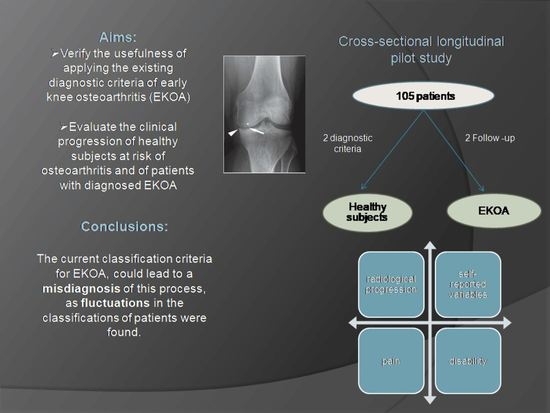
Early Knee Osteoarthritis Classification and Clinical Evolution: A Longitudinal Observational Pilot Study
Abstract
:1. Introduction
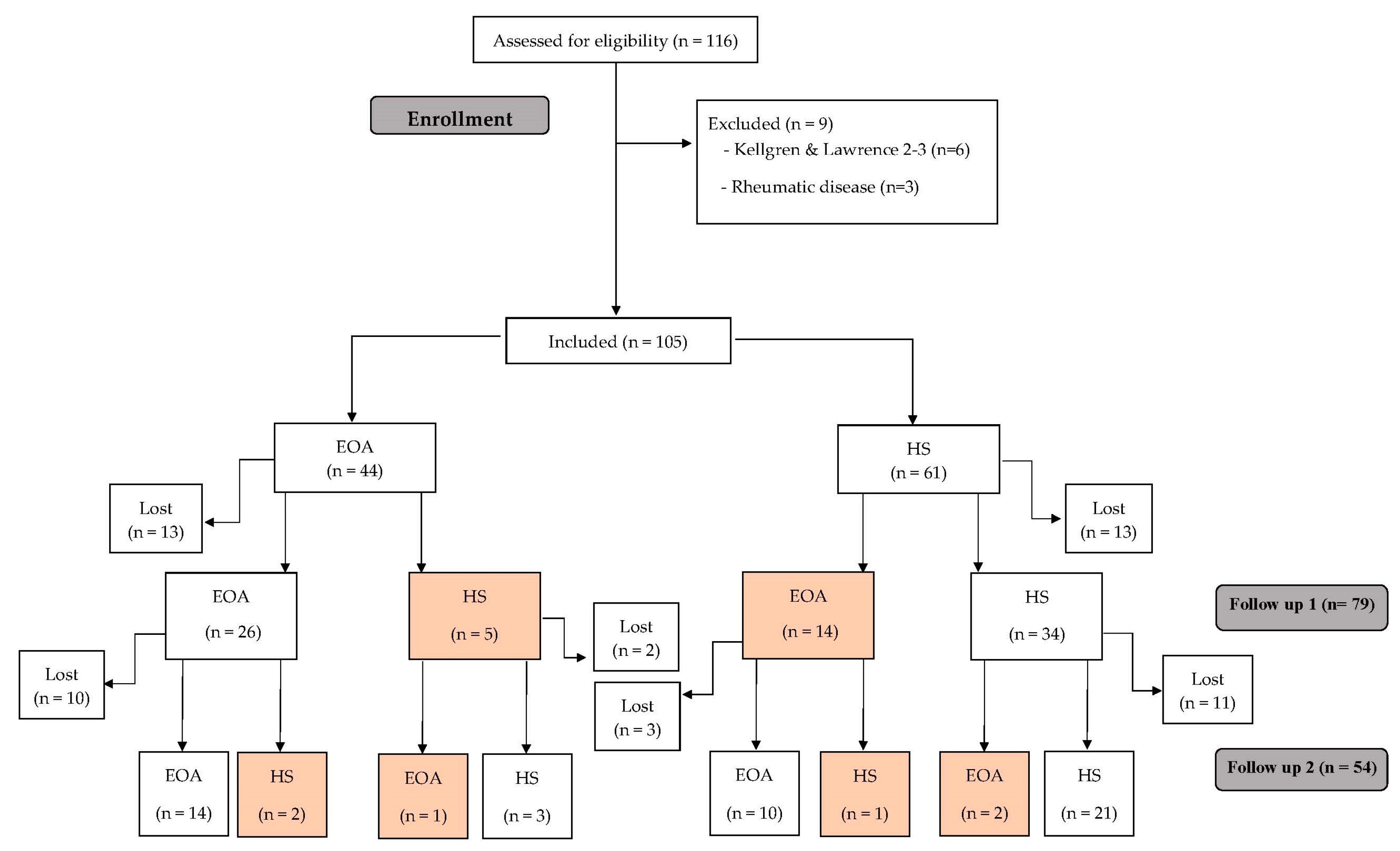
2. Materials and Methods
2.1. Study Design
2.2. Participants
- Patient-based questionnaires such as Knee Injury and Osteoarthritis Outcome score (KOOS) were used; 2 out of the 4 KOOS subscales (Symptoms, Knee pain, Function, and Knee-related quality of life) needed to score “positive” (≤85%);
- In the clinical examination, participants had to show crepitus or joint line tenderness;
- Regarding X-rays, patients with Kellgren and Lawrence (KL) grade 0–1 on standing weight-bearing radiographs (AP, lateral, AP fixed flexion, and skyline for patellofemoral OA) were included [9].
- 4.
- Patient-based questionnaires such as KOOS 4 score: the average of 4 of the 5 KOOS subscale averages, including pain, symptoms, ADL, and QOL (Pain, Symptoms, Function, and Knee-related quality of life) needed to score “positive” (≤80%);
- 5.
- In the clinical examination, participants had to show crepitus or joint line tenderness.
- 6.
- Regarding X-rays, patients with KL grade 0–1 on standing weight-bearing radiographs (AP, lateral, AP fixed flexion, and skyline for patellofemoral OA) were included.
- -
- Kellgren and Lawrence grade 0–1.
- -
- Regarding age limit, patients were required to be 40 years old or over.
- -
- Participants had to be overweight, with a body mass index of at least 25.
2.3. Outcome Measures
2.3.1. Demographic Data and Control Variables
2.3.2. Pain and Disability Variables
Pain Intensity
Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)
Knee Injury and Osteoarthritis Outcome Score (KOOS)
2.3.3. Psychological Variables
Anxiety and Depression Symptoms
2.4. Procedures
2.5. Statistical Analysis
3. Results
3.1. Evolution Classification
3.2. Clinical Progression
3.2.1. Pain Intensity
3.2.2. Disability
3.2.3. Psychological Variables
4. Discussion
4.1. Diagnosis Criteria of EOA
4.2. Clinical Progression of OA
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uchio, Y.Y. Early knee osteoarthritis—Definition, pathogenesis, diagnosis, treatment, and prevention. Ann. Jt. 2019, 4, 35. [Google Scholar] [CrossRef]
- Kanamoto, T.; Mae, T.; Yokoyama, T.; Tanaka, H.; Ebina, K.; Nakata, K. Significance and definition of early knee osteoarthritis. Ann. Jt. 2020, 5, 4. [Google Scholar] [CrossRef]
- Chiba, D.; Nakamura, T.; Fu, F.H. Early osteoarthritis—Definition, pathogenesis, diagnosis, management and prevention: Management. Ann. Jt. 2019, 4, 5. [Google Scholar] [CrossRef]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Rondanelli, M.; Braschi, V.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; Peroni, G.; Naso, M.; Iannello, G.; Spadaccini, D.; Miraglia, N.; et al. Effectiveness of Non-Animal Chondroitin Sulfate Supplementation in the Treatment of Moderate Knee Osteoarthritis in a Group of Overweight Subjects: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyten, F.P.; Denti, M.; Filardo, G.; Kon, E.; Engebretsen, L. Definition and classification of early osteoarthritis of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felson, D.T.; Hodgson, R. Identifying and treating preclinical and early osteoarthritis. Rheum. Dis. Clin. N. Am. 2014, 40, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Favero, M.; Ramonda, R.; Goldring, M.B.; Goldring, S.R.; Punzi, L. Early knee osteoarthritis. RMD Open 2015, 1, e000062. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Bierma-Zeinstra, S.; Dell’Accio, F.; Kraus, V.B.; Nakata, K.; Sekiya, I.; Arden, N.K.; Lohmander, L.S. Toward classification criteria for early osteoarthritis of the knee. Semin. Arthritis Rheum. 2018, 47, 457–463. [Google Scholar] [CrossRef]
- Mahmoudian, A.; Lohmander, L.S.; Jafari, H.; Luyten, F.P. Towards classification criteria for early-stage knee osteoarthritis: A population-based study to enrich for progressors. Semin. Arthritis Rheum. 2021, 51, 285–291. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; McWilliams, D.F.; Ingham, S.L.; Doherty, S.A.; Muthuri, S.; Muir, K.R.; Doherty, M. Nottingham knee osteoarthritis risk prediction models. Ann. Rheum. Dis. 2011, 70, 1599–1604. [Google Scholar] [CrossRef] [Green Version]
- Bijur, P.E.; Silver, W.; Gallagher, E.J. Reliability of the visual analog scale for measurement of acute pain. Acad. Emerg. Med. 2001, 8, 1153–1157. [Google Scholar] [CrossRef]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting Change Scores for Pain and Functional Status in Low Back Pain. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Escobar, A.; Quintana, J.M.; Bilbao, A.; Azkárte, J.; Güenaga, L.I. Validation of the Spanish version of the WOMAC questionnaire for patients with hip or knee osteoarthritis. Clin. Rheumatol. 2002, 21, 466–471. [Google Scholar] [CrossRef]
- Vaquero, J.; Longo, U.G.; Forriol, F.; Martinelli, N.; Vethencourt, R.; Denaro, V. Reliability, validity and responsiveness of the Spanish version of the Knee Injury and Osteoarthritis Outcome Score (KOOS) in patients with chondral lesion of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 104–108. [Google Scholar] [CrossRef]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the Hospital Anxiety and Depression Scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Publishers: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Holla, J.F.; van der Leeden, M.; Heymans, M.W.; Roorda, L.D.; Bierma-Zeinstra, S.M.; Boers, M.; Lems, W.F.; Steultjens, M.P.; Dekker, J. Three trajectories of activity limitations in early symptomatic knee osteoarthritis: A 5-year follow-up study. Ann. Rheum. Dis. 2014, 73, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verkleij, S.P.; Hoekstra, T.; Rozendaal, R.M.; Waarsing, J.H.; Koes, B.W.; Luijsterburg, P.A.; Bierma-Zeinstra, S.M. Defining discriminative pain trajectories in hip osteoarthritis over a 2-year time period. Ann. Rheum. Dis. 2012, 71, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Coffman, C.J.; Golightly, Y.M.; Stechuchak, K.M.; Keefe, F.J. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthr. Cartil. 2009, 17, 1275–1282. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A.; Stewart, L.; French, M.R.; Cibere, J.; Jordan, J.M.; March, L.; Suarez-Almazor, M.; Gooberman-Hill, R. Understanding the pain experience in hip and knee osteoarthritis—An OARSI/OMERACT initiative. Osteoarthr. Cartil. 2008, 16, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Lluch, E.; Torres, R.; Nijs, J.; Van Oosterwijck, J. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur. J. Pain 2014, 18, 1367–1375. [Google Scholar] [CrossRef]
- Villafañe, J.H.; Pedersini, P.; Berjano, P. Epigenetics in osteoarthritis related pain: An update. Arch. Rheumatol. 2020, 35, 456–457. [Google Scholar] [CrossRef]
- Johnson, S.R.; Archibald, A.; Davis, A.M.; Badley, E.; Wright, J.G.; Hawker, G.A. Is self-reported improvement in osteoarthritis pain and disability reflected in objective measures? J. Rheumatol. 2007, 34, 159–164. [Google Scholar] [PubMed]
- Massardo, L.; Watt, I.; Cushnaghan, J.; Dieppe, P. Osteoarthritis of the knee joint: An eight-year prospective study. Ann. Rheum. Dis. 1989, 48, 893–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholls, E.; Thomas, E.; van der Windt, D.A.; Croft, P.R.; Peat, G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: Findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthr. Cartil. 2014, 22, 2041–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| EOA (n = 54) | HS (n = 51) | p Value | |
|---|---|---|---|
| Age (years) | 51.85 ± 5.72 | 50.49 ± 6.21 | 0.46 |
| BMI (kg/m2) | 27.40 ± 4.19 | 26.80 ± 3.68 | 0.43 |
| Gender | 0.17 | ||
| Women | 35 (64.8) | 35 (68.6) | |
| Men | 19 (35.2) | 16 (31.4) | |
| Marital status | 0.95 | ||
| Single | 5 | 7 | |
| Married | 31 | 30 | |
| Widowed | 2 | 2 | |
| Divorced | 6 | 6 | |
| Level of formal education | 0.18 | ||
| Primary | 6 | 9 | |
| Secondary | 20 | 12 | |
| College | 18 | 24 | |
| Smoking | 0.27 | ||
| No | 18 | 23 | |
| Yes | 9 | 4 | |
| Ex | 21 | 19 | |
| Alcohol | 0.65 | ||
| Never | 8 | 4 | |
| Seldom | 13 | 18 | |
| 1–2 times/month | 11 | 9 | |
| 1–2 times/week | 15 | 15 | |
| 1 time per day | 2 | 2 | |
| More than 1 per day | 1 | 0 |
| Baseline | Follow-Up 1 | Follow-Up 2 | |
|---|---|---|---|
| VAS Rest | |||
| Luyten et al. [9] | 2.04 ± 1.56 | 1.29 ± 2.05 | 2.21 ± 2.67 |
| Mahmoudian et al. [10] | 1.88 ± 2.31 | 1.50 ± 2.06 | 2.44 ± 2.78 |
| VAS Walking | |||
| Luyten et al. [9] | 2.67 ± 2.73 | 2.42 ± 2.02 | 2.46 ± 2.40 |
| Mahmoudian et al. [10] | 2.69 ± 2.77 | 2.94 ± 2.11 | 2.63 ± 2.55 |
| * WOMAC | |||
| Luyten et al. [9] | 18.55 ± 10.15 | 16.56 ± 15.66 * | 22.47 ± 17.23 * |
| Mahmoudian et al. [10] | 17.62 ± 9.47 | 22.04 ± 16.75 * | 25.26 ± 18.75 * |
| KOOS Pain | |||
| Luyten et al. [9] | 80.48 ± 13.44 | 78.62 ± 16.24 | 80.52 ± 19.97 |
| Mahmoudian et al. [10] | 86.17 ± 14.31 | 75.08 ± 19.40 | 74.25 ± 23.42 |
| KOOS Symptoms | |||
| Luyten et al. [9] | 85.00 ± 12.71 | 79.62 ± 15.81 | 83.61 ± 12.45 |
| Mahmoudian et al. [10] | 85.92 ± 12.41 | 74.24 ± 16.58 | 77.67 ± 12.07 |
| KOOS ADL | |||
| Luyten et al. [9] | 83.29 ± 16.94 | 79.62 ± 18.53 | 81.95 ± 18.67 |
| Mahmoudian et al. [10] | 86.83 ± 18.66 | 73.67 ± 21.62 | 73.92 ± 20.91 |
| KOOS QQL | |||
| Luyten et al. [9] | 62.29 ± 26.56 | 51.86 ± 20.45 | 61.33 ± 24.59 |
| Mahmoudian et al. [10] | 65.17 ± 32.80 | 45.42 ± 18.64 | 50.08 ± 22.37 |
| KOOS Sport | |||
| Luyten et al. [9] | 60.71 ± 30.83 | 55.71 ± 29.43 | 57.61 ± 26.77 |
| Mahmoudian et al. [10] | 58.75 ± 37.54 | 44.58 ± 30.63 | 46.25 ± 24.23 |
| HAD Anxiety | |||
| Luyten et al. [9] | 5.42 ± 4.14 | 4.17 ± 3.81 | 5.29 ± 4.60 |
| Mahmoudian et al. [10] | 4.94 ± 3.35 | 4.75 ± 2.46 | 4.88 ± 3.20 |
| HAD Depression | |||
| Luyten et al. [9] | 3.38 ± 3.07 | 3.13 ± 2.91 | 2.96 ± 3.32 |
| Mahmoudian et al. [10] | 2.81 ± 2.34 | 3.31 ± 2.85 | 2.50 ± 2.68 |
| Baseline | Follow-Up 1 | Follow-Up 2 | |
|---|---|---|---|
| VAS Rest | |||
| Luyten et al. [9] | 0.1 ± 0.29 | 0.74 ± 1.36 | 0.91 ± 1.41 |
| Mahmoudian et al. [10] | 0.68 ± 1.85 | 0.77 ± 1.54 | 1.13 ± 1.77 |
| VAS Walking | |||
| Luyten et al. [9] | 0.52 ± 1.76 | 1.3 ± 2.01 | 1.52 ± 1.86 |
| Mahmoudian et al. [10] | 1.06 ± 2.23 | 1.32 ± 1.85 | 1.68 ± 1.92 |
| * WOMAC | |||
| Luyten et al. [9] | 5.86 ± 4.3 | 9.57 ± 8.20 * | 12.30 ± 10.30 * |
| Mahmoudian et al. [10] | 9.32 ± 8.89 | 12.44 ± 8.45 * | 12.77 ± 10.42 * |
| KOOS Pain | |||
| Luyten et al. [9] | 88.80 ± 13.05 | 87.60 ± 9.65 | 88.96 ± 12.28 |
| Mahmoudian et al. [10] | 84.10 ± 14.31 | 86.90 ± 9.31 | 87.47 ± 11.61 |
| KOOS Symptoms | |||
| Luyten et al. [9] | 90.24 ± 10.83 | 91.80 ± 6.79 | 92.60 ± 8.80 |
| Mahmoudian et al. [10] | 88.20 ± 12.20 | 90.53 ± 7.84 | 91.30 ± 8.51 |
| KOOS ADL | |||
| Luyten et al. [9] | 89.88 ± 14.37 | 90.32 ± 7.84 | 92.01 ± 9.78 |
| Mahmoudian et al. [10] | 86.13 ± 15.54 | 89.83 ± 7.66 | 91.20 ± 9.06 |
| KOOS QQL | |||
| Luyten et al. [9] | 76.88 ± 22.29 | 75.12 ± 20.62 | 79.64 ± 21.88 |
| Mahmoudian et al. [10] | 71.57 ± 22.72 | 71.97 ± 20.53 | 75.93 ± 20.81 |
| KOOS Sport | |||
| Luyten et al. [9] | 71.40 ± 26.71 | 71.60 ± 22.16 | 72.60 ± 23.29 |
| Mahmoudian et al. [10] | 67.67 ± 25.72 | 71.33 ± 21.01 | 69.83 ± 22.03 |
| HAD Anxiety | |||
| Luyten et al. [9] | 2.83 ± 3.34 | 3.04 ± 3.34 | 2.87 ± 2.79 |
| Mahmoudian et al. [10] | 3.74 ± 4.21 | 3.45 ± 4.09 | 3.71 ± 4.31 |
| HAD Depression | |||
| Luyten et al. [9] | 1.17 ± 2.05 | 1.52 ± 2.27 | 1.26 ± 1.18 |
| Mahmoudian et al. [10] | 2.03 ± 3.04 | 1.84 ± 2.54 | 1.94 ± 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Manley, L.; Alabajos-Cea, A.; Suso-Martí, L.; Viosca-Herrero, E.; Vazquez-Arce, I. Early Knee Osteoarthritis Classification and Clinical Evolution: A Longitudinal Observational Pilot Study. Biomedicines 2023, 11, 1670. https://doi.org/10.3390/biomedicines11061670
Herrero-Manley L, Alabajos-Cea A, Suso-Martí L, Viosca-Herrero E, Vazquez-Arce I. Early Knee Osteoarthritis Classification and Clinical Evolution: A Longitudinal Observational Pilot Study. Biomedicines. 2023; 11(6):1670. https://doi.org/10.3390/biomedicines11061670
Chicago/Turabian StyleHerrero-Manley, Luz, Ana Alabajos-Cea, Luis Suso-Martí, Enrique Viosca-Herrero, and Isabel Vazquez-Arce. 2023. "Early Knee Osteoarthritis Classification and Clinical Evolution: A Longitudinal Observational Pilot Study" Biomedicines 11, no. 6: 1670. https://doi.org/10.3390/biomedicines11061670
APA StyleHerrero-Manley, L., Alabajos-Cea, A., Suso-Martí, L., Viosca-Herrero, E., & Vazquez-Arce, I. (2023). Early Knee Osteoarthritis Classification and Clinical Evolution: A Longitudinal Observational Pilot Study. Biomedicines, 11(6), 1670. https://doi.org/10.3390/biomedicines11061670