The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases
Abstract
1. Introduction
2. Historical Perspective of Light Therapy
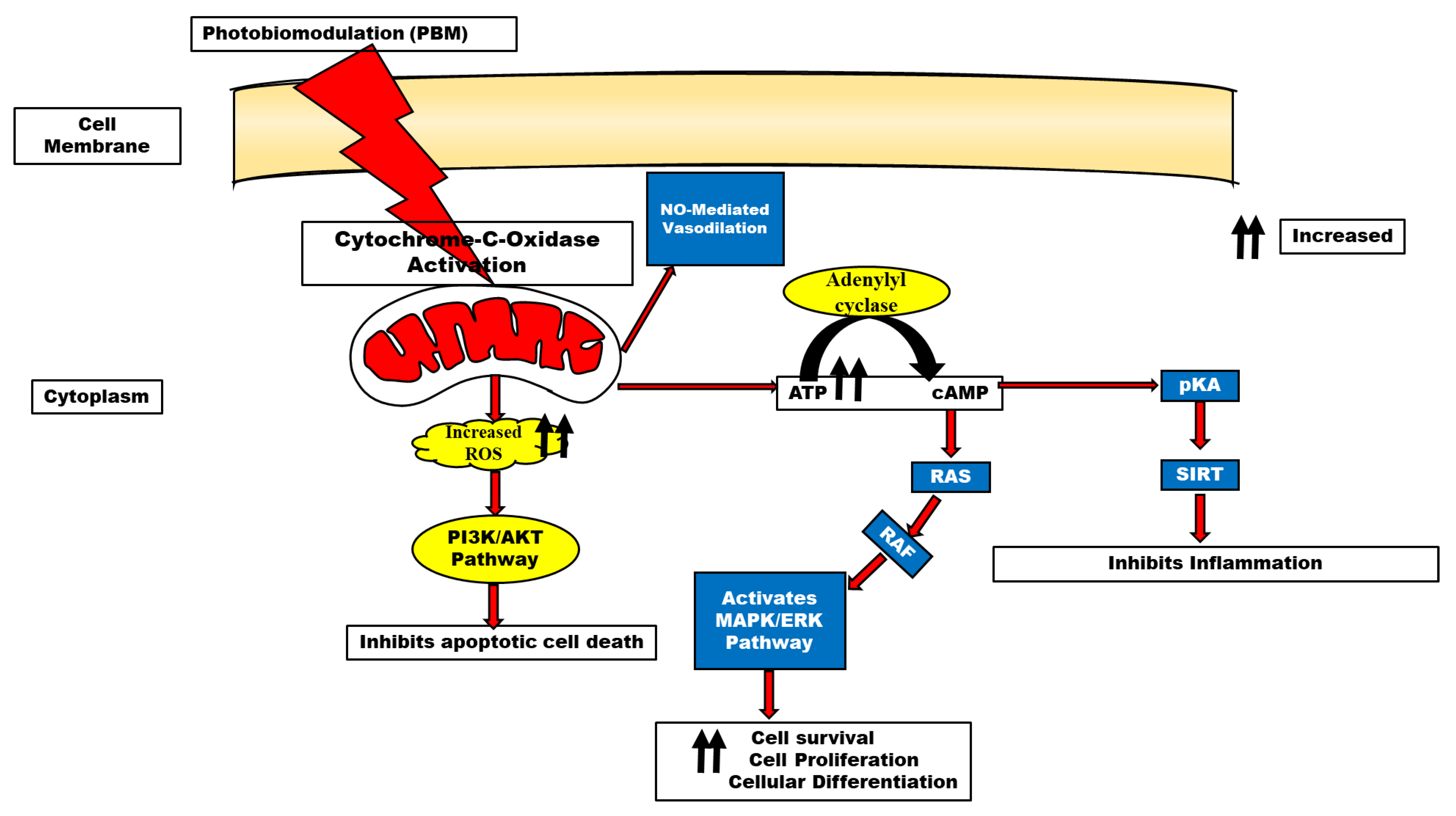
3. Photobiomodulation Mechanisms
4. Role of Gated Channels in Photobiomodulation
5. ATP and Mitochondrial Function in PBM
6. Reactive Oxygen Species in PBM
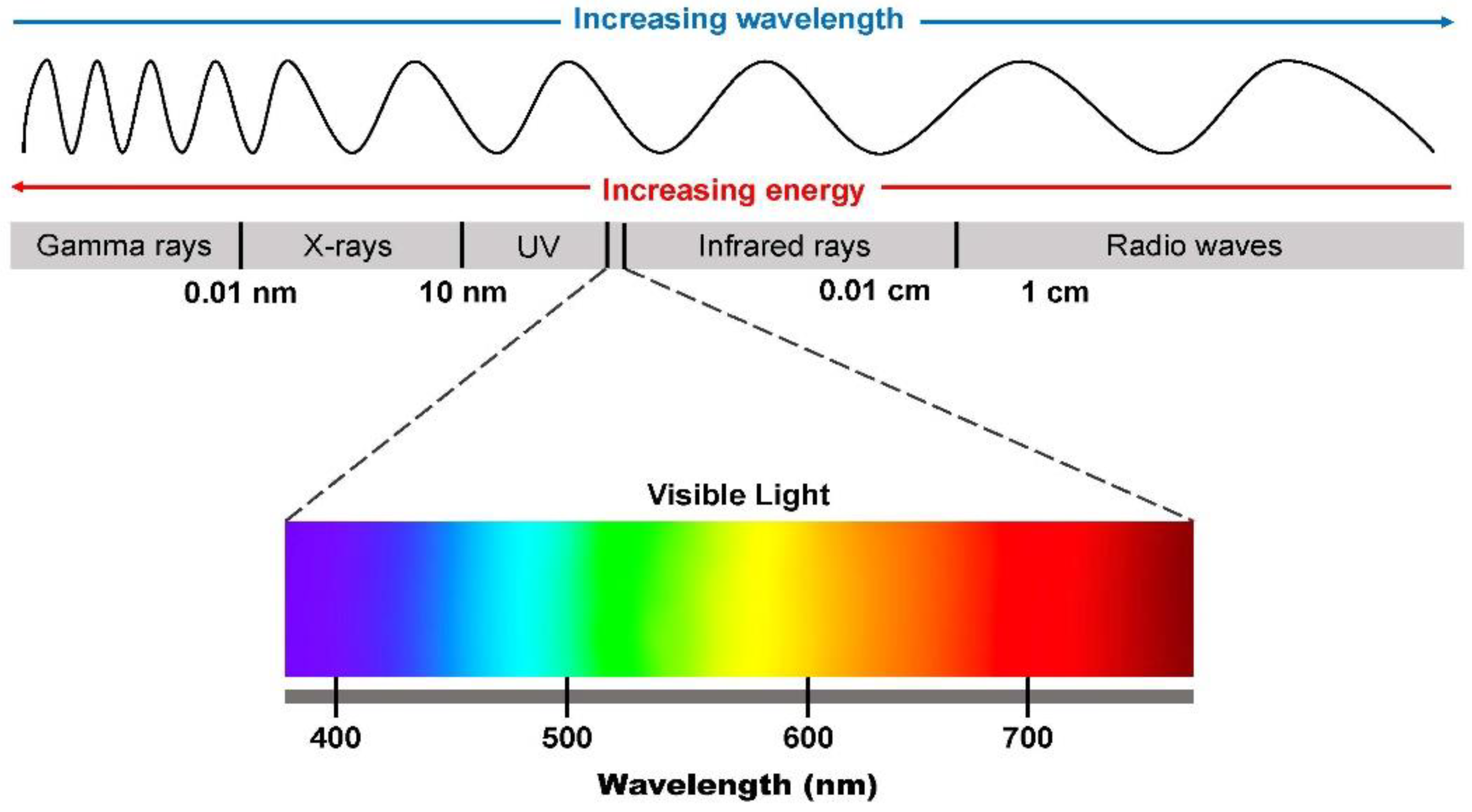
7. Light Properties as Consideration in Photobiomodulation
7.1. Wavelength
7.2. Photons (Energy)
7.3. Interaction between Light and Tissue in Photobiomodulation
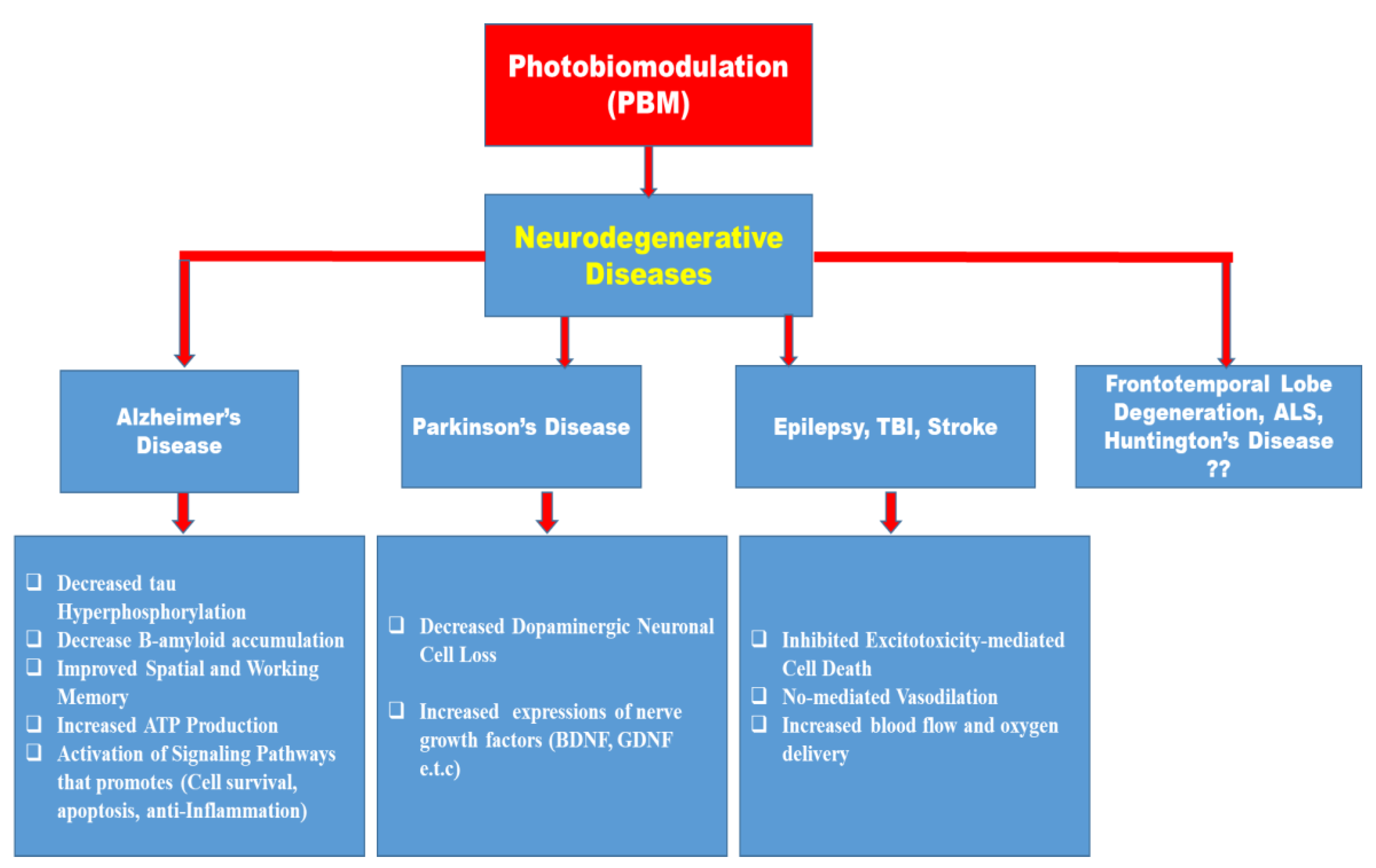
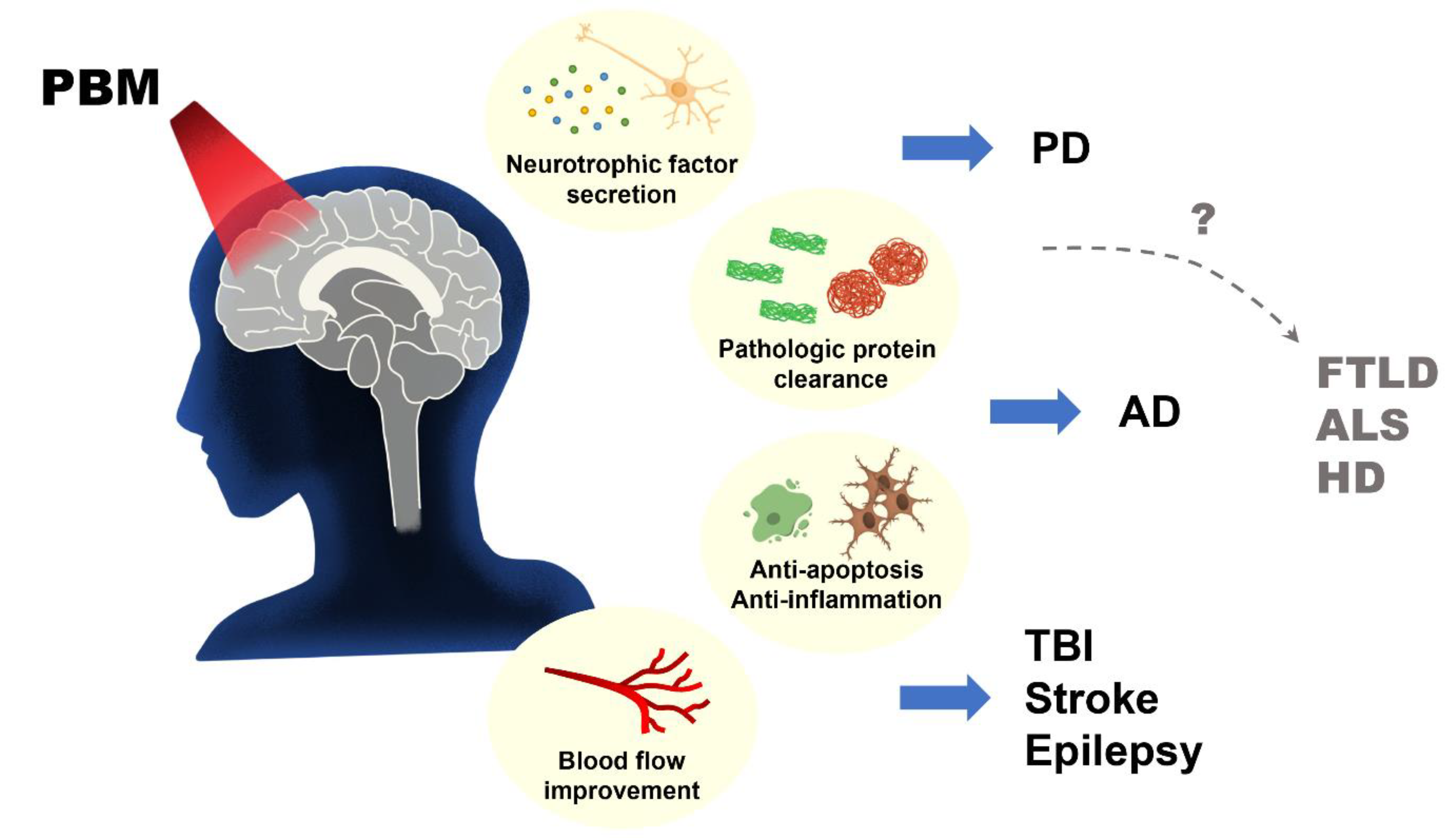
8. Photobiostimulation in Neurodegenerative Diseases
8.1. Alzheimer’s Disease (AD)
8.2. Parkinson’s Disease (PD)
8.3. Traumatic Brain Injury (TBI)
8.4. Stroke
8.5. Epilepsy
8.6. Major Depressive Disorders (MDD)
8.7. Involvement of Remote (Systemic) Photobiostimulation
8.8. Effect of Polarization in Photobiomodulation
8.9. PBM in Other Degenerative Diseases
9. Nanotechnology and Photobiomodulation Combination as Cutting-Edge Techniques in the Management of Neurodegenerative Diseases: A Consideration?
Strategies for the Nano-Based Photo (Light)-Controlled System
10. Clinical Trial Studies on Photobiomodulation
11. Conclusions
12. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamblin, M.R. Shining Light on the Head: Photobiomodulation for Brain Disorders. BBA Clin. 2016, 6, 113–124. [Google Scholar] [CrossRef]
- McGuff, P.E.; Deterling, R.A., Jr.; Gottlieb, L.S. Tumoricidal effect of laser energy on experimental and human malignant tumors. N. Engl. J. Med. 1965, 273, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Maiman, T.H. Stimulated optical radiation in ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Mester, E.; Szende, B.; Gärtner, P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. 1968, 9, 621–626. [Google Scholar]
- Mester, E.L.G.S.M.; Ludany, G.; Selyei, M.; Szende, B. The Stimulating Effect of Low Power Laser Rays on Biological Systems; Medical University: Budapest, Hungary, 1968. [Google Scholar]
- Bathini, M.; Raghushaker, C.R.; Mahato, K.K. The Molecular Mechanisms of Action of Photobiomodulation Against Neurodegenerative Diseases: A Systematic Review. Cell. Mol. Neurobiol. 2022, 42, 955–971. [Google Scholar] [CrossRef]
- Hamblin, M.R. Low-level light therapy: Photobiomodulation. In Society of Photo-Optical Instrumentation Engineers; SPIE: Bellingham, WA, USA, 2018. [Google Scholar]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Meng, C.; He, Z.; Xing, D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer’s disease. J. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef]
- Liang, J.; Liu, L.; Xing, D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radic. Biol. Med. 2012, 53, 1459–1467. [Google Scholar] [CrossRef]
- Song, S.; Zhou, F.; Chen, W.R. Low-level laser therapy regulates microglial function through Src-mediated signaling pathways: Implications for neurodegenerative diseases. J. NeuroInflamm. 2012, 9, 707. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Q.; Wu, X.; Zhang, D.; Xing, D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell 2020, 19, e13054sana. [Google Scholar] [CrossRef]
- Lipko, N.B. Photobiomodulation: Evolution and Adaptation. Photobiomodulation Photomed. Laser Surg. 2022, 40, 213–233. [Google Scholar] [CrossRef]
- Jarrett, P.; Scragg, R. A short history of phototherapy, vitamin D and skin disease. Photochem. Photobiol. Sci. 2017, 16, 283–290. [Google Scholar] [CrossRef]
- Dillon, K.J. Healing Photons—The Science and Art of Blood Irradiation Therapy; Scientia Press: Washington, DC, USA, 1998. [Google Scholar]
- Liebert, A.; Kiat, H. The history of light therapy in hospital physiotherapy and medicine with emphasis on Australia: Evolution into novel areas of practice. Physiother. Theory Pract. 2021, 37, 389–400. [Google Scholar] [CrossRef]
- Zhang, D.; Spielmann, A.; Wang, L.; Ding, G.; Huang, F.; Gu, Q.; Schwarz, W. Mast-cell degranulation induced by physical stimuli involves the activation of transient-receptor-potential channel TRPV2. Physiol. Res. 2012, 61, 113–124. [Google Scholar] [CrossRef]
- Yang, W.Z.; Chen, J.Y.; Yu, J.T.; Zhou, L.W. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem. Photobiol. 2007, 83, 979–984. [Google Scholar] [CrossRef]
- Ryu, J.J.; Yoo, S.; Kim, K.Y.; Park, J.S.; Bang, S.; Lee, S.H.; Yang, T.-J.; Cho, H.; Hwang, S.W. Laser modulation of heat and capsaicin receptor TRPV1 leads to thermal antinociception. J. Dent. Res. 2010, 89, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.S.; Bec, J.M.; Desmadryl, G.; Chekroud, K.; Travo, C.; Gaboyard, S.; Bardin, F.; Marc, I.; Dumas, M.; Lenaers, G.; et al. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J. Neurophysiol. 2012, 107, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, L.; Huang, F.; Schwarz, W. Stimulation of TRPV1 by green laser light. Evid.-Based Complement. Altern. Med. 2012, 2012, 857123. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 2010, 62, 607–610. [Google Scholar] [CrossRef]
- Anders, J.J.; Romanczyk, T.B.; Ilev, I.K.; Waynant, R. Light supports neurite outgrowth of human neural progenitor cells in vitro: The role of P2Y receptors. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 118–125. [Google Scholar] [CrossRef]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B Biol. 1995, 27, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Karu, T. Ten Lectures on Basic Science of Laser Phototherapy; Prima Books AB: Grängesberg, Sweden, 2007. [Google Scholar]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. A Publ. IEEE Lasers Electro-Opt. Soc. 2016, 22, 7000417. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.K.; Noto, C.; Rizzo, L.B.; Rios, A.C.; Nunes, S.O.; Barbosa, D.S.; Sethi, S.; Zeni, M.; Mansur, R.B.; Maes, M.; et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 134–144. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, C.; Perluigi, M.; Butterfield, D.A. Oxidative stress, protein modification and Alzheimer disease. Brain Res. Bull. 2017, 133, 88–96. [Google Scholar] [CrossRef]
- Kumar Rajendran, N.; George, B.P.; Chandran, R.; Tynga, I.M.; Houreld, N.; Abrahamse, H. The Influence of Light on Reactive Oxygen Species and NF-κB in Disease Progression. Antioxidants 2019, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Smith, K.A.; Schumacker, P.T. O2 sensing, mitochondria, and ROS signaling: The fog is lifting. Mol. Asp. Med. 2016, 47–48, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Chen, H.; Aaron, C.H.; Arany, P.R.; Hamblin, M.R. Role of reactive oxygen species in low-level light therapy. In Mechanisms for Low-Light Therapy IV; Michael, R.H., Ronald, W.W., Juanita, A., Eds.; SPIE: San Jose, CA, USA, 2009. [Google Scholar]
- Tseng, S.H.; Bargo, P.; Durkin, A.; Kollias, N. Chromophore concentrations, absorption and scattering properties of human skin in-vivo. Opt. Express 2009, 17, 14599–14617. [Google Scholar] [CrossRef]
- Rukmini, A.; Milea, D.; Aung, T.; Gooley, J.J. Pupillary responses to short-wavelength light are preserved in aging. Sci. Rep. 2017, 7, 43832. [Google Scholar] [CrossRef]
- Jagdeo, J.; Austin, E.; Mamalis, A.; Wong, C.; Ho, D.; Siegel, D.M. Light-emitting diodes in dermatology: A systematic review of randomized controlled trials. Lasers Surg. Med. 2018, 50, 613–628. [Google Scholar] [CrossRef]
- Jacques, S.L. Origins of tissue optical properties in the UVA, visible, and NIR regions. In Advances in Optical Imaging and Photon Migration; Optica Publishing Group: Washington, DC, USA, 1996; p. OPC364. [Google Scholar]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef]
- Keiser, G. Light-Tissue Interactions. In Biophotonics. Graduate Texts in Physics; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Adelodun, S.T.; Ishola, O.A.; Abijo, A.Z.; Olatunji, S.Y.; Owolabi, J.O.; Olanrewaju, J.A.; Adekomi, D.A. Aluminium chloride-induced hippocampal damage: CA3 hippocampal subfield involvement and the neuroprotective role of Buchholzia coriacea ethanolic seed extract. Phytomedicine Plus 2021, 1, 100104. [Google Scholar] [CrossRef]
- Nelson, L.; Tabet, N. Slowing the progression of Alzheimer’s disease; what works? Ageing Res. Rev. 2015, 23 Pt B, 193–209. [Google Scholar] [CrossRef]
- Hayashi, Y.; Lin, H.T.; Lee, C.C.; Tsai, K.-J. Effects of neural stem cell transplantation in Alzheimer’s disease models. J. Biomed. Sci. 2020, 27, 29. [Google Scholar] [CrossRef]
- Michalikova, S.; Ennaceur, A.; van Rensburg, R.; Chazot, P.L. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiol. Learn. Mem. 2008, 89, 480–488. [Google Scholar] [CrossRef]
- De Taboada, L.; Yu, J.; El-Amouri, S.; Gattoni-Celli, S.; Richieri, S.; McCarthy, T.; Streeter, J.; Kindy, M.S. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J. Alzheimer’s Dis. JAD 2011, 23, 521–535. [Google Scholar] [CrossRef]
- Grillo, S.L.; Duggett, N.A.; Ennaceur, A.; Chazot, P.L. Non-invasive infra-red therapy (1072 nm) reduces β-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J. Photochem. Photobiol. B Biol. 2013, 123, 13–22. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex-evidence from two transgenic mouse models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Eersel, J.; Ittner, L.M.; Mitrofanis, J.; Stone, J. Near infrared light mitigates cerebellar pathology in transgenic mouse models of dementia. Neurosci. Lett. 2015, 591, 155–159. [Google Scholar] [CrossRef]
- Da Luz Eltchechem, C.; Salgado, A.S.I.; Zângaro, R.A.; Pereira, M.C.D.S.; Kerppers, I.I.; da Silva, L.A.; Parreira, R.B. Transcranial LED therapy on amyloid-β toxin 25–35 in the hippocampal region of rats. Lasers Med. Sci. 2017, 32, 749–756. [Google Scholar] [CrossRef]
- Sommer, A.P.; Bieschke, J.; Friedrich, R.P.; Zhu, D.; Wanker, E.E.; Fecht, H.J.; Mereles, D.; Hunstein, W. 670 nm laser light and EGCG complementarily reduce amyloid-β aggregates in human neuroblastoma cells: Basis for treatment of Alzheimer’s disease? Photomed. Laser Surg. 2012, 30, 54–60. [Google Scholar] [CrossRef]
- Heo, J.C.; Park, J.A.; Kim, D.K.; Lee, J.H. Photobiomodulation (660 nm) therapy reduces oxidative stress and induces BDNF expression in the hippocampus. Sci. Rep. 2019, 9, 10114. [Google Scholar] [CrossRef]
- Fang, H.; Chartier, J.; Sodja, C.; Desbois, A.; Ribecco-Lutkiewicz, M.; Walker, P.R.; Sikorska, M. Transcriptional activation of the human brain-derived neurotrophic factor gene promoter III by dopamine signaling in NT2/N neurons. J. Biol. Chem. 2003, 278, 26401–26409. [Google Scholar] [CrossRef]
- Ou, L.C.; Gean, P.W. Transcriptional regulation of brain-derived neurotrophic factor in the amygdala during consolidation of fear memory. Mol. Pharmacol. 2007, 72, 350–358. [Google Scholar] [CrossRef]
- Ma, Q.L.; Harris-White, M.E.; Ubeda, O.J.; Simmons, M.; Beech, W.; Lim, G.P.; Teter, B.; Frautschy, S.A.; Cole, G.M. Evidence of Aβ-and transgene-dependent defects in ERK-CREB signaling in Alzheimer’s models. J. Neurochem. 2007, 103, 1594–1607. [Google Scholar] [CrossRef]
- Beurel, E.; Jope, R.S. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 2006, 79, 173–189. [Google Scholar] [CrossRef]
- Gómez-Sintes, R.; Hernández, F.; Lucas, J.J.; Avila, J. GSK-3 mouse models to study neuronal apoptosis and neurodegeneration. Front. Mol. Neurosci. 2011, 4, 45. [Google Scholar] [CrossRef]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118659. [Google Scholar] [CrossRef]
- He, P.; Shen, Y. Interruption of β-catenin signaling reduces neurogenesis in Alzheimer’s disease. J. Neurosci. 2009, 29, 6545–6557. [Google Scholar] [CrossRef]
- Guarente, L. Sirtuins as potential targets for metabolic syndrome. Nature 2006, 444, 868–874. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in neurodevelopment and brain senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Donmez, G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol. Sci. 2012, 33, 494–501. [Google Scholar] [CrossRef]
- Jorge, G.-U. Update on Parkinson’s Disease. Am. J. Biomed. Sci. Res. 2019, 2, AJBSR.MS.ID.000614. [Google Scholar]
- Lázaro, D.F.; Dias, M.C.; Carija, A.; Navarro, S.; Madaleno, C.S.; Tenreiro, S.; Ventura, S.; Outeiro, T.F. The effects of the novel A53E alpha-synuclein mutation on its oligomerization and aggregation. Acta Neuropathol. Commun. 2016, 4, 128. [Google Scholar] [CrossRef]
- Rizek, P.; Kumar, N.; Jog, M.S. An update on the diagnosis and treatment of Parkinson disease. CMAJ 2016, 188, 1157–1165. [Google Scholar] [CrossRef]
- Kobylecki, C.; Jones, T.; Lim, C.K.; Miller, C.; Thomson, A.M. Phenomenology and outcomes of in-patients with Parkinson’s disease during the coronavirus disease 2019 pandemic. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1295–1296. [Google Scholar] [CrossRef]
- Prachi, B.; Vaishnavi, C.; Anna, P.N. A Brief Review on Parkinson’s Disease. In EC Pharmacology & Toxicology–Review Article, Maharshtra; ResearchGate: Berlin, Germany, 2018. [Google Scholar]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Hu, S.; Hu, M.; Liu, J.; Zhang, B.; Zhang, Z.; Zhou, F.H.; Wang, L.; Dong, J. Phosphorylation of Tau and α-Synuclein Induced Neurodegeneration in MPTP Mouse Model of Parkinson’s Disease. Neuropsychiatr. Dis. Treat. 2020, 16, 651–663. [Google Scholar] [CrossRef]
- Xu, L.; Pu, J. Alpha-Synuclein in Parkinson’s Disease: From Pathogenetic Dysfunction to Potential Clinical Application. Park. Dis. 2016, 2016, 1720621. [Google Scholar] [CrossRef]
- Nag, N.; Jelinek, G.A. A Narrative Review of Lifestyle Factors Associated with Parkinson’s Disease Risk and Progression. Neuro-Degener. Dis. 2019, 19, 51–59. [Google Scholar] [CrossRef]
- Gómez-Benito, M.; Granado, N.; García-Sanz, P.; Michel, A.; Dumoulin, M.; Moratalla, R. Modeling Parkinson’s Disease with the Alpha-Synuclein Protein. Front. Pharmacol. 2020, 11, 356. [Google Scholar] [CrossRef]
- El Massri, N.; Lemgruber, A.P.; Rowe, I.J.; Moro, C.; Torres, N.; Reinhart, F.; Chabrol, C.; Benabid, A.L.; Mitrofanis, J. Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: Changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res. 2017, 235, 1861–1874. [Google Scholar] [CrossRef]
- Darlot, F.; Moro, C.; El Massri, N.; Chabrol, C.; Johnstone, D.M.; Reinhart, F.; Agay, D.; Torres, N.; Bekha, D.; Auboiroux, V.; et al. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann. Neurol. 2016, 79, 59–75. [Google Scholar] [CrossRef]
- Xuan, W.; Agrawal, T.; Huang, L.; Gupta, G.K.; Hamblin, M.R. Low-level laser therapy for traumatic brain injury in mice increases brain-derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics. 2015, 8, 502–511. [Google Scholar] [CrossRef]
- Shaw, V.E.; Spana, S.; Ashkan, K.; Benabid, A.-L.; Stone, J.; Baker, G.E.; Mitrofanis, J. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 2010, 518, 25–40. [Google Scholar] [CrossRef]
- El Massri, N.; Moro, C.; Torres, N.; Darlot, F.; Agay, D.; Chabrol, C.; Johnstone, D.M.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. Near-infrared light treatment reduces astrogliosis in MPTP-treated monkeys. Exp. Brain Res. 2016, 234, 3225–3232. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Austin, P.J. Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague-Dawley Rat. Biomolecules 2019, 9, 381. [Google Scholar] [CrossRef]
- Gordon, L.; Kim, B.; Petrucco, C.; Kim, J.Y.; Benson, P.; Stone, J.; Johnstone, D.M. Remote photobiomodulation as a neuroprotective intervention–harnessing the indirect effects of PBM. In Photobiomodulation in the Brain; Hamblin, M.R., Huang, Y.Y., Eds.; Elsevier: Cambridge, MA, USA, 2019. [Google Scholar]
- Kim, B.; Brandli, A.; Mitrofanis, J.; Stone, J.; Purushothuman, S.; Johnstone, D.M. Remote tissue conditioning—An emerging approach for inducing body-wide protection against diseases of ageing. Ageing Res. Rev. 2017, 37, 69–78. [Google Scholar] [CrossRef]
- Johnstone, D.M.; Coleman, K.; Moro, C.; Torres, N.; Eells, J.T.; Baker, G.E.; Ashkan, K.; Stone, J.; Benabid, A.L.; Mitrofanis, J. The potential of light therapy in Parkinson’s disease. ChronoPhysiology Ther. 2014, 4, 1. [Google Scholar]
- Kim, B.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Remote tissue conditioning is neuroprotective against MPTP insult in mice. IBRO Rep. 2018, 4, 14–17. [Google Scholar] [CrossRef]
- Ganeshan, V.; Skladnev, N.V.; Kim, J.Y.; Mitrofanis, J.; Stone, J.; Johnstone, D.M. Pre-conditioning with remote photobiomodulation modulates the brain transcriptome and protects against MPTP insult in mice. Neuroscience 2019, 400, 85–97. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. North Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain Photobiomodulation Therapy: A Narrative Review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Saltmarche, A.; Krengel, M.H.; Hamblin, M.R.; Knight, J.A. Improved Cognitive Function after Transcranial, Light-Emitting Diode Treatments in Chronic, Traumatic Brain Injury: Two Case Reports. Photomed. Laser Surg. 2011, 29, 351–358. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.H.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.A.; Meehan, W.P., III; Baker, E.H. Significant Improvements in Cognitive Performance Post-Transcranial, Red/Near-Infrared Light-Emitting Diode Treatments in Chronic, Mild Traumatic Brain Injury: Open-Protocol Study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Henry, M.M.; Summerfelt, P.; Wong–Riley, M.T.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Whelan, H.T. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3439–3444.33. [Google Scholar] [CrossRef]
- Wong–Riley, M.T.; Liang, H.L.; Eells, J.T.; Chance, B.; Henry, M.M.; Buchmann, E.; Kane, M.; Whelan, H.T. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005, 280, 4761–4771. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000, 93, 815–820. [Google Scholar] [CrossRef]
- Lifshitz, J.; Sullivan, P.G.; Hovda, D.A.; Wieloch, T.; McIntosh, T.K. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 2004, 4, 705–713. [Google Scholar] [CrossRef]
- Cardoso, F.D.S.; Salehpour, F.; Coimbra, N.C.; Gonzalez-Lima, F.; Gomes da Silva, S. Photobiomodulation for the treatment of neuroinflammation: A systematic review of controlled laboratory animal studies. Front. Neurosci. 2022, 16, 1610. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Nawashiro, H.; Wada, K.; Nakai, K.; Sato, S. Focal increase in cerebral blood flow after treatment with near-infrared light to the forehead in a patient in a persistent vegetative state. Photomed. Laser Surg. 2012, 30, 231–233. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, E.; Sadigh-Eteghad, S.; Mohaddes, G.; Rasta, S.H. Transcranial Photobiomodulation Prevents Anxiety and Depression via Changing Serotonin and Nitric Oxide Levels in Brain of Depression Model Mice: A Study of Three Different Doses of 810 nm Laser. Lasers Surg. Med. 2019, 51, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Naeser, M.A.; Ho, M.D.; Martin, P.I.; Hamblin, M.R.; Koo, B.-B. Increased Functional Connectivity within Intrinsic Neural Networks in Chronic Stroke Following Treatment with Red/Near-Infrared Transcranial Photobiomodulation: Case Series with Improved Naming in Aphasia. Photobiomodulation Photomed. Laser Surg. 2019, 38, 115–131. [Google Scholar] [CrossRef]
- Stephan, W.; Banas, L.; Misiak, M.; Brierley, W.; Hamblin, M. Photobiomodulation with Super-Pulsed Laser Shows Efficacy for Stroke and Aphasia: Case Studies. World J. Neurosci. 2023, 13, 12–20. [Google Scholar] [CrossRef]
- Oron, A.; Oron, U.; Chen, J.; Eilam, A.; Zhang, C.; Sadeh, M.; Lampl, Y.; Streeter, J.; DeTaboada, L.; Chopp, M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke 2006, 37, 2620–2624. [Google Scholar] [CrossRef]
- Lampl, Y. Laser treatment for stroke. Expert Rev. Neurother. 2007, 7, 961–965. [Google Scholar] [CrossRef]
- Fisher, R.S.; van Emde Boas, W.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J., Jr. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Olatunji, S.Y.; Ogunnaike, P.O.; Owolabi, J.O.; Abijo, A.Z.; Alabi, A.S.; Adelodun, S.T.; Olanrewaju, J.A.; Adelabi, A.S. Investigating the Effects of Allium sativum on the Prefrontal Cortex in Lithium Chloride Pilocarpine-Induced Epilepsy in Wistar Rat. Nig. J. Neurosci. 2021, 12, 56–66. [Google Scholar]
- Ogunsanya, S.T.; Ayannuga, O.A.; Owolabi, A.R.; Olusola, T.; Abijo, A.Z. Progressive evaluation of cortical histomorphology and histomorphometric parameters in adult Wistar rats following lithium-pilocarpine-induced temporal lobe epilepsy. Eur. J. Med. Res. 2022, 9, 45–56. [Google Scholar]
- Moshi, M.J.; Kagashe, G.A.; Mbwambo, Z.H. Plants used to treat epilepsy by Tanzanian traditional healers. J. Ethnopharmacol. 2005, 97, 327–336. [Google Scholar] [CrossRef]
- Bertram, E.H. Temporal lobe epilepsy: Where do the seizures really begin? Epilepsy Behav. 2009, 14, 32–37. [Google Scholar] [CrossRef]
- Ahmed, N.A.; Radwan, N.M.; Ibrahim, K.M.; Khedr, M.E.; El Aziz, M.A.; Khadrawy, Y.A. Effect of three different intensities of infrared laser energy on the levels of amino acid neurotransmitters in the cortex and hippocampus of rat brain. Photomed. Laser Surg. 2008, 26, 479–488. [Google Scholar] [CrossRef]
- Radwan, N.M.; El Hay Ahmed, N.A.; Ibrahim, K.M.; Khedr, M.E.; Aziz, M.A.; Khadrawy, Y.A. Effect of infrared laser irradiation on amino acid neurotransmitters in an epileptic animal model induced by pilocarpine. Photomed. Laser Surg. 2009, 27, 401–409. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; Hamblin, M.R. Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro. J. Biophotonics 2014, 7, 656–664. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Koretz, D.; Merikangas, K.R.; Rush, A.J.; Walters, E.E.; Wang, P.S. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003, 289, 3095–3105. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Yang, J.; Feng, X.; Zhao, F.; Lyu, J. Changes in the global burden of depression from 1990 to 2017: Findings from the Global Burden of Disease study. J. Psychiatr. Res. 2020, 126, 134–140. [Google Scholar] [CrossRef]
- Chambers, C.D.; Hernandez-Diaz, S.; Van Marter, L.J.; Werler, M.M.; Louik, C.; Jones, K.L.; Mitchell, A.A. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 2006, 354, 579–587. [Google Scholar] [CrossRef]
- Mrazek, D.A.; Hornberger, J.C.; Altar, C.A.; Degtiar, I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr. Serv. 2014, 65, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S. Transcranial low-level infrared laser irradiation ameliorates depression induced by reserpine in rats. Lasers Med. Sci. 2016, 31, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Guo, X.; Yang, Y.; Tucker, D.; Lu, Y.; Xin, N.; Zhang, G.; Yang, L.; Li, J.; Du, X.; et al. Low-level laser irradiation improves depression-like behaviors in mice. Mol. Neurobiol. 2017, 54, 4551–4559. [Google Scholar] [CrossRef]
- Tanaka, Y.; Akiyoshi, J.; Kawahara, Y.; Ishitobi, Y.; Hatano, K.; Hoaki, N.; Mori, A.; Goto, S.; Tsuru, J.; Matsushita, H.; et al. Infrared radiation has potential antidepressant and anxiolytic effects in animal model of depression and anxiety. Brain Stimul. 2011, 4, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Rasta, S.H.; Mohaddes, G.; Sadigh-Eteghad, S.; Salarirad, S. Therapeutic effects of 10-HzPulsed wave lasers in rat depression model: A comparison between near-infrared and red wavelengths. Lasers Surg. Med. 2016, 48, 695–705. [Google Scholar] [CrossRef]
- Salehpour, F.; Rasta, S.H.; Mohaddes, G.; Sadigh-Eteghad, S.; Salarirad, S. A comparison between antidepressant effects of transcranial near-infrared laser and citalopram in a rat model of depression. In Clinical and Translational Neurophotonics; SPIE: Bellingham, WA, USA, 2017; Volume 10050, pp. 28–36. [Google Scholar]
- Salehpour, F.; Farajdokht, F.; Cassano, P.; Sadigh-Eteghad, S.; Erfani, M.; Hamblin, M.R.; Salimi, M.M.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 2019, 144, 213–222. [Google Scholar] [CrossRef]
- Muili, K.A.; Gopalakrishnan, S.; Meyer, S.L.; Eells, J.T.; Lyons, J.A. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS ONE 2012, 7, e30655. [Google Scholar] [CrossRef]
- Hennessy, M.; Hamblin, M.R. Photobiomodulation and the brain: A new paradigm. J. Opt. 2017, 19, 013003. [Google Scholar] [CrossRef]
- Braverman, B.; McCarthy, R.J.; Ivankovich, A.D.; Forde, D.E.; Overfield, M.; Bapna, M.S. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg. Med. 1989, 9, 50–58. [Google Scholar] [CrossRef]
- Abe, M.; Fujisawa, K.; Suzuki, H.; Sugimoto, T.; Kanno, T. Role of 830nm low reactive level laser on the growth of an implanted glioma in mice. Keio J. Med. 1993, 42, 177–179. [Google Scholar] [CrossRef]
- Whelan, H.T.; Connelly, J.F.; Hodgson, B.D.; Barbeau, L.; Post, A.C.; Bullard, G.; Buchmann, E.V.; Kane, M.; Whelan, N.T.; Warwick, A.; et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J. Clin. Laser Med. Surg. 2002, 20, 319–324. [Google Scholar] [CrossRef]
- Hopkins, J.T.; McLoda, T.A.; Seegmiller, J.G.; Baxter, G.D. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J. Athl. Train. 2004, 39, 223. [Google Scholar]
- Kharbanda, R.K.; Nielsen, T.T.; Redington, A.N. Translation of remote ischaemic preconditioning into clinical practice. Lancet 2009, 374, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.A.; Loukogeorgakis, S.; Yannopoulos, F.; Rimpiläinen, E.; Petzold, A.; Tuominen, H.; Lepola, P.; MacAllister, R.J.; Deanfield, J.E.; Mäkelä, T.; et al. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation 2011, 123, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, N.; Feehan, J.; Husaric, M.; Kiatos, D.; Sidiroglou, F.; Fraser, S.; Apostolopoulos, V. Good, better, best? The effects of polarization on photobiomodulation therapy. J. Biophotonics 2020, 13, e201960230. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Biphasic dose response in low level light therapy. Dose-Response A Publ. Int. Hormesis Soc. 2009, 7, 358–383. [Google Scholar] [CrossRef]
- Hadis, M.A.; Zainal, S.A.; Holder, M.J.; Carroll, J.D.; Cooper, P.R.; Milward, M.R.; Palin, W.M. The dark art of light measurement: Accurate radiometry for low-level light therapy. Lasers Med. Sci. 2016, 31, 789–809. [Google Scholar] [CrossRef]
- Aragona, S.E.; Grassi, F.R.; Nardi, G.; Lotti, J.; Mereghetti, G.; Canavesi, E.; Equizi, E.; Puccio, A.M.; Lotti, T. Photobiomodulation with polarized light in the treatment of cutaneous and mucosal ulcerative lesions. J. Biol. Regul. Homeost. Agents 2017, 31 (Suppl. S2), 213–218. [Google Scholar]
- Kumar, A.; Ghatak, A.K. Polarization of Light with Applications in Optical Fibers; SPIE Press: Bellingham, WA, USA, 2011; Volume 246. [Google Scholar]
- Ando, T.; Sato, S.; Kobayashi, H.; Nawashiro, H.; Ashida, H.; Hamblin, M.R.; Obara, M. Low-level laser therapy for spinal cord injury in rats: Effects of polarization. J. Biomed. Opt. 2013, 18, 098002. [Google Scholar] [CrossRef]
- Tada, K.; Ikeda, K.; Tomita, K. Effect of polarized light emitting diode irradiation on wound healing. J. Trauma 2009, 67, 1073–1079. [Google Scholar] [CrossRef]
- Hamblin, M.R. Role of Polarized Light in Photobiomodulation. Photobiomodulation Photomed. Laser Surg. 2022, 40, 775–776. [Google Scholar] [CrossRef]
- Tripodi, N.; Sidiroglou, F.; Fraser, S.; Husaric, M.; Kiatos, D.; Apostolopoulos, V.; Feehan, J. The effects of polarized photobiomodulation on cellular viability, proliferation, mitochondrial membrane potential and apoptosis in human fibroblasts: Potential applications to wound healing. J. Photochem. Photobiol. B Biol. 2022, 236, 112574. [Google Scholar] [CrossRef]
- Calixto, G.M.; Bernegossi, J.; de Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced graphene oxide nanosheet for chemo-photothermal therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef]
- Croissant, J.G.; Guardado-Alvarez, T.M. Photocracking silica: Tuning the plasmonic photothermal degradation of mesoporous silica encapsulating gold nanoparticles for cargo release. Inorganics 2019, 7, 72. [Google Scholar] [CrossRef]
- Álvarez, Y.D.; Pellegrotti, J.V.; Stefani, F.D. In Use of Nanoparticles in Neuroscience Neuromethods Ch; Chapter 16; Humana Press: New York, NY, USA, 2018; pp. 269–291. [Google Scholar]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona-Ule, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnology 2018, 16, 1–16. [Google Scholar] [CrossRef]
- Sagar, V.; Nair, M. Near-infrared biophotonics-based nanodrug release systems and their potential application for neuro-disorders. Expert Opin. Drug Deliv. 2018, 15, 137–152. [Google Scholar] [CrossRef]
- Wen, G.; Li, X.; Zhang, Y.; Han, X.; Xu, X.; Liu, C.; Chan, K.W.Y.; Lee, C.-S.; Yin, C.; Bian, L.; et al. Effective phototheranostics of brain tumor assisted by near-infrared-II light-responsive semiconducting polymer nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 33492–33499. [Google Scholar] [CrossRef]
- Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules 2020, 25, 5294. [Google Scholar] [CrossRef]
- Liu, W.; Dong, X.; Liu, Y.; Sun, Y. Photoresponsive materials for intensified modulation of Alzheimer’s amyloid-β protein aggregation: A review. Acta Biomater. 2021, 123, 93–109. [Google Scholar] [CrossRef]
- Bae, K.H.; Chung, H.J.; Park, T.G. Nanomaterials for cancer therapy and imaging. Mol. Cells 2011, 31, 295–302. [Google Scholar] [CrossRef]
- Chanana, M.; Rivera_Gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical Properties of Protein-Coated Gold Nanoparticles in Biological Fluids and Cells before and after Proteolytic Digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef]
- Wang, P.; Lux, L.; Jin, M.; Wan, Y.; Wang, W.; Hung, C.T.; Albaqami, F.H.; El-Toni, A.M.; Alhoshan, M.S.; Li, X.; et al. Au/Ag nanobox-based near-infrared surface-enhanced Raman scattering for hydrogen sulfide sensing. ACS Appl. Bio Mater. 2018, 2, 417–423. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, H.; Cho, H.H.; Lee, J.H.; Nam, Y. Gold nanostar-mediated neural activity control using plasmonic photothermal effects. Biomaterials 2018, 153, 59–69. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-based nanomaterials for biomedical applications: A recent study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.; Zhen, X.; Ding, D.; Pu, K. Intraparticle molecular orbital engineering of semiconducting polymer nanoparticles as amplified theranostics for in vivo photoacoustic imaging and photothermal therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef]
- Chang, K.; Liu, Y.; Hu, D.; Qi, Q.; Gao, D.; Wang, Y.; Li, D.; Zhang, X.; Zheng, H.; Sheng, Z.; et al. Highly stable conjugated polymer dots as multifunctional agents for photoacoustic imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 7012–7021. [Google Scholar] [CrossRef]
- Paviolo, C.; Stoddart, P.R. Gold nanoparticles for modulating neuronal behavior. Nanomaterials 2017, 7, 92. [Google Scholar] [CrossRef]
- Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-based nanomaterials for delivery of biologicals and therapeutics: A cutting-edge technology. C 2021, 7, 19. [Google Scholar] [CrossRef]
- Pan, W.-T.; Liu, P.-M.; Ma, D.; Yang, J.-J. Advances in photobiomodulation for cognitive improvement by near-infrared derived multiple strategies. J. Transl. Med. 2023, 21, 135. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Cheng, Z.; Hou, Z.; Huang, S.; Lin, J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater. Sci. 2016, 4, 890–909. [Google Scholar] [CrossRef]
- Jain, K.K. Nanobiotechnology-based strategies for crossing the blood-brain barrier. Nanomedicine 2012, 7, 1225–1233. [Google Scholar] [CrossRef]
- Agrahari, V. The exciting potential of nanotherapy in brain-tumor targeted drug delivery approaches. Neural Regen. Res. 2017, 12, 197–200. [Google Scholar] [CrossRef]
- Bansal, A.; Zhang, Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. 2014, 47, 3052–3060. [Google Scholar] [CrossRef]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef]
- Geng, J.; Sun, C.; Liu, J.; Liao, L.D.; Yuan, Y.; Thakor, N.; Wang, J.; Liu, B. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. Small 2015, 11, 1603–1610. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Zhao, X.; Du, W.; Jiang, J.; Han, Y. Brain Photobiomodulation Improves Sleep Quality in Subjective Cognitive Decline: A Randomized, Sham-Controlled Study. J. Alzheimer’s Dis. JAD 2022, 87, 1581–1589. [Google Scholar] [CrossRef]
- Van Harten, A.C.; Mielke, M.M.; Swenson-Dravis, D.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology 2018, 91, e300–e312. [Google Scholar] [CrossRef]
- Zlatar, Z.Z.; Muniz, M.C.; Espinoza, S.G.; Gratianne, R.; Gollan, T.H.; Galasko, D.; Salmon, D.P. Subjective Cognitive Decline, Objective Cognition, and Depression in Older Hispanics Screened for Memory Impairment. J. Alzheimer’s Dis. JAD 2018, 63, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.H.; Halper, J.P.; Nichols, T.W.; Jarrett, H.; Lundy, A.; Huang, J.H. Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition. J. Neurol. Neurosci. 2017, 8, 176. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodulation Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abijo, A.; Lee, C.-Y.; Huang, C.-Y.; Ho, P.-C.; Tsai, K.-J. The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases. Biomedicines 2023, 11, 1828. https://doi.org/10.3390/biomedicines11071828
Abijo A, Lee C-Y, Huang C-Y, Ho P-C, Tsai K-J. The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases. Biomedicines. 2023; 11(7):1828. https://doi.org/10.3390/biomedicines11071828
Chicago/Turabian StyleAbijo, Ayodeji, Chun-Yuan Lee, Chien-Ying Huang, Pei-Chuan Ho, and Kuen-Jer Tsai. 2023. "The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases" Biomedicines 11, no. 7: 1828. https://doi.org/10.3390/biomedicines11071828
APA StyleAbijo, A., Lee, C.-Y., Huang, C.-Y., Ho, P.-C., & Tsai, K.-J. (2023). The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases. Biomedicines, 11(7), 1828. https://doi.org/10.3390/biomedicines11071828







