Real-World Endoscopic and Histologic Outcomes in Ulcerative Colitis Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Evaluation of Disease Activity
2.3. Statistical Analysis
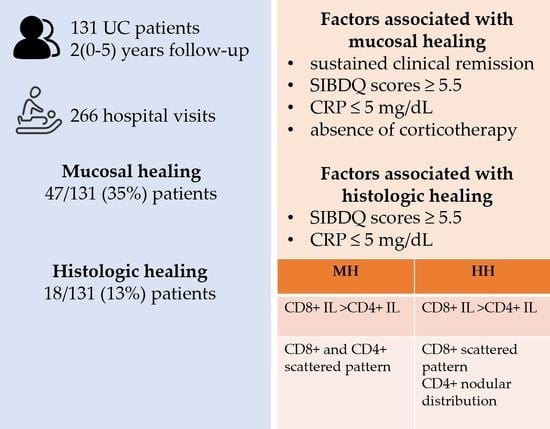
3. Results
3.1. Patients
3.2. Endoscopic Activity
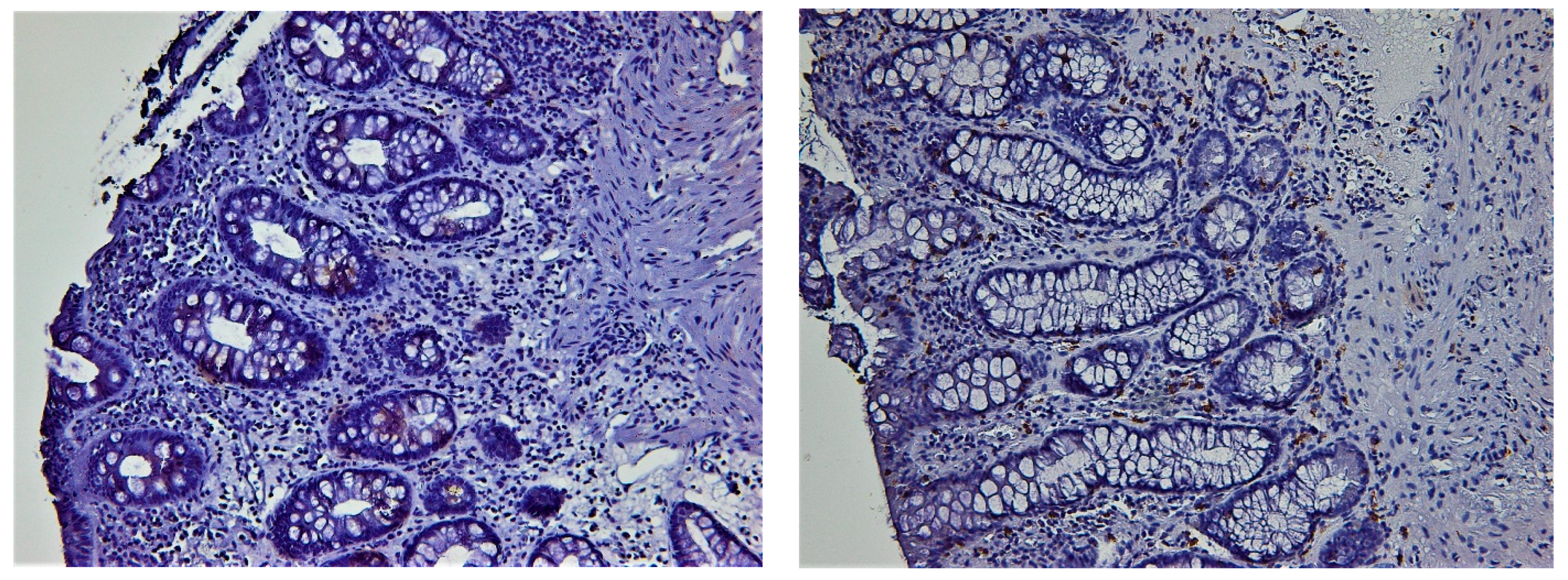
3.3. Histologic Healing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Actis, G.C. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Parker, C.E.; Nelson, S.A.; Baker, K.A.; MacDonald, J.K.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Levesque, B.G.; Jairath, V. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2017, 5, CD011256. [Google Scholar] [CrossRef] [PubMed]
- Geboes, K.; Riddell, R.; Ost, A.; Jensfelt, B.; Persson, T.; Löfberg, R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000, 47, 404–409. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670, Erratum in J. Crohn’s Colitis 2023, 17, 149. [Google Scholar] [CrossRef]
- Magro, F.; Sabino, J.; Rosini, F.; Tripathi, M.; Borralho, P.; Baldin, P.; Danese, S.; Driessen, A.; Gordon, I.O.; Iacucci, M.; et al. ECCO Position on Harmonisation of Crohn’s Disease Mucosal Histopathology. J. Crohn’s Colitis 2022, 16, 876–883. [Google Scholar] [CrossRef]
- Magro, F.; Lopes, J.; Borralho, P.; Lopes, S.; Coelho, R.; Cotter, J.; De Castro, F.D.; Sousa, H.T.; Salgado, M.; Andrade, P.; et al. Comparison of different histological indexes in the assessment of UC activity and their accuracy regarding endoscopic outcomes and faecal calprotectin levels. Gut 2018, 68, 594–603. [Google Scholar] [CrossRef]
- Nozaki, K.; Mochizuki, W.; Matsumoto, Y.; Matsumoto, T.; Fukuda, M.; Mizutani, T.; Watanabe, M.; Nakamura, T. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 2016, 51, 206–213. [Google Scholar] [CrossRef]
- Regner, E.H.; Ohri, N.; Stahly, A.; Gerich, M.E.; Fennimore, B.P.; Ir, D.; Jubair, W.K.; Görg, C.; Siebert, J.; Robertson, C.E.; et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res. Ther. 2018, 20, 149. [Google Scholar] [CrossRef]
- Hu, M.D.; Edelblum, K.L. Sentinels at the frontline: The role of intraepithelial lymphocytes in inflammatory bowel disease. Curr. Pharmacol. Rep. 2017, 3, 321–334. [Google Scholar] [CrossRef]
- Bryant, R.V.; Winer, S.; SPL, T.; Riddell, R.H. Systematic review: Histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J. Crohn’s Colitis 2014, 8, 1582–1597. [Google Scholar] [CrossRef] [PubMed]
- Voiosu, T.; Benguş, A.; Dinu, R.; Voiosu, A.M.; Bălănescu, P.; Băicuş, C.; Diculescu, M.; Voiosu, R.; Mateescu, B. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: A prospective study. J. Gastrointestin Liver Dis. 2014, 23, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Chuai, S.; Nessel, L.; Lichtenstein, G.R.; Aberra, F.N.; Ellenberg, J.H. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm. Bowel Dis. 2008, 14, 1660–1666. [Google Scholar] [CrossRef]
- Ruscio, M.D.; Cedola, M.; Mangone, M.; Brighi, S. How to assess endoscopic disease activity in ulcerative colitis in 2022. Ann. Gastroenterol. 2022, 35, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sedano, R.; Almradi, A.; Vande Casteele, N.; Parker, C.E.; Guizzetti, L.; Schaeffer, D.F.; Riddell, R.H.; Pai, R.K.; Battat, R.; et al. An International Consensus to Standardize Integration of Histopathology in Ulcerative Colitis Clinical Trials. Gastroenterology 2021, 160, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.K.; Lo, B.; Bendtsen, F.; Vind, I.; Vester-Andersen, M.K.; Burisch, J. Health-related quality of life in inflammatory bowel disease in a Danish population-based inception cohort. United Eur. Gastroenterol. J. 2019, 7, 942–954. [Google Scholar] [CrossRef]
- Aslam, N.; Lo, S.W.; Sikafi, R.; Barnes, T.; Segal, J.; Smith, P.J.; Limdi, J.K. A review of the therapeutic management of ulcerative colitis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221138160. [Google Scholar] [CrossRef]
- Rosenberg, L.; Nanda, K.S.; Zenlea, T.; Gifford, A.; Lawlor, G.O.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Goldsmith, J.D.; Moss, A.C. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin. Gastroenterol. Hepatol. 2013, 11, 991–996. [Google Scholar] [CrossRef]
- Bessissow, T.; Lemmens, B.; Ferrante, M.; Bisschops, R.; Van Steen, K.; Geboes, K.; Van Assche, G.; Vermeire, S.; Rutgeerts, P.; De Hertogh, G. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am. J. Gastroenterol. 2012, 107, 1684–1692. [Google Scholar] [CrossRef]
- Colombel, J.F.; D’haens, G.; Lee, W.J.; Petersson, J.; Panaccione, R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J. Crohn’s Colitis 2020, 14, 254–266. [Google Scholar] [CrossRef]
- Chey, W.Y. Infliximab for patients with refractory ulcerative colitis. Inflamm. Bowel Dis. 2001, 7 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Molander, P.; Sipponen, T.; Kemppainen, H.; Jussila, A.; Blomster, T.; Koskela, R.; Nissinen, M.; Rautiainen, H.; Kuisma, J.; Kolho, K.L.; et al. Achievement of deep remission during scheduled maintenance therapy with TNFα-blocking agents in IBD. J. Crohn’s Colitis 2013, 7, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Moortgat, L.; Van Assche, G.; Caenepeel, P.; Vergauwe, P.; De Vos, M.; Stokkers, P.; Hommes, D.; Rutgeerts, P.; Vermeire, S.; et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010, 138, 463–468, quiz e10-1. [Google Scholar] [CrossRef] [PubMed]
- Riley, S.A.; Mani, V.; Goodman, M.J.; Dutt, S.; Herd, M.E. Microscopic activity in ulcerative colitis: What does it mean? Gut 1991, 32, 174–178. [Google Scholar] [CrossRef]
- Azad, S.; Sood, N.; Sood, A. Biological and histological parameters as predictors of relapse in ulcerative colitis: A prospective study. Saudi J. Gastroenterol. 2011, 17, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: A cohort study. Gastroenterology 2007, 133, 1099–1105, quiz 1340-1. [Google Scholar] [CrossRef]
- Christensen, B.; Erlich, J.; Gibson, P.R.; Turner, J.R.; Hart, J.; Rubin, D.T. Histologic Healing Is More Strongly Associated with Clinical Outcomes in Ileal Crohn’s Disease than Endoscopic Healing. Clin. Gastroenterol. Hepatol. 2020, 18, 2518–2525.e1. [Google Scholar] [CrossRef]
- Battat, R.; Duijvestein, M.; Guizzetti, L.; Choudhary, D.; Boland, B.S.; Dulai, P.S.; Parker, C.E.; Nguyen, T.M.; Singh, S.; Vande Casteele, N.; et al. Histologic Healing Rates of Medical Therapies for Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2019, 114, 733–745. [Google Scholar] [CrossRef]
- State, M.; Negreanu, L.; Voiosu, T.; Voiosu, A.; Balanescu, P.; Mateescu, R.B. Surrogate markers of mucosal healing in inflammatory bowel disease: A systematic review. World J. Gastroenterol. 2021, 27, 1828–1840. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Longhi, M.S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018, 103, 38–45. [Google Scholar] [CrossRef]
| Ulcerative Colitis | |
|---|---|
| Gender, male/female, n (%) | 83 (63)/48 (37) |
| Age, mean (SD), years | 43 (14) |
| Smoker/non-smoker, n (%) | 16 (12)/105 (80) |
| Disease extension, n (%) | E1 29 (22), E2 34 (26), E3 58 (44) |
| Disease duration, mean (SD), years | 5.0 (6.2) |
| Clinical activity, n (%) | 75 (57) |
| Active disease at endoscopy, n (%) | 100 (76) |
| Mayo endoscopic score 0/1/2/3, n (%) | 23 (17)/43 (32)/29 (22)/26 (19) |
| Mayo endoscopic score, median | 1 (0–3) |
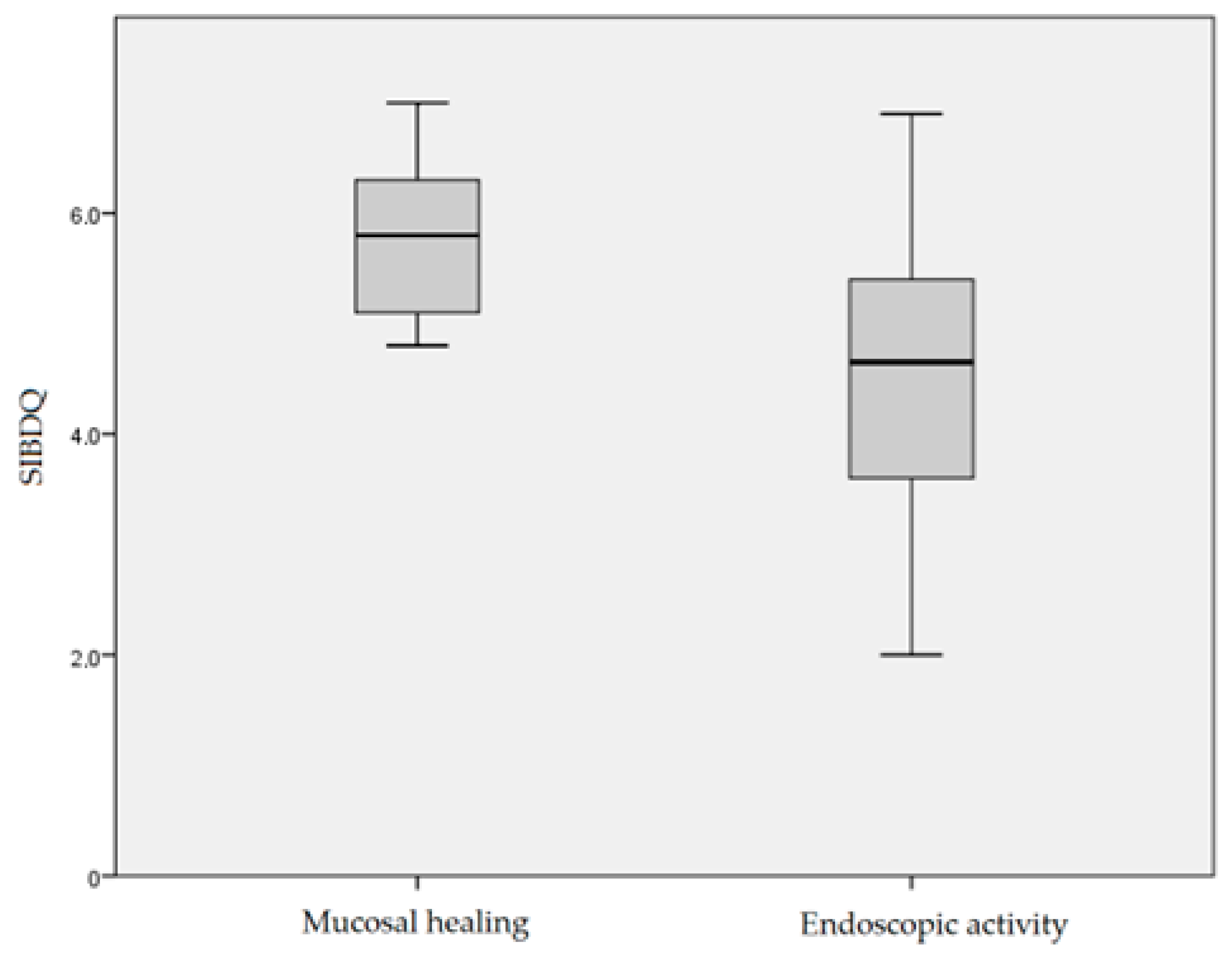
| SIBDQ, median (min, max) | 4.9 (2.0–7.0) |
| CRP, median (mg/dL) | 3.55 |
| Ongoing biologics, n (%) | 27 (20%) |
| Previous exposure to biologics, n (%) | 5 (0.3%) |
| Ongoing corticosteroids, n (%) | 32 (24%) |
| Parameter | Mucosal Healing | Endoscopic Activity | p Value |
|---|---|---|---|
| Gender (M/F) | 18/65 | 12/36 | 0.32 |
| Sustained clinical remission (yes/no) | 24/4 | 27/70 | <0.001 * |
| Ongoing corticosteroids (yes/no) | 2/26 | 30/68 | 0.004 * |
| Ongoing biologics (yes/no) | 7/21 | 22/76 | 0.08 |
| Smoking status (yes/no) | 22/3 | 81/12 | 0.90 |
| SIBDQ < 5.5 (yes/no) | 11/16 | 54/22 | 0.01 * |
| CRP > 5 mg/dL (yes/no) | 4/48 | 40/22 | 0.01 * |
| Variable | Unstandardized Coefficients | Standardized Coefficients | t | p | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| Age | 0.003 | 0.006 | 0.061 | 0.404 | 0.688 |
| Gender | −0.086 | 0.133 | −0.095 | −0.647 | 0.521 |
| Disease extension | 0.079 | 0.085 | 0.154 | 0.936 | 0.355 |
| Corticotherapy | 0.005 | 0.080 | 0.011 | 0.062 | 0.951 |
| Biologics | 0.020 | 0.142 | 0.024 | 0.138 | 0.891 |
| Smoking status | −0.191 | 0.213 | −0.134 | −0.895 | 0.376 |
| Disease duration | −0.016 | 0.010 | −0.248 | −1.660 | 0.104 |
| Parameter | Histologic Healing | Histologic Activity | p Value |
|---|---|---|---|
| Gender (M/F) | 14/6 | 16/12 | 0.36 |
| Endoscopic activity (yes/no) | 1/17 | 0/26 | 0.40 |
| Ongoing corticosteroids (yes/no) | 1/11 | 1/14 | 0.82 |
| Ongoing biologics (yes/no) | 7/10 | 9/16 | 0.91 |
| Smoking status (yes/no) | 2/16 | 3/23 | 0.96 |
| SIBDQ < 5.5 (yes/no) | 6/13 | 15/12 | 0.01 * |
| CRP > 5 mg/dL (yes/no) | 0/13 | 8/18 | 0.05 * |
| Variable | Ulcerative Colitis with MH at Follow-Up |
|---|---|
| Gender, male/female, n | 9/11 |
| Age, mean (SD), years | 49 (15) |
| Smoker/non-smoker, n | 4/15 |
| Disease extension, E1/E2/E3, n | 4/10/6 |
| Disease duration, mean (SD), years | 5.1 (8.3) |
| Corticosteroids, n | 0 |
| Biologics, n | 12 |
| Partial Mayo score, median | 1.5 |
| Endoscopic Mayo score, median | 1 |
| SIBDQ, median (min max) | 5.3 (2.8, 6.8) |
| CRP, median (mg/dL) | 5.86 |
| Parameter | Endoscopic Activity | Mucosal Healing | p Value |
|---|---|---|---|
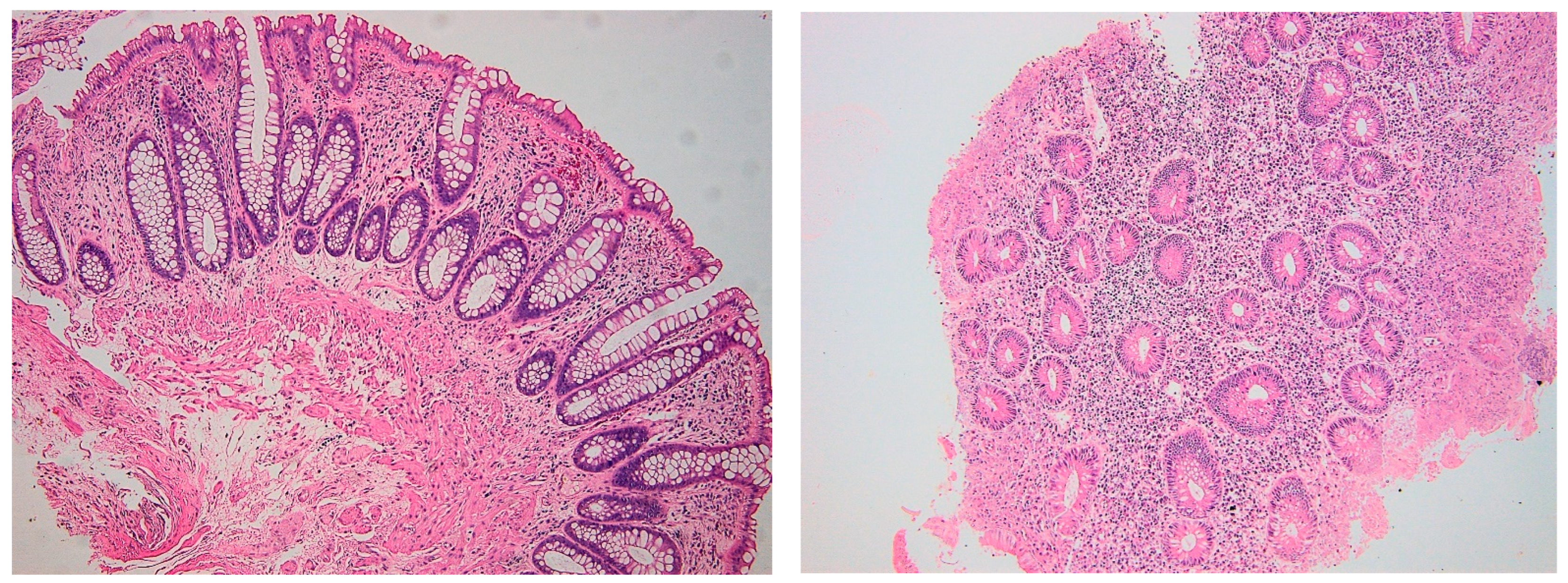
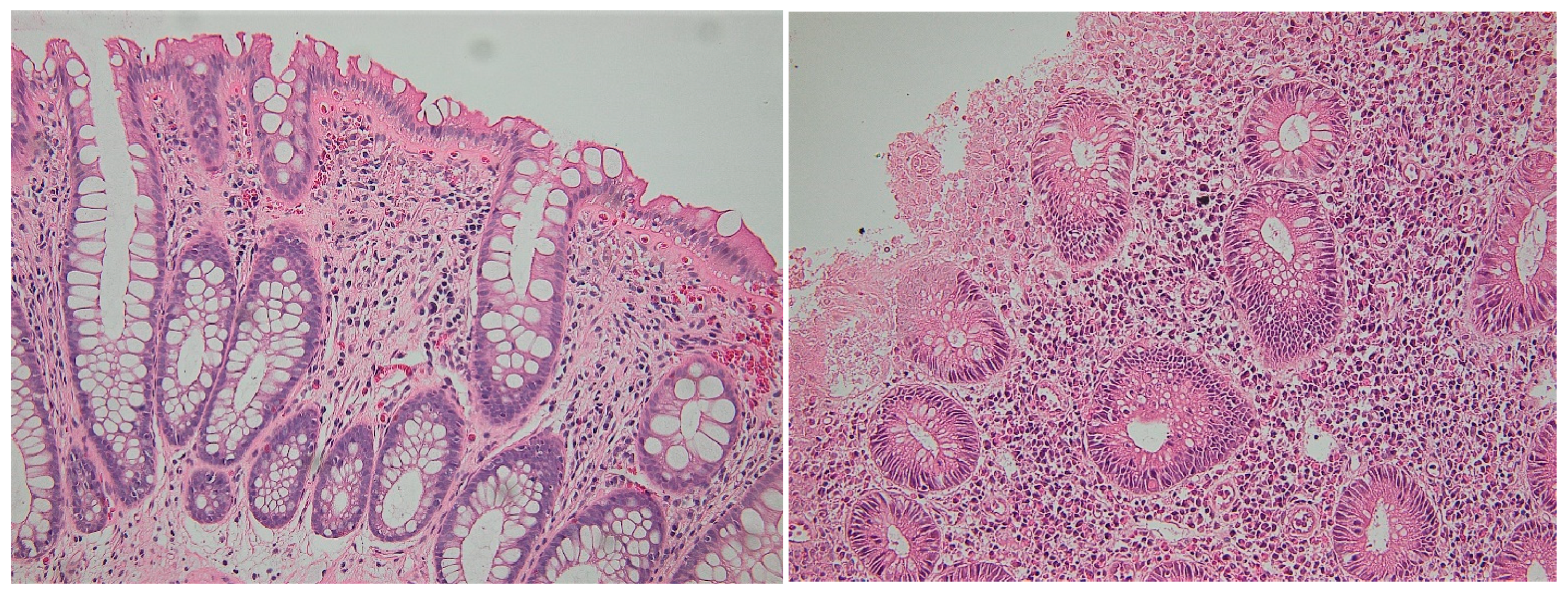
| Geboes score, median (min–max) | 4.3 (1–5) | 2.1 (0–5) | <0.01 |
| CD4+ IELs, median (min–max) | 6 (0–25) | 4 (0–13) | p < 0.01 |
| CD8+ IELs, Median (min–max) | 16 (5–42) | 10.5 (0–26) | p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
State, M.; Balanescu, P.; Voiosu, T.; Bengus, A.; Voiosu, A.; Coman, A.; Mustatea, P.; Negreanu, L.; Mateescu, R.B.; Popp, C. Real-World Endoscopic and Histologic Outcomes in Ulcerative Colitis Patients: A Retrospective Cohort Study. Biomedicines 2023, 11, 1860. https://doi.org/10.3390/biomedicines11071860
State M, Balanescu P, Voiosu T, Bengus A, Voiosu A, Coman A, Mustatea P, Negreanu L, Mateescu RB, Popp C. Real-World Endoscopic and Histologic Outcomes in Ulcerative Colitis Patients: A Retrospective Cohort Study. Biomedicines. 2023; 11(7):1860. https://doi.org/10.3390/biomedicines11071860
Chicago/Turabian StyleState, Monica, Paul Balanescu, Theodor Voiosu, Andreea Bengus, Andrei Voiosu, Andrei Coman, Petronel Mustatea, Lucian Negreanu, Radu Bogdan Mateescu, and Cristiana Popp. 2023. "Real-World Endoscopic and Histologic Outcomes in Ulcerative Colitis Patients: A Retrospective Cohort Study" Biomedicines 11, no. 7: 1860. https://doi.org/10.3390/biomedicines11071860
APA StyleState, M., Balanescu, P., Voiosu, T., Bengus, A., Voiosu, A., Coman, A., Mustatea, P., Negreanu, L., Mateescu, R. B., & Popp, C. (2023). Real-World Endoscopic and Histologic Outcomes in Ulcerative Colitis Patients: A Retrospective Cohort Study. Biomedicines, 11(7), 1860. https://doi.org/10.3390/biomedicines11071860