A Comprehensive Review on Monkeypox Viral Disease with Potential Diagnostics and Therapeutic Options
Abstract
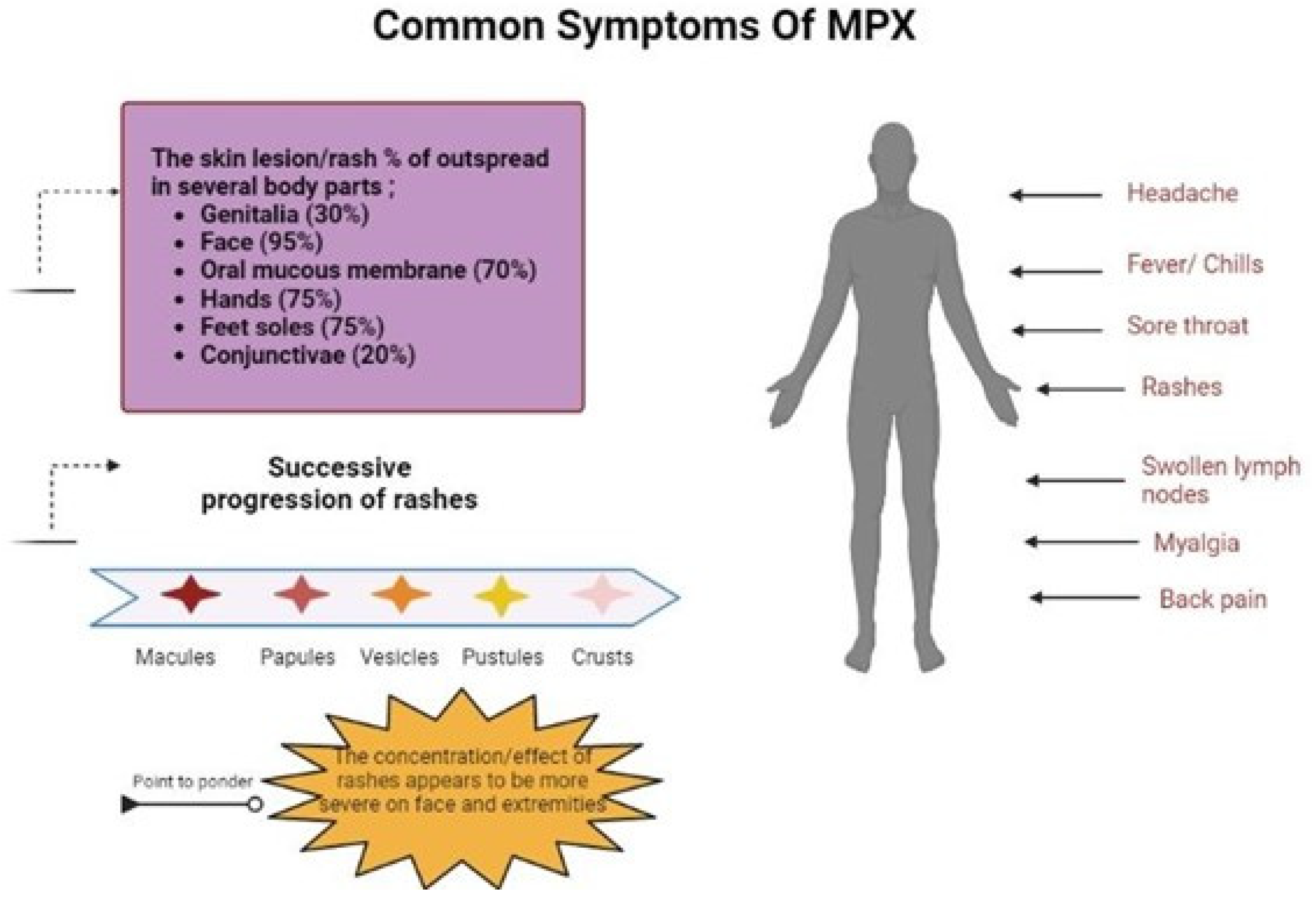
:1. Introduction
2. Transmission
3. Pathophysiology of Monkeypox
3.1. Bronchopneumonia
3.2. Clinical Manifestation Comparison
3.3. Secondary Co-Infections
4. Outbreak History of Monkeypox Virus Disease
Suspected Cases
5. Diagnostic Strategies
5.1. Polymerase Chain Reaction (PCR)
5.2. Cell Culture
5.3. Immunohistological Techniques
5.4. ELISA
6. Treatment and Management
6.1. Therapeutic Measures
- Cidofovir can be used to inhibit viral replication by inactivation of DNA polymerase which can be administered intravenously, which could be nephrotoxic.
- Brincidofovir (CMX-001) is an orthopoxvirus nucleotide analogue DNA polymerase inhibitor and a lipid conjugate of the nucleotide analogue cidofovir and is indicated for the treatment of human smallpox disease (Figure 4).
6.2. Vaccination
6.3. Prevention
Public Responsibility
6.4. Clinical Management
Healthcare Workers’ Response
6.5. Outbreak Surveillance
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ladnyj, I.; Ziegler, P.; Kima, E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar]
- Okyay, R.A.; Bayrak, E.; Kaya, E.; Şahin, A.R.; Koçyiğit, B.F.; Taşdoğan, A.M.; Avcı, A.; Sümbül, H.E. Another Epidemic in the Shadow of Covid 19 Pandemic: A Review of Monkeypox. Proteins 2022, 7, 10. [Google Scholar] [CrossRef]
- Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Abdelaal, A.; Serhan, H.A.; Mahmoud, M.A.; Rodriguez-Morales, A.J.; Sah, R. Ophthalmic manifestations of monkeypox virus. Eye 2023, 37, 383–385. [Google Scholar] [CrossRef]
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human monkeypox: Epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019, 33, 1027–1043. [Google Scholar] [CrossRef] [Green Version]
- Adnan, N.; ul Haq, Z.; Malik, A.; Mehmood, A.; Ishaq, U.; Faraz, M.; Malik, J.; Mehmoodi, A. Human monkeypox virus: An updated review. Medicine 2022, 101, e30406. [Google Scholar] [CrossRef]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J. Clinical features and management of human monkeypox: A retrospective observational study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791. [Google Scholar] [CrossRef] [Green Version]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox virus in Nigeria: Infection biology, epidemiology, and evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Wang, J.; Shahed-AI-Mahmud, M.; Chen, A.; Li, K.; Tan, H.; Joyce, R. An overview of antivirals against monkeypox virus and other orthopoxviruses. J. Med. Chem. 2023, 66, 4468–4490. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Alonso-Cadenas, J.A.; Roguera-Sopena, M.; Lorusso, N.; San Miguel, L.G.; Calvo, C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc. Health 2022, 6, e22–e23. [Google Scholar] [CrossRef]
- Alakunle, E.F.; Okeke, M.I. Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat. Rev. Microbiol. 2022, 20, 507–508. [Google Scholar] [CrossRef]
- Mauldin, M.R.; McCollum, A.M.; Nakazawa, Y.J.; Mandra, A.; Whitehouse, E.R.; Davidson, W.; Zhao, H.; Gao, J.; Li, Y.; Doty, J. Exportation of monkeypox virus from the African continent. J. Infect. Dis. 2022, 225, 1367–1376. [Google Scholar]
- Dye, C.; Kraemer, M.U. Investigating the monkeypox outbreak. BMJ 2022, 377, o1314. [Google Scholar] [CrossRef]
- McAndrew, T.; Majumder, M.S.; Lover, A.A.; Venkatramanan, S.; Boccini, P.; Codi, A.; Besiroglu, T.; Braun, D.; Dempsey, G.; Abbott, S. Human judgment forecasts of human monkeypox transmission and burden in non-endemic countries. BMJ 2022. [Google Scholar] [CrossRef]
- Alshahrani, N.Z.; Algethami, M.R.; Alarifi, A.M.; Alzahrani, F.; Sheerah, H.A.; Abdelaal, A.; Sah, R.; Rodriguez-Morales, A.J. Knowledge and attitude regarding monkeypox virus among physicians in Saudi Arabia, a cross-sectional study. Vaccines 2022, 10, 2099. [Google Scholar] [CrossRef]
- Sood, A.; Sui, Y.; McDonough, E.; Santamaría-Pang, A.; Al-Kofahi, Y.; Pang, Z.; Jahrling, P.B.; Kuhn, J.H.; Ginty, F. Comparison of multiplexed immunofluorescence imaging to chromogenic immunohistochemistry of skin biomarkers in response to monkeypox virus infection. Viruses 2020, 12, 787. [Google Scholar] [CrossRef]
- Peter, O.J.; Kumar, S.; Kumari, N.; Oguntolu, F.A.; Oshinubi, K.; Musa, R. Transmission dynamics of Monkeypox virus: A mathematical modelling approach. Model. Earth Syst. Environ. 2022, 8, 3423–3434. [Google Scholar] [CrossRef]
- Yang, Z.-S.; Lin, C.-Y.; Urbina, A.N.; Wang, W.-H.; Assavalapsakul, W.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; Yu, M.-L.; Wang, S.-F. The first case of monkeypox virus infection detected in Taiwan: Awareness and preparation. Int. J. Infect. Dis. 2022, 122, 991–995. [Google Scholar] [CrossRef]
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Jain, A.; Paweska, J.T.; Tripathi, A.K.; Abdel-Moneim, A.S. Re-emerging human monkeypox: A major public-health debacle. J. Med. Virol. 2023, 95, e27902. [Google Scholar] [CrossRef]
- Parvin, R.; Ali, A.; Abdou Nagy, Z.Z.; Zhao, S.; Paul, A.K.; Hafez, M. Monkeypox virus: A comprehensive review of taxonomy, evolution, epidemiology, diagnosis, prevention, and control regiments so far. Ger. J. Microbiol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Spicknall, I.H.; Pollock, E.D.; Clay, P.A.; Oster, A.M.; Charniga, K.; Masters, N.; Nakazawa, Y.J.; Rainisch, G.; Gundlapalli, A.V.; Gift, T.L. Modeling the Impact of Sexual Networks in the Transmission of Monkeypox Virus among Gay, Bisexual, and Other Men Who Have Sex with Men—United States, 2022 v. 71. 2022. Available online: https://stacks.cdc.gov/view/cdc/120698. (accessed on 12 May 2023).
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.-J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef]
- Al-Musa, A.; Chou, J.; LaBere, B. The resurgence of a neglected orthopoxvirus: Immunologic and clinical aspects of monkeypox virus infections over the past six decades. Clin. Immunol. 2022, 243, 109108. [Google Scholar] [CrossRef]
- Baxevanis, A.D. An overview of gene identification: Approaches, strategies, and considerations. Curr. Protoc. Bioinform. 2004, 6, 4.1.1–4.1.9. [Google Scholar] [CrossRef]
- Charniga, K.; Masters, N.B.; Slayton, R.B.; Gosdin, L.; Minhaj, F.S.; Philpott, D.; Smith, D.; Gearhart, S.; Alvarado-Ramy, F.; Brown, C. Estimating the incubation period of monkeypox virus during the 2022 multi-national outbreak. medRxiv 2022, 2022–06. [Google Scholar] [CrossRef]
- Duque, M.P.; Ribeiro, S.; Martins, J.V.; Casaca, P.; Leite, P.P.; Tavares, M.; Mansinho, K.; Duque, L.M.; Fernandes, C.; Cordeiro, R. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance 2022, 27, 2200424. [Google Scholar]
- Rao, A.K.; Schulte, J.; Chen, T.-H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 509. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Dhama, K.; Chakraborty, C. Recently spreading human monkeypox virus infection and its transmission during COVID-19 pandemic period: A travelers’ prospective. Travel Med. Infect. Dis. 2022, 49, 102398. [Google Scholar] [CrossRef]
- de Jonge, E.F.; Peterse, C.M.; Koelewijn, J.M.; van der Drift, A.-M.R.; van der Beek, R.F.; Nagelkerke, E.; Lodder, W.J. The detection of monkeypox virus DNA in wastewater samples in the Netherlands. Sci. Total Environ. 2022, 852, 158265. [Google Scholar] [CrossRef]
- Assessment, R.R. Monkeypox multi-country outbreak. Eur. Cent. Dis. Prev. Control. 2022. Available online: http://www.sepexpal.org/wp-content/uploads/2022/05/23-mayo.-ECDC.-Monkeypox-multi-country-outbreak.pdf. (accessed on 12 May 2023).
- Remichkova, M. Poxviruses: Smallpox vaccine, its complications and chemotherapy. Virus Adapt. Treat. 2010, 2, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Sklenovska, N.; Van Ranst, M. Emergence of monkeypox as the most important orthopoxvirus infection in humans. Front. Public Health 2018, 6, 241. [Google Scholar] [CrossRef] [Green Version]
- Sv, P.; Ittamalla, R. What concerns the general public the most about monkeypox virus?–A text analytics study based on Natural Language Processing (NLP). Travel Med. Infect. Dis. 2022, 49, 102404. [Google Scholar] [CrossRef]
- Petersen, B.; Damon, I. Smallpox, monkeypox, and other poxvirus infections. In Goldman-Cecil Medicine, 26th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Peiró-Mestres, A.; Fuertes, I.; Camprubí-Ferrer, D.; Marcos, M.Á.; Vilella, A.; Navarro, M.; Rodriguez-Elena, L.; Riera, J.; Català, A.; Martínez, M.J. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance 2022, 27, 2200503. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Carroll, D.S.; Karem, K.L. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr. Opin. Virol. 2012, 2, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Shchelkunov, S.; Totmenin, A.; Safronov, P.; Mikheev, M.; Gutorov, V.; Ryazankina, O.; Petrov, N.; Babkin, I.; Uvarova, E.; Sandakhchiev, L. Analysis of the monkeypox virus genome. Virology 2002, 297, 172–194. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Haider, F.; Khan, S.; Ahmad, I.; Ahmed, N.; Imran, M.; Rashid, S.; Ren, Z.-G.; Khattak, S.; Ji, X.-Y. Clinical Manifestation, Transmission, Pathogenesis, and Diagnosis of Monkeypox Virus: A Comprehensive Review. Life 2023, 13, 522. [Google Scholar] [CrossRef]
- Verreault, D.; Killeen, S.Z.; Redmann, R.K.; Roy, C.J. Susceptibility of monkeypox virus aerosol suspensions in a rotating chamber. J. Virol. Methods 2013, 187, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.N.; Whitehill, F.; Doty, J.B.; Schulte, J.; Matheny, A.; Stringer, J.; Delaney, L.J.; Esparza, R.; Rao, A.K.; McCollum, A.M. Environmental Persistence of Monkeypox Virus on Surfaces in Household of Person with Travel-Associated Infection, Dallas, Texas, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1982–1989. [Google Scholar] [CrossRef]
- Riopelle, J.C.; Munster, V.J.; Port, J.R. Atypical and unique transmission of monkeypox virus during the 2022 outbreak: An overview of the current state of knowledge. Viruses 2022, 14, 2012. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.-C.; Davidson, W.B.; Karem, K.L. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539. [Google Scholar] [CrossRef]
- Ward, T.; Christie, R.; Paton, R.S.; Cumming, F.; Overton, C.E. Transmission dynamics of monkeypox in the United Kingdom: Contact tracing study. BMJ 2022, 379, e073153. [Google Scholar] [CrossRef]
- Miura, F.; van Ewijk, C.E.; Backer, J.A.; Xiridou, M.; Franz, E.; de Coul, E.O.; Brandwagt, D.; van Cleef, B.; van Rijckevorsel, G.; Swaan, C. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Eurosurveillance 2022, 27, 2200448. [Google Scholar] [CrossRef]
- Ježek, Z.; Grab, B.; Szczeniowski, M.; Paluku, K.; Mutombo, M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull. World Health Organ. 1988, 66, 459. [Google Scholar]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.-J.T. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S. Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.G.; Doty, J.B.; McCollum, A.M.; Olson, V.A.; Nakazawa, Y. Monkeypox re-emergence in Africa: A call to expand the concept and practice of One Health. Expert Rev. Anti-Infect. Ther. 2019, 17, 129–139. [Google Scholar] [CrossRef]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J. Outbreak of human monkeypox in Nigeria in 2017–18: A clinical and epidemiological report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Zhu, F.; Li, L.; Che, D. Monkeypox virus under COVID-19: Caution for sexual transmission–Correspondence. Int. J. Surg. 2022, 104, 106768. [Google Scholar] [CrossRef]
- Uwishema, O.; Adekunbi, O.; Peñamante, C.A.; Bekele, B.K.; Khoury, C.; Mhanna, M.; Nicholas, A.; Adanur, I.; Dost, B.; Onyeaka, H. The burden of monkeypox virus amidst the COVID-19 pandemic in Africa: A double battle for Africa. Ann. Med. Surg. 2022, 80, 104197. [Google Scholar] [CrossRef]
- Veintimilla, C.; Catalán, P.; Alonso, R.; de Viedma, D.G.; Pérez-Lago, L.; Palomo, M.; Cobos, A.; Aldamiz-Echevarria, T.; Muñoz, P. The relevance of multiple clinical specimens in the diagnosis of monkeypox virus, Spain, June 2022. Eurosurveillance 2022, 27, 2200598. [Google Scholar] [CrossRef]
- Shaheen, N.; Diab, R.A.; Meshref, M.; Shaheen, A.; Ramadan, A.; Shoib, S. Is there a need to be worried about the new monkeypox virus outbreak? A brief review on the monkeypox outbreak. Ann. Med. Surg. 2022, 81, 104396. [Google Scholar] [CrossRef]
- Shafaati, M.; Zandi, M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med. Infect. Dis. 2022, 49, 102414. [Google Scholar] [CrossRef]
- Thakur, V.; Thakur, P.; Srivastava, S.; Kumar, P. Monkeypox virus (MPX) in humans a concern: Trespassing the global boundaries–Correspondence. Int. J. Surg. 2022, 104, 106703. [Google Scholar] [CrossRef]
- Reynolds, M.G.; McCollum, A.M.; Nguete, B.; Shongo Lushima, R.; Petersen, B.W. Improving the care and treatment of monkeypox patients in low-resource settings: Applying evidence from contemporary biomedical and smallpox biodefense research. Viruses 2017, 9, 380. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782. [Google Scholar] [CrossRef]
- Vouga, M.; Nielsen-Saines, K.; Dashraath, P.; Baud, D. The monkeypox outbreak: Risks to children and pregnant women. Lancet. Child Adolesc. Health 2022, 6, 751–753. [Google Scholar] [CrossRef]
- Sepehrinezhad, A.; Ashayeri Ahmadabad, R.; Sahab-Negah, S. Monkeypox virus from neurological complications to neuroinvasive properties: Current status and future perspectives. J. Neurol. 2022, 270, 101–108. [Google Scholar] [CrossRef]
- Nolasco, S.; Vitale, F.; Geremia, A.; Tramuto, F.; Maida, C.M.; Sciuto, A.; Coco, C.; Manuele, R.; Frasca, E.; Frasca, M. First case of monkeypox virus, SARS-CoV-2 and HIV co-infection. J. Infect. 2022, 86, e21–e23. [Google Scholar] [CrossRef]
- Doshi, R.H.; Guagliardo, S.A.J.; Doty, J.B.; Babeaux, A.D.; Matheny, A.; Burgado, J.; Townsend, M.B.; Morgan, C.N.; Satheshkumar, P.S.; Ndakala, N. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerg. Infect. Dis. 2019, 25, 273. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, Y.; Mauldin, M.R.; Emerson, G.L.; Reynolds, M.G.; Lash, R.R.; Gao, J.; Zhao, H.; Li, Y.; Muyembe, J.-J.; Kingebeni, P.M. A phylogeographic investigation of African monkeypox. Viruses 2015, 7, 2168–2184. [Google Scholar] [CrossRef] [Green Version]
- Nörz, D.; Brehm, T.T.; Tang, H.T.; Grewe, I.; Hermanussen, L.; Matthews, H.; Pestel, J.; Degen, O.; Günther, T.; Grundhoff, A. Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J. Clin. Virol. 2022, 155, 105254. [Google Scholar] [CrossRef]
- Alshahrani, N.Z.; Alzahrani, F.; Alarifi, A.M.; Algethami, M.R.; Alhumam, M.N.; Ayied, H.A.M.; Awan, A.Z.; Almutairi, A.F.; Bamakhrama, S.A.; Almushari, B.S. Assessment of knowledge of monkeypox viral infection among the general population in Saudi Arabia. Pathogens 2022, 11, 904. [Google Scholar] [CrossRef]
- Khallafallah, O.; Grose, C. Reassessment of Evidence about Coinfection of Chickenpox and Monkeypox (Mpox) in African Children. Viruses 2022, 14, 2800. [Google Scholar] [CrossRef]
- Prabaker, K.K.; de St Maurice, A.; Uslan, D.Z.; Yen, G.C.; Manavi, S.; Gray, H.K.; Caldera, J.R.; Chan, J.L.; Garner, O.B.; Yang, S. Case Report: Symptomatic Herpes Simplex Virus Type 2 and Monkeypox Coinfection in an Adult Male. Am. J. Trop. Med. Hygeine 2022, 107, 1258–1260. [Google Scholar] [CrossRef]
- Ola, P. Monkeypox Is the Manifestation of Spectral Diseases and Not of Monkeypox Virus Transmission; OSF Preprints: Charlottesville, VA, USA, 2022. [Google Scholar]
- Murphy, H.; Ly, H. The potential risks posed by inter-and intraspecies transmissions of monkeypox virus. Virulence 2022, 13, 1681–1683. [Google Scholar] [CrossRef] [PubMed]
- Ježek, Z.; Szczeniowski, M.; Paluku, K.; Mutombo, M. Human monkeypox: Clinical features of 282 patients. J. Infect. Dis. 1987, 156, 293–298. [Google Scholar] [CrossRef]
- Mucker, E.M.; Shamblin, J.D.; Raymond, J.L.; Twenhafel, N.A.; Garry, R.F.; Hensley, L.E. Effect of Monkeypox Virus Preparation on the Lethality of the Intravenous Cynomolgus Macaque Model. Viruses 2022, 14, 1741. [Google Scholar] [CrossRef]
- Echekwube, P.; Mbaave, P.; Abidakun, O.; Utoo, B.; Swende, T. Human Monkeypox and Human Immunodeficiency Virus Co-infection: A Case Series in Makurdi, Benue State, Nigeria. J. BioMedical Res. Clin. Pract. 2020, 3, 375–381. [Google Scholar] [CrossRef]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2022, 23, 74–80. [Google Scholar] [CrossRef]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Wawina-Bokalanga, T.; Sklenovska, N.; Vanmechelen, B.; Bloemen, M.; Vergote, V.; Laenen, L.; Andre, E.; Van Ranst, M.; Muyembe, J.-J.T.; Maes, P. An accurate and rapid Real-time PCR approach for human Monkeypox virus diagnosis. medRxiv 2022, 2022-06. [Google Scholar] [CrossRef]
- Cohen, J. Is an old virus up to new tricks? Science 1997, 277, 312–313. [Google Scholar] [CrossRef]
- World Health Organization. Monkeypox in the Democratic Republic of the Congo (former Zaire). Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 1997, 72, 258. [Google Scholar]
- Reynolds, M.G.; Emerson, G.L.; Pukuta, E.; Karhemere, S.; Muyembe, J.J.; Bikindou, A.; McCollum, A.M.; Moses, C.; Wilkins, K.; Zhao, H. Detection of human monkeypox in the Republic of the Congo following intensive community education. Am. J. Trop. Med. Hyg. 2013, 88, 982. [Google Scholar] [CrossRef] [Green Version]
- Murugesu, J.A. Monkeypox on The Rise; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chakraborty, C.; Bhattacharya, M.; Nandi, S.S.; Mohapatra, R.K.; Dhama, K.; Agoramoorthy, G. Appearance and re-appearance of zoonotic disease during the pandemic period: Long-term monitoring and analysis of zoonosis is crucial to confirm the animal origin of SARS-CoV-2 and monkeypox virus. Vet. Q. 2022, 42, 119–124. [Google Scholar] [CrossRef]
- Vusirikala, A.; Charles, H.; Balasegaram, S.; Macdonald, N.; Kumar, D.; Barker-Burnside, C.; Cumiskey, K.; Dickinson, M.; Watson, M.; Olufon, O. Epidemiology of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg. Infect. Dis. 2022, 28, 2082–2086. [Google Scholar] [CrossRef]
- Costello, V.; Sowash, M.; Gaur, A.; Cardis, M.; Pasieka, H.; Wortmann, G.; Ramdeen, S. Imported Monkeypox from International Traveler, Maryland, USA, 2021. Emerg. Infect. Dis. 2022, 28, 1002. [Google Scholar] [CrossRef]
- Wei, Q. Is China ready for monkeypox? Anim. Model. Exp. Med. 2022, 5, 397. [Google Scholar] [CrossRef]
- Xiang, Y.; White, A. Monkeypox virus emerges from the shadow of its more infamous cousin: Family biology matters. Emerg. Microbes Infect. 2022, 11, 1768–1777. [Google Scholar] [CrossRef]
- Chadha, J.; Khullar, L.; Gulati, P.; Chhibber, S.; Harjai, K. Insights into the monkeypox virus: Making of another pandemic within the pandemic? Environ. Microbiol. 2022, 24, 4547–4560. [Google Scholar] [CrossRef]
- Forni, D.; Molteni, C.; Cagliani, R.; Sironi, M. Geographic structuring and divergence time frame of monkeypox virus in the endemic region. J. Infect. Dis. 2022, 227, 742–751. [Google Scholar] [CrossRef]
- Le Page, M. Monkeypox: Key Questions Answered; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Durski, K.N.; McCollum, A.M.; Nakazawa, Y.; Petersen, B.W.; Reynolds, M.G.; Briand, S.; Djingarey, M.H.; Olson, V.; Damon, I.K.; Khalakdina, A. Emergence of monkeypox—West and central Africa, 1970–2017. Morb. Mortal. Wkly. Rep. 2018, 67, 306. [Google Scholar] [CrossRef]
- Petersen, E.; Zumla, A.; Hui, D.; Blumberg, L.; Valdoleiros, S.; Amao, L.; Ntoumi, F.; Asogun, D.; Simonsen, L.; Haider, N. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int. J. Infect. Dis. 2022, 122, 569–571. [Google Scholar] [CrossRef]
- Pfeiffer, J.A. High-contact object and surface contamination in a household of persons with monkeypox virus infection—Utah, June 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 1092. [Google Scholar] [CrossRef]
- World Health Organization. Disease Outbreak News; Multi-Country Monkeypox Outbreak in Non-Endemic Countries. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 12 May 2022).
- Sharma, R.; Jasrotia, T.; Umar, A.; Sharma, M.; Sharma, S.; Kumar, R.; Alkhanjaf, A.A.M.; Vats, R.; Beniwal, V.; Kumar, R. Effective removal of Pb (II) and Ni (II) ions by Bacillus cereus and Bacillus pumilus: An experimental and mechanistic approach. Environ. Res. 2022, 212, 113337. [Google Scholar] [CrossRef]
- World Health Organization. Disease Outbreak News; Multi-Country Monkeypox Outbreak in Non-Endemic Countries: Update. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396 (accessed on 27 June 2022).
- Kanji, J.N.; Dieu, P.; Wong, A.; Pabbaraju, K.; Shokoples, S.; Smyczek, P.; Gratrix, J.; Singh, A.E.; Charlton, C.L.; Zhou, H.Y.; et al. Retrospective testing for the presence of monkeypox virus in a high-risk population from February-June 2022 in Alberta, Canada. J. Assoc. Med. Microbiol. Infect. Dis. Can. 2023, 8, 85–89. [Google Scholar] [CrossRef]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef]
- Altindis, M.; Puca, E.; Shapo, L. Diagnosis of monkeypox virus–An overview. Travel Med. Infect. Dis. 2022, 50, 102459. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Zhang, L.; Lai, A. Laboratory diagnostics for monkeypox: An overview of sensitivities from various published tests. In Travel Medicine and Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Angahar, L.T. An Overview of Monkey-pox Disease. Am. J. Curr. Microbiol. 2018, 6, 39–51. [Google Scholar]
- Ahmad, A.; Baig, A.A.; Hussain, M.; Saeed, M.U.; Bilal, M.; Ahmed, N.; Chopra, H.; Hassan, M.; Rachamalla, M.; Putnala, S.K. Narrative on Hydrogen Therapy and its Clinical Applications: Safety and Efficacy. Curr. Pharm. Des. 2022, 28, 2519–2537. [Google Scholar]
- Harapan, H.; Wagner, A.L.; Yufika, A.; Setiawan, A.M.; Anwar, S.; Wahyuni, S.; Asrizal, F.W.; Sufri, M.R.; Putra, R.P.; Wijayanti, N.P. Acceptance and willingness to pay for a hypothetical vaccine against monkeypox viral infection among frontline physicians: A cross-sectional study in Indonesia. Vaccine 2020, 38, 6800–6806. [Google Scholar] [CrossRef]
- Gordon, S.N.; Cecchinato, V.; Andresen, V.; Heraud, J.-M.; Hryniewicz, A.; Parks, R.W.; Venzon, D.; Chung, H.-k.; Karpova, T.; McNally, J. Smallpox vaccine safety is dependent on T cells and not B cells. J. Infect. Dis. 2011, 203, 1043–1053. [Google Scholar] [CrossRef] [Green Version]
- Atukorale, V.N.; Weir, J.P.; Meseda, C.A. Stability of the HSV-2 US-6 gene in the del II, del III, CP77, and I8R-G1L sites in modified vaccinia virus ankara after serial passage of recombinant vectors in cells. Vaccines 2020, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Meyer, H.; Ehmann, R.; Smith, G.L. Smallpox in the post-eradication era. Viruses 2020, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E. Smallpox vaccine–induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]
- Roess, A.A.; Monroe, B.P.; Kinzoni, E.A.; Gallagher, S.; Ibata, S.R.; Badinga, N.; Molouania, T.M.; Mabola, F.S.; Mombouli, J.V.; Carroll, D.S. Assessing the effectiveness of a community intervention for monkeypox prevention in the Congo basin. PLoS Negl. Trop. Dis. 2011, 5, e1356. [Google Scholar] [CrossRef] [Green Version]
- Shapovalova, V. Monkeypox virus–new challenges of modernity: Experimental organizational and legal, clinical and pharmacological studies. SSP Mod. Pharm. Med. 2022, 2, 1–15. [Google Scholar] [CrossRef]



| Character | Smallpox | Monkeypox | Varicella |
|---|---|---|---|
| Viral cycle completion | 28 days | 28 days | 21 days |
| Incubation time | 2 weeks | 2 weeks | 3 weeks |
| Lesion inflammatory cycle | 2–4 weeks | 2–4 weeks | 1–3 weeks |
| Body temperature | >40 °C | 38.5–40.5 °C | 38 ± 8 °C |
| Lymphadenopathy | Often | Often | Rare |
| Lesion | Centrifugal | Centrifugal | Centripetal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabaan, A.A.; Al-Shwaikh, S.A.; Alfouzan, W.A.; Al-Bahar, A.M.; Garout, M.; Halwani, M.A.; Albayat, H.; Almutairi, N.B.; Alsaeed, M.; Alestad, J.H.; et al. A Comprehensive Review on Monkeypox Viral Disease with Potential Diagnostics and Therapeutic Options. Biomedicines 2023, 11, 1826. https://doi.org/10.3390/biomedicines11071826
Rabaan AA, Al-Shwaikh SA, Alfouzan WA, Al-Bahar AM, Garout M, Halwani MA, Albayat H, Almutairi NB, Alsaeed M, Alestad JH, et al. A Comprehensive Review on Monkeypox Viral Disease with Potential Diagnostics and Therapeutic Options. Biomedicines. 2023; 11(7):1826. https://doi.org/10.3390/biomedicines11071826
Chicago/Turabian StyleRabaan, Ali A., Seham A. Al-Shwaikh, Wadha A. Alfouzan, Ali M. Al-Bahar, Mohammed Garout, Muhammad A. Halwani, Hawra Albayat, Norah B. Almutairi, Mohammed Alsaeed, Jeehan H. Alestad, and et al. 2023. "A Comprehensive Review on Monkeypox Viral Disease with Potential Diagnostics and Therapeutic Options" Biomedicines 11, no. 7: 1826. https://doi.org/10.3390/biomedicines11071826
APA StyleRabaan, A. A., Al-Shwaikh, S. A., Alfouzan, W. A., Al-Bahar, A. M., Garout, M., Halwani, M. A., Albayat, H., Almutairi, N. B., Alsaeed, M., Alestad, J. H., Al-Mozaini, M. A., Ashgar, T. M. A., Alotaibi, S., Abuzaid, A. A., Aldawood, Y., Alsaleh, A. A., Al-Afghani, H. M., Altowaileb, J. A., Alshukairi, A. N., ... Imran, M. (2023). A Comprehensive Review on Monkeypox Viral Disease with Potential Diagnostics and Therapeutic Options. Biomedicines, 11(7), 1826. https://doi.org/10.3390/biomedicines11071826








