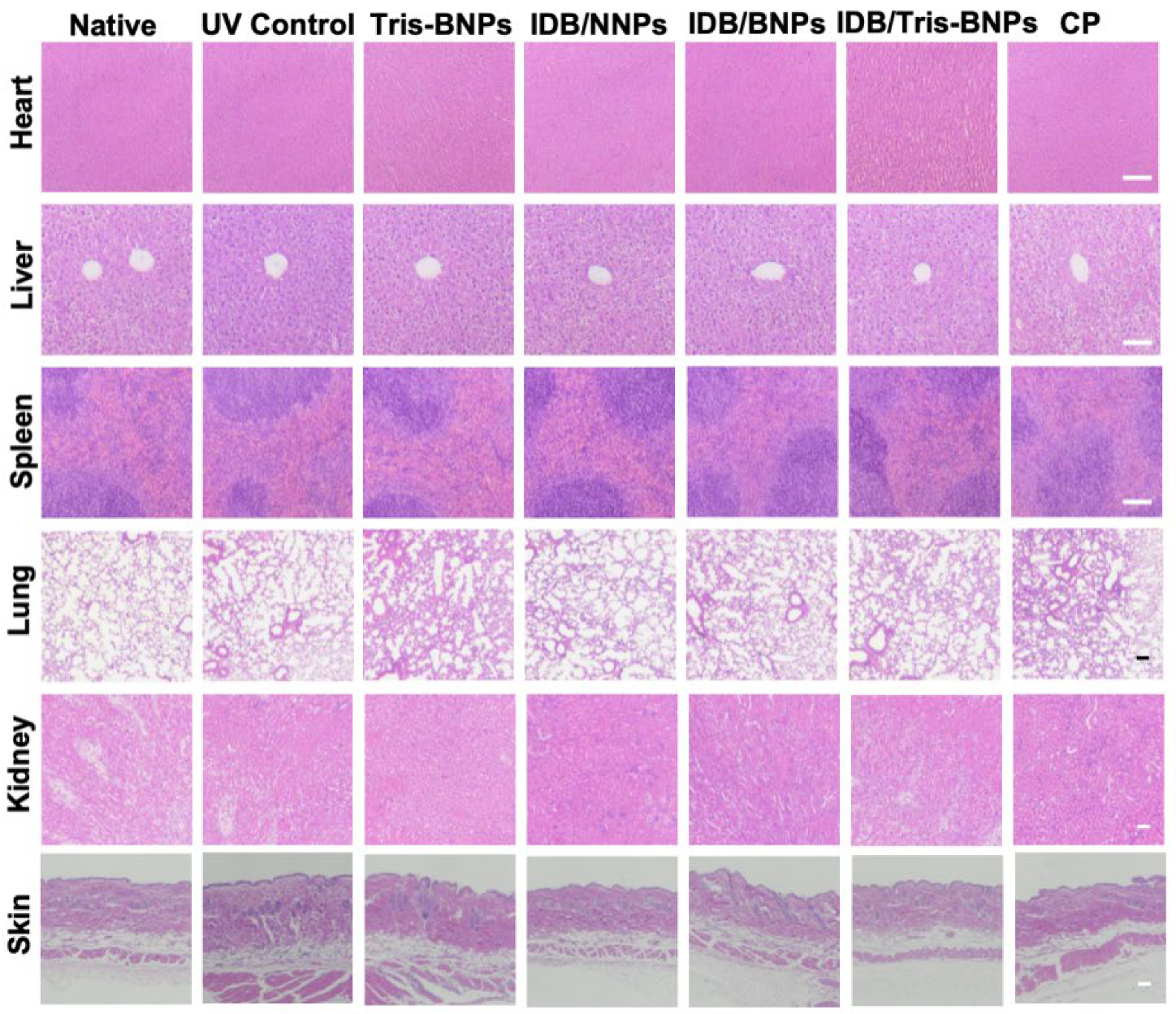
3.7. Assessments of the Toxicity of NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croce, R.; van Amerongen, H. Natural strategies for photosynthetic light harvesting. Nat. Chem. Biol. 2014, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N.; Junge, W. Structure and Energy Transfer in Photosystems of Oxygenic Photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef] [PubMed]
- Gies, P.; Roy, C.; Udelhofen, P. Solar and ultraviolet radiation. In Prevention of Skin Cancer; Springer: Berlin/Heidelberg, Germany, 2004; pp. 21–54. [Google Scholar]
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. J. Biophotonics 2018, 11, e201700377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chen, X.-Z.; Gowrisankar, Y.V.; Yen, H.-R.; Chuang, J.-Y.; Yang, H.-L. The Skin-Whitening Effects of Ectoine via the Suppression of α-MSH-Stimulated Melanogenesis and the Activation of Antioxidant Nrf2 Pathways in UVA-Irradiated Keratinocytes. Antioxidants 2020, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Yu, A.X.; Xu, M.L.; Yao, P.; Kwan, K.K.; Liu, Y.X.; Duan, R.; Dong, T.T.; Ko, R.K.; Tsim, K.W. Corylin, a flavonoid derived from Psoralea Fructus, induces osteoblastic differentiation via estrogen and Wnt/β-catenin signaling pathways. FASEB J. 2020, 34, 4311–4328. [Google Scholar] [CrossRef] [Green Version]
- Baumann, L. How to Use Oral and Topical Cosmeceuticals to Prevent and Treat Skin Aging. Facial Plast. Surg. Clin. N. Am. 2018, 26, 407–413. [Google Scholar] [CrossRef]
- Kumari, S.; Thng, S.T.G.; Verma, N.K.; Gautam, H.K. Melanogenesis Inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, D.H.; Neudecker, B.A.; DiNardo, J.C.; Lewis, J.A., 2nd; Maibach, H.I. Clinical efficacy assessment in photodamaged skin of 0.5% and 1.0% idebenone. J. Cosmet. Dermatol. 2005, 4, 167–173. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, D.H.; Neudecker, B.A.; DiNardo, J.C.; Lewis, J.A., 2nd; Maibach, H.I. Idebenone: A new antioxidant—Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J. Cosmet. Dermatol. 2005, 4, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Amorim Cde, M.; Couto, A.G.; Netz, D.J.; de Freitas, R.A.; Bresolin, T.M. Antioxidant idebenone-loaded nanoparticles based on chitosan and N-carboxymethylchitosan. Nanomedicine 2010, 6, 745–752. [Google Scholar] [CrossRef]
- Palumbo, M.; Russo, A.; Cardile, V.; Renis, M.; Paolino, D.; Puglisi, G.; Fresta, M. Improved Antioxidant Effect of Idebenone-Loaded Polyethyl-2-Cyanoacrylate Nanocapsules Tested on Human Fibroblasts. Pharm. Res. 2002, 19, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Liu, Z.; Song, L.; Wang, X.; Zhang, Y.; Wu, N.; Lin, J.; Liu, Y.; Liu, Z. Idebenone Alleviates Neuroinflammation and Modulates Microglial Polarization in LPS-Stimulated BV2 Cells and MPTP-Induced Parkinson’s Disease Mice. Front. Cell. Neurosci. 2019, 12, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Ediriwickrema, A.; Yang, F.; Lewis, J.; Girardi, M.; Saltzman, W.M. A sunblock based on bioadhesive nanoparticles. Nat. Mater. 2015, 14, 1278–1285. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Yang, F.; Cocco, E.; Song, E.; Zhang, J.; Cui, J.; Mohideen, M.; Bellone, S.; Santin, A.D.; Saltzman, W.M. Improved i.p. drug delivery with bioadhesive nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 11453–11458. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Gaudin, A.; King, A.R.; Seo, Y.-E.; Suh, H.-W.; Deng, Y.; Cui, J.; Tietjen, G.T.; Huttner, A.; Saltzman, W.M. Surface chemistry governs cellular tropism of nanoparticles in the brain. Nat. Commun. 2017, 8, 15322. [Google Scholar] [CrossRef] [Green Version]
- Mohideen, M.; Quijano, E.; Song, E.; Deng, Y.; Panse, G.; Zhang, W.; Clark, M.R.; Saltzman, W.M. Degradable bioadhesive nanoparticles for prolonged intravaginal delivery and retention of elvitegravir. Biomaterials 2017, 144, 144–154. [Google Scholar] [CrossRef]
- Thavarajah, R.; Mudimbaimannar, V.K.; Elizabeth, J.; Rao, U.K.; Ranganathan, K. Chemical and physical basics of routine formaldehyde fixation. J. Oral Maxillofac. Pathol. 2012, 16, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Mai, Y.; Ouyang, Y.; Yu, M.; Qin, Y.; Girardi, M.; Saltzman, W.M.; Cocco, E.; Zhao, C.; Yu, L.; Jia, Y.; et al. Topical formulation based on disease-specific nanoparticles for single-dose cure of psoriasis. J. Control. Release 2022, 349, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.N.; Al Ramahi, R. Depigmentation and Anti-aging Treatment by Natural Molecules. Curr. Pharm. Des. 2019, 25, 2292–2312. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Microneedles for transdermal drug delivery using clay-based composites. Expert Opin. Drug Deliv. 2022, 19, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.-S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef]
- Mai, Y.; Ouyang, Y.; Qin, Y.; Jia, C.; McCoubrey, L.E.; Basit, A.W.; Nie, Y.; Jia, Y.; Yu, L.; Dou, L.; et al. Poly(lactic acid)-hyperbranched polyglycerol nanoparticles enhance bioadhesive treatment of esophageal disease and reduce systemic drug exposure. Nanoscale 2022, 14, 8418–8428. [Google Scholar] [CrossRef]
- Chan, C.-F.; Wu, C.-T.; Huang, W.-Y.; Lin, W.-S.; Wu, H.-W.; Huang, T.-K.; Chang, M.-Y.; Lin, Y.-S. Antioxidation and Melanogenesis Inhibition of Various Dendrobium tosaense Extracts. Molecules 2018, 23, 1810. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ku, H.J.; Lee, J.H.; Park, J.W. IDH2 deficiency accelerates skin pigmentation in mice via enhancing melanogenesis. Redox Biol. 2018, 17, 16–24. [Google Scholar] [CrossRef]
- Deng, Y.; Saucier-Sawyer, J.K.; Hoimes, C.J.; Zhang, J.; Seo, Y.-E.; Andrejecsk, J.W.; Saltzman, W.M. The effect of hyperbranched polyglycerol coatings on drug delivery using degradable polymer nanoparticles. Biomaterials 2014, 35, 6595–6602. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Song, W.; Wu, H.; Wang, J.; Wang, Y.; Zhang, Z.; Lv, H. Lecithins-Zein nanoparticles for antifungal treatment: Enhancement and prolongation of drug retention in skin with reduced toxicity. Int. J. Pharm. 2020, 590, 119894. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Tomar, Y.; Sharma, S.; Roy, A.; Singhvi, G. Apremilast loaded lyotropic liquid crystalline nanoparticles embedded hydrogel for improved permeation and skin retention: An effective approach for psoriasis treatment. Biomed. Pharmacother. 2023, 162, 114634. [Google Scholar] [CrossRef]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Ye, J.; Ouyang, Y.; Gong, J.; Li, C.; Deng, Y.; Mai, Y.; Liu, Y.; Deng, W. Microneedle-Assisted Topical Delivery of Idebenone-Loaded Bioadhesive Nanoparticles Protect against UV-Induced Skin Damage. Biomedicines 2023, 11, 1649. https://doi.org/10.3390/biomedicines11061649
Xie Y, Ye J, Ouyang Y, Gong J, Li C, Deng Y, Mai Y, Liu Y, Deng W. Microneedle-Assisted Topical Delivery of Idebenone-Loaded Bioadhesive Nanoparticles Protect against UV-Induced Skin Damage. Biomedicines. 2023; 11(6):1649. https://doi.org/10.3390/biomedicines11061649
Chicago/Turabian StyleXie, Yuan, Jingping Ye, Yaqi Ouyang, Jianing Gong, Chujie Li, Yang Deng, Yang Mai, Yang Liu, and Wenbin Deng. 2023. "Microneedle-Assisted Topical Delivery of Idebenone-Loaded Bioadhesive Nanoparticles Protect against UV-Induced Skin Damage" Biomedicines 11, no. 6: 1649. https://doi.org/10.3390/biomedicines11061649






