Is Spontaneous Preterm Prelabor of Membrane Rupture Irreversible? A Review of Potentially Curative Approaches
Abstract
:1. Introduction
2. Materials and Methods
Assessment of Reporting Quality
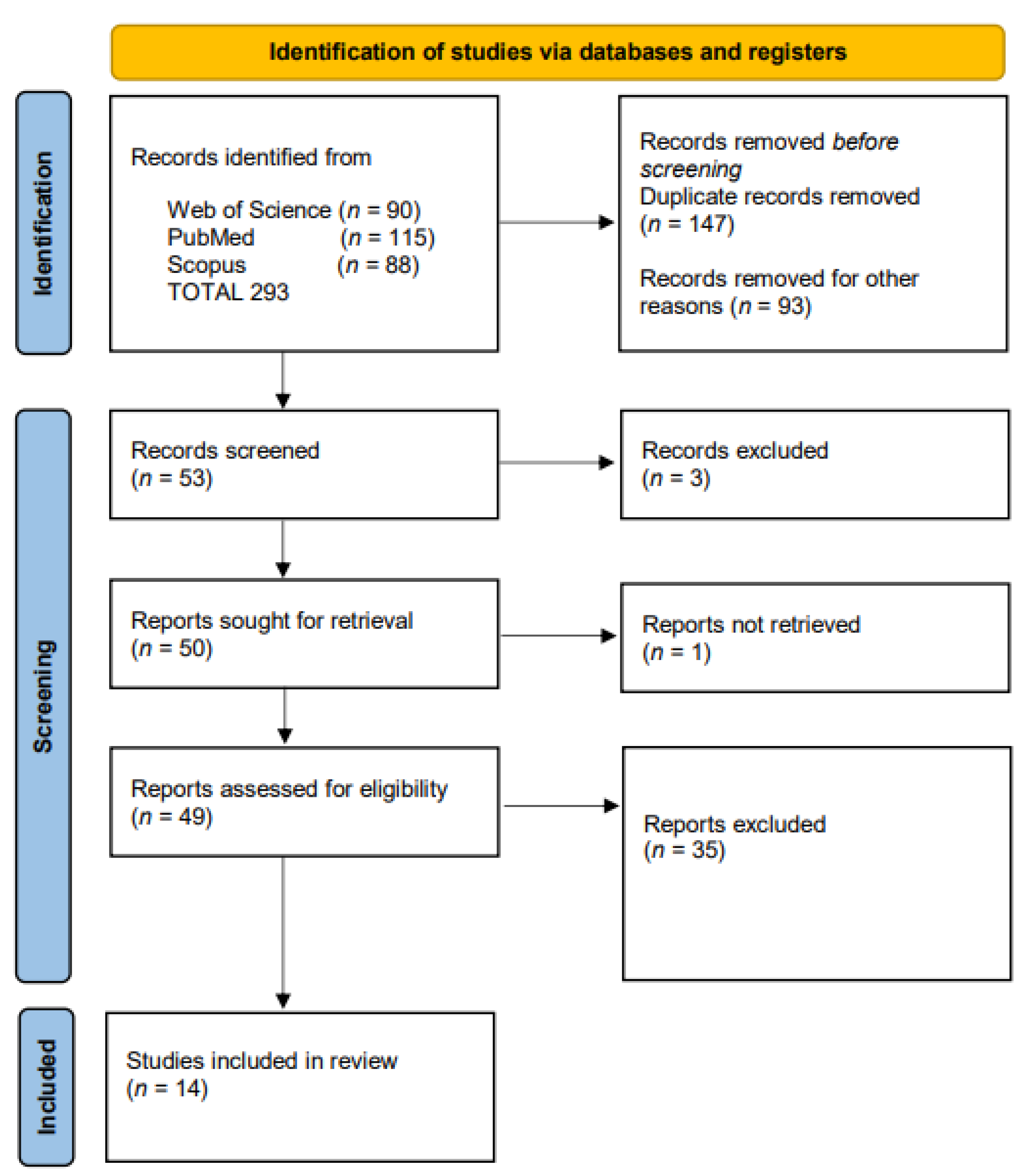
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bond, D.M.; Middleton, P.; Levett, K.M.; van der Ham, D.P.; Crowther, C.A.; Buchanan, S.L.; Morris, J. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 2017, 3, CD004735. [Google Scholar] [CrossRef]
- Dinsmoor, M.J.; Bachman, R.; Haney, E.I.; Goldstein, M.; MacKendrick, W. Outcomes after expectant management of extremely preterm premature rupture of the membranes. Am. J. Obstet. Gynecol. 2004, 190, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Lorthe, E.; Torchin, H.; Delorme, P.; Ancel, P.Y.; Marchand-Martin, L.; Foix-L’Hélias, L.; Benhammou, V.; Gire, C.; d’Ercole, C.; Winer, N.; et al. Preterm premature rupture of membranes at 22-25 weeks’ gestation: Perinatal and 2-year outcomes within a national population-based study (EPIPAGE-2). Am. J. Obstet. Gynecol. 2018, 219, 298.e1–298.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsakiridis, I.; Mamopoulos, A.; Chalkia-Prapa, E.M.; Athanasiadis, A.; Dagklis, T. Preterm Premature Rupture of Membranes: A Review of 3 National Guidelines. Obstet. Gynecol. Surv. 2018, 73, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Sklar, A.; Sheeder, J.; Davis, A.R.; Wilson, C.; Teal, S.B. Maternal morbidity after preterm premature rupture of membranes at <24 weeks’ gestation. Am. J. Obstet. Gynecol. 2022, 226, 558.e1–558.e11. [Google Scholar] [CrossRef]
- Elçi, G.; Çakmak, A.; Elçi, E.; Sayan, S. The effect of advanced maternal age on perinatal outcomes in nulliparous pregnancies. J. Perinat. Med. 2022, 50, 1087–1095. [Google Scholar] [CrossRef]
- Linehan, L.A.; Walsh, J.; Morris, A.; Kenny, L.; O’Donoghue, K.; Dempsey, E.; Russell, N. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: A retrospective cohort study. BMC Pregnancy Childbirth 2016, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.M.; Roberts, C.L.; Bowen, J.R.; Patterson, J.A.; Bond, D.M.; Algert, C.S.; Thornton, J.G.; Crowther, C.A.; PPROMT Collaboration. Immediate delivery compared with expectant management after preterm pre-labour rupture of the membranes close to term (PPROMT trial): A randomised controlled trial. Lancet 2016, 387, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Peaceman, A.M.; Lai, Y.; Rouse, D.J.; Spong, C.Y.; Mercer, B.M.; Varner, M.W.; Thorp, J.M.; Ramin, S.M.; Malone, F.D.; O’Sullivan, M.J.; et al. Length of latency with preterm premature rupture of membranes before 32 weeks’ gestation. Am. J. Perinatol. 2015, 32, 57–62. [Google Scholar] [CrossRef]
- Dale, P.O.; Tanbo, T.; Bendvold, E.; Moe, N. Duration of the latency period in preterm premature rupture of the membranes. Maternal and neonatal consequences of expectant management. Eur. J. Obstet. Gynecol. Reprod. Biol. 1989, 30, 257–262. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Kuckelman, J.P.; Kononchik, J.; Smith, J.; Kniery, K.R.; Kay, J.T.; Hoffer, Z.S.; Steele, S.R.; Sohn, V. Human-Derived Amniotic Membrane Is Associated with Decreased Postoperative Intraperitoneal Adhesions in a Rat Model. Dis. Colon. Rectum. 2018, 61, 484–490. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyoshi, S.; Ikegami, Y.; Hida, N.; Asada, H.; Togashi, I.; Suzuki, J.; Satake, M.; Nakamizo, H.; Tanaka, M.; et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ. Res. 2010, 106, 1613–1623. [Google Scholar] [CrossRef]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyce, E.M.; Diaz, P.; Tamarkin, S.; Moore, R.; Strohl, A.; Stetzer, B.; Kumar, D.; Sacks, M.S.; Moore, J.J. In-vivo stretch of term human fetal membranes. Placenta 2016, 38, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Rautava, S.; Aakko, J.; Isolauri, E.; Salminen, S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep. 2016, 6, 23129. [Google Scholar] [CrossRef] [Green Version]
- Combs, C.A.; Gravett, M.; Garite, T.J.; Hickok, D.E.; Lapidus, J.; Porreco, R.; Rael, J.; Grove, T.; Morgan, T.K.; Clewell, W.; et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am. J. Obstet. Gynecol. 2014, 210, 125.e1–125.e15. [Google Scholar] [CrossRef]
- Dutta, E.H.; Behnia, F.; Boldogh, I.; Saade, G.R.; Taylor, B.D.; Kacerovský, M.; Menon, R. Oxidative stress damage-associated molecular signaling pathways differentiate spontaneous preterm birth and preterm premature rupture of the membranes. Mol. Hum. Reprod. 2016, 22, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Lavery, J.P.; Miller, C.E.; Knight, R.D. The effect of labor on the rheologic response of chorioamniotic membranes. Obstet. Gynecol. 1982, 60, 87–92. [Google Scholar] [PubMed]
- Devlieger, R.; Millar, L.K.; Bryant-Greenwood, G.; Lewi, L.; Deprest, J.A. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: A review of current evidence. Am. J. Obstet. Gynecol. 2006, 195, 1512–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erez, O.; Romer, R.; Vaisbuch, E.; Chaiworapongsa, T.; Kusanovic, J.P.; Mazaki-Tovi, S.; Gotsch, F.; Gomez, R.; Maymon, E.; Pacora, P.; et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: Evidence of an increased thrombin generation. J. Matern. Fetal Neonatal Med. 2009, 22, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Gomez, R.; Romero, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Berry, S.M. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol. 1998, 179, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, P.E.; Parry, S.; Sammel, M.; Macones, G.A.; Kuivaniemi, H.; Romero, R.; Strauss, J.F., 3rd. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol. Hum. Reprod. 2002, 8, 494–501. [Google Scholar] [CrossRef] [Green Version]
- Severi, F.M.; Bocchi, C.; Voltolini, C.; Borges, L.E.; Florio, P.; Petraglia, F. Thickness of fetal membranes: A possible ultrasound marker for preterm delivery. Ultrasound Obstet. Gynecol. 2008, 32, 205–209. [Google Scholar] [CrossRef]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem. Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef]
- Mohammed, Y.A.; Farouk, H.K.; Gbreel, M.I.; Ali, A.M.; Salah, A.A.; Nourelden, A.Z.; Gawad, M.M.A. Human amniotic membrane products for patients with diabetic foot ulcers. do they help? A systematic review and meta-analysis. J. Foot Ankle Res. 2022, 15, 71. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, L.; Yan, J. Amniotic membrane for treating skin graft donor sites: A systematic review and meta-analysis. Burns 2020, 46, 621–629. [Google Scholar] [CrossRef]
- Corrêa, M.E.A.B.; Mendes, C.; Bittencourt, J.V.S.; Takejima, A.; de Souza, I.C.; de Carvalho, S.C.D.; Orlandini, I.G.; de Andrade, T.A.M.; Guarita-Souza, L.C.; Silveira, P.C.L. Effects of the Application of Decellularized Amniotic Membrane Solubilized with Hyaluronic Acid on Wound Healing. Ann. Biomed. Eng. 2022, 50, 1895–1910. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Goetzinger, K.; Javinani, A.; Espinoza, J.; Aghajani, F.; Harman, C.; Shamshirsaz, A.A.; Sanz Cortes, M.; Donepudi, R.V.; Krispin, E.; et al. Surgery for TTTS- Systematic review and Meta-analysis. Am. J. Obstet. Gynecol. 2022, 26, S174–S175. [Google Scholar] [CrossRef]
- Micheletti, T.; Eixarch, E.; Bennasar, M.; Torres, X.; Martinez-Crespo, J.M.; Deprest, J.; Gratacos, E. Risk Factors Associated with Preterm Prelabor Rupture of Membranes after Cord Occlusion in Monochorionic Diamniotic Twins. Fetal Diagn. Ther. 2021, 48, 457–463. [Google Scholar] [CrossRef]
- Harrison, M.R.; Mychaliska, G.B.; Albanese, C.T.; Jennings, R.W.; Farrell, J.A.; Hawgood, S.; Sandberg, P.; Levine, A.H.; Lobo, E.; Filly, R.A. Correction of congenital diaphragmatic hernia in utero IX: Fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J. Pediatr. Surg. 1998, 33, 1017–1022; discussion 1022-3. [Google Scholar] [CrossRef] [PubMed]
- Graves, C.E.; Harrison, M.R.; Padilla, B.E. Minimally invasive fetal surgery. Clin. Perinatol. 2017, 44, 729–751. [Google Scholar] [CrossRef]
- Beck, V.; Lewi, P.; Gucciardo, L.; Devlieger, R. Preterm prelabor rupture of membranes and fetal survival after minimally invasive fetal surgery: A systematic review of the literature. Fetal Diagn. Ther. 2012, 31, 1–9. [Google Scholar] [CrossRef]
- Gratacós, E.; Sanin-Blair, J.; Lewi, L.; Toran, N.; Verbist, G.; Cabero, L.; Deprest, J. A histological study of fetoscopic membrane defects to document membrane healing. Placenta 2006, 27, 452–456. [Google Scholar] [CrossRef]
- Chang, J.; Tracy, T.F.; Carr, S.R.; Sorrells, D.L.; Luks, F.I. Port insertion and removal techniques to minimize premature rupture of the membranes in endoscopic fetal surgery. J. Pediatr. Surg. 2006, 41, 905–909. [Google Scholar] [CrossRef]
- Engels, A.C.; Van Calster, B.; Richter, J.; DeKoninck, P.; Lewi, L.; De Catte, L.; Devlieger, R.; Deprest, J.A. Collagen plug sealing of iatrogenic fetal membrane defects after fetoscopic surgery for congenital diaphragmatic hernia. Ultrasound Obstet. Gynecol. 2014, 43, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Sorano, S.; Fukuoka, M.; Kawakami, K.; Momohara, Y. Prognosis of preterm premature rupture of membranes between 20 and 24 weeks of gestation: A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 5, 100102. [Google Scholar] [CrossRef] [PubMed]
- Tchirikov, M.; Schlabritz-Loutsevitch, N.; Maher, J.; Buchmann, J.; Naberezhnev, Y.; Winarno, A.S.; Seliger, G. Mid-trimester preterm premature rupture of membranes (PPROM): Etiology, diagnosis, classification, international recommendations of treatment options and outcome. J. Perinat. Med. 2018, 46, 465–488. [Google Scholar] [CrossRef] [PubMed]
- Herzlich, J.; Mangel, L.; Halperin, A.; Lubin, D.; Marom, R. Neonatal outcomes in women with preterm premature rupture of membranes at periviable gestational age. Sci. Rep. 2022, 12, 11999. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Kringe, L.; Sena, E.S.; Motschall, E.; Bahor, Z.; Wang, Q.; Herrmann, A.M.; Mülling, C.; Meckel, S.; Boltze, J. Quality and validity of large animal experiments in stroke: A systematic review. J. Cereb. Blood Flow Metab. 2020, 40, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Kamel, H.H.; Sanad, A.S.; Mahram, A.E.; AbdAllah, A.A.; Elkhateeb, R.; Bhaa, H.A.; Hussein, E.A.; Essam, A.; Ibrahim, S. The value of amniopatch in pregnancies associated with spontaneous preterm premature rupture of fetal membranes: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2021, 34, 267–273. [Google Scholar] [CrossRef]
- Ibirogba, E.R.; Shazly, S.A.; Narang, K.; Wahood, W.; Trad, A.T.A.; Tsimis, M.E.; Ruano, R. Interventional resealing of preterm premature rupture of the membranes: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 5978–5992. [Google Scholar] [CrossRef]
- Kwak, H.M.; Choi, H.J.; Cha, H.H.; Yu, H.J.; Lee, J.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R.; Kim, J.H. Amniopatch treatment for spontaneous previable, preterm premature rupture of membranes associated or not with incompetent cervix. Fetal Diagn. Ther. 2013, 33, 47–54. [Google Scholar] [CrossRef]
- Crowley, A.E.; Grivell, R.M.; Dodd, J.M. Sealing procedures for preterm prelabour rupture of membranes. Cochrane Database Syst. Rev. 2016, 7, CD010218. [Google Scholar] [CrossRef]
- Sung, J.H.; Kuk, J.Y.; Cha, H.H.; Choi, S.J.; Oh, S.Y.; Roh, C.R.; Kim, J.H. Amniopatch treatment for preterm premature rupture of membranes before 23 weeks’ gestation and factors associated with its success. Taiwan J. Obstet. Gynecol. 2017, 56, 599–605. [Google Scholar] [CrossRef]
- Ferianec, V.; Križko, M.; Gábor, M.; Papcun, P.; Alföldi, M.; Feriancová, M. Amniopatch as an active treatment of spontaneous previable rupture of membranes. J. Matern. Fetal Neonatal Med. 2022, 35, 9900–9906. [Google Scholar] [CrossRef] [PubMed]
- Kivelio, A.; Dekoninck, P.; Perrini, M.; Brubaker, C.E.; Messersmith, P.B.; Mazza, E.; Deprest, J.; Zimmermann, R.; Ehrbar, M.; Ochsenbein-Koelble, N. Mussel mimetic tissue adhesive for fetal membrane repair: Initial in vivo investigation in rabbits. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 240–245. [Google Scholar] [CrossRef]
- Mogami, H.; Hari Kishore, A.; Akgul, Y.; Word, R.A. Healing of Preterm Ruptured Fetal Membranes. Sci. Rep. 2017, 7, 13139. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Kim, H.; Ha, D.H.; Shin, J.C.; Kim, A.; Ko, H.S.; Cho, D.W. Amnion-Analogous Medical Device for Fetal Membrane Healing: A Preclinical Long-Term Study. Adv. Healthc. Mater. 2018, 7, e1800673. [Google Scholar] [CrossRef] [PubMed]
- Engels, A.C.; Joyeux, L.; Van der Merwe, J.; Jimenez, J.; Pranpanus, S.; Barrett, D.W.; Connon, C.; Chowdhury, T.T.; David, A.L.; Deprest, J. Tissuepatch is biocompatible and seals iatrogenic membrane defects in a rabbit model. Prenat. Diagn. 2018, 38, 99–105. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, C.; Xu, T.; Lin, S.; Wang, Z.; Zhu, Y. Subaqueous Bioprinting: A Novel Strategy for Fetal Membrane Repair with 7-Axis Robot-Assisted Minimally Invasive Surgery. Adv. Funct. Mater. 2022, 32, 2207496. [Google Scholar] [CrossRef]
- Avilla-Royo, E.; Seehusen, F.; Devaud, Y.R.; Monné Rodriguez, J.M.; Strübing, N.; Weisskopf, M.; Messersmith, P.B.; Vonzun, L.; Moehrlen, U.; Ehrbar, M.; et al. In vivo Sealing of Fetoscopy-Induced Fetal Membrane Defects by Mussel Glue. Fetal Diagn. Ther. 2022, 49, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Ryu, K.J.; Ahn, K.H.; Kang, D.; Geum, D.H.; Kim, B.S.; Cho, G.J.; Oh, M.J.; Kim, H.J.; Hong, S.C. Spontaneous healing of human amnion in the premature rupture of membrane model. Placenta 2020, 97, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, E.; Kawamura, Y.; Chigusa, Y.; Mogami, H.; Ueda, A.; Hamanishi, J.; Mandai, M. Intracervical elastomeric sealant in an ex vivo model. J. Matern. Fetal Neonatal Med. 2021, 34, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.W.; Okesola, B.O.; Costa, E.; Thrasivoulou, C.; Becker, D.L.; Mata, A.; Deprest, J.A.; David, A.L.; Chowdhury, T.T. Potential sealing and repair of human FM defects after trauma with peptide amphiphiles and Cx43 antisense. Prenat. Diagn. 2021, 41, 89–99. [Google Scholar] [CrossRef]
- Meuwese, R.T.C.; Versteeg, E.M.M.; van Drongelen, J.; de Hoog, D.; Bouwhuis, D.; Vandenbussche, F.P.H.A.; van Kuppevelt, T.H.; Daamen, W.F. A collagen plug with shape memory to seal iatrogenic fetal membrane defects after fetoscopic surgery. Bioact. Mater. 2022, 20, 463–471. [Google Scholar] [CrossRef]
- Vaitkiene, D.; Bergström, S. Management of amniocentesis in women with oligohydramnios due to membrane rupture: Evaluation of a cervical adapter. Gynecol. Obstet. Invest. 1995, 40, 28–31. [Google Scholar] [CrossRef]
- Dam, P.; Somnath, L.; Parnamita, B.; Pallavi, D. Role of amnioseal in premature rupture of membranes. J. Obstet. Gynaecol. India 2011, 61, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.W.; Egerman, R.S.; Moorhead, J. Cases with ruptured membranes that “reseal”. Am. J. Obstet. Gynecol. 1990, 163, 1024–1030; discussion 1030–1022. [Google Scholar] [CrossRef]
- Bilic, G.; Ochsenbein-Kölble, N.; Hall, H.; Huch, R.; Zimmermann, R. In vitro lesion repair by human amnion epithelial and mesenchymal cells. Am. J. Obstet. Gynecol. 2004, 190, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, M.S.; Kim, Y.G.; Kang, D.; Choi, H.Y.; Pae, J.Y.; Wie, J.H.; Park, I.Y. Characteristics of amniotic mesenchymal stromal cells derived from term and preterm labor. Taiwan J. Obstet. Gynecol. 2022, 61, 51–56. [Google Scholar] [CrossRef]
- Mogami, H.; Word, R.A. Healing Mechanism of Ruptured Fetal Membrane. Front. Physiol. 2020, 11, 623. [Google Scholar] [CrossRef] [PubMed]
- Mogami, H.; Kishore, A.H.; Word, R.A. Collagen Type 1 Accelerates Healing of Ruptured Fetal Membranes. Sci. Rep. 2018, 8, 696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.L.; Chen, Y.; Xu, D.X.; Chen, D.Z. Possible important roles of galectins in the healing of human fetal membranes. Front. Endocrinol. 2022, 13, 941029. [Google Scholar] [CrossRef] [PubMed]
- Vintzileos, A.M.; Campbell, W.A.; Nochimson, D.J. Degree of oligohydramnios and pregnancy outcome in patients with preterm premature rupture of the membranes. Obstet. Gynecol. 1985, 66, 162–167. [Google Scholar]
- Vermillion, S.; Kooba, A. Amniotic fluid index value after prom and subsequent perinatal infection. Am. J. Obstet. Gynecol. 2000, 183, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Deruelle, P.; Behal, H.; Rakza, T.; Balagny, S.; Subtil, D.; Clouqueur, E.; Garabedian, C. Preterm premature rupture of membranes: Which criteria contraindicate home care management? Acta. Obstet. Gynecol. Scand. 2018, 97, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Benson-Martin, J.; Zammaretti, P.; Bilic, G.; Schweizer, T.; Portmann-Lanz, B.; Burkhardt, T.; Zimmermann, R.; Ochsenbein-Kölble, N. The Young’s modulus of fetal preterm and term amniotic membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 103–107. [Google Scholar] [CrossRef] [PubMed]
| Article (Author *, Year, Country) | Type of Study | Gestational Age at PPROM | Number of Subjects/ Procedures | Treatment and/or Evaluation before Sealing Method | Technique Used and Sealing Material | Treatment and Evaluation after Sealing Method | Outcomes |
|---|---|---|---|---|---|---|---|
| Kwak, 2013 [47], South Korea | Case–control (amniopatch versus conservative management group) | 17–23 weeks | 7/8 | Antibioprophylaxis for 7 days and Amniotic fluid | Amniopatch with autologous platelet concentrates and cryoprecipitate inserted by ultrasound-guided amnioinfusion technique after amnioinfusion after 7 days | Corticotherapy for pulmonary maturation 24 h after sealing | Lower incidence of respiratory distress syndrome and lower incidence of neonatal sepsis in the amniopatch group. |
| Crowley, 2016 [48], India, Lituania | Randomized and quasi-randomized control trial | less than 37 weeks | 59/16 cases cervical adapter and 43 cases oral immunological membrane sealing | Cervical bacteriology before cervical adapter Amnioseal: antibioprophylaxy, tocolysis and prophylactic corticosteroids | Cervical adapter (mechanical sealing) in the 24 h after PPROM. Oral immunological membrane Amnioseal (TNS Meryl Pharma) (a combination of matrix metalloproteinases inhibitors, cytokines and defensins)- two capsules after 3 h, followed by two capsules 8 hourly for up to 72 h. Maintenance dose was one capsule twice daily for 15 days and one capsule daily for another 15 days | Cervical adapter: Evaluation for AFI and chorioamnionitis Amnioseal: evaluation for chorioamnionitis, AFI and liver function | Cervical adapter is useful for an increase in amniotic fluid volume |
| Sung, 2018 [49] South Korea | Cohort, retrospective | 15–23 weeks | 17/21 | Antibiotherapy for 2 days Measurement of amniotic fluid volume by MVP | Amniopatch autologous after 2 days of conservative treatment, ultrasound-guided amnioinfusion, using 20–22 gauge needles, of the platelet concentrate followed by cryoprecipitate | Antibiotic therapy Daily measurement of amniotic fluid +/− Corticotherapy or tocolytic | Lower incidence of respiratory distress syndrome and early neonatal sepsis in the amniopatch group |
| Ferianec, 2022 [50], Slovakia | Descriptive | 19 + 3–22 weeks. Cervical length of more than 25 mm | 53 | NS | Amniopatch platelets and fresh frozen plasma from donors, transamniotic, after minimum 10 days post rupture, amnioinfusion | NS | No maternal/fetal complications directly related to the amniopatch procedure |
| Article (Author *, Year, Country) | Preclinical Model/Type of Study | Length of Gestation | Intervention/Mechanism | Diameter of Defect | Performance of the Materiel Used | Pregnancy Outcomes |
|---|---|---|---|---|---|---|
| Kivelio, 2013, [51] Switzerland, Belgium | Mid gestational model, case–control study | 31 days | Mussel glue (MG) alone or combined with decellularized amnion membrane (DAM), and fibrin glue (FG) combined with decellularized amnion membrane | Large amniotic defect of 2.1 mm | Short-term outcomes, after 7 days 75% sealing membrane | 80% of fetal survival for MG + DAM, 60% for MG and 40% for FG + DAM |
| Mogami, 2017 [52], US | Mouse model of sterile membrane rupture mechanism healing | 21 days | Arg1-positive M2-macrophages. Amniotic fluid macrophages of fetal origin at the level of amnion | Small (0.47 mm) and large rupture (0.91 mm) | Mid-term evaluation at 15 days of gestation Small ruptures of the amnion closed by 24–72 h, >50% of large ruptures remained open | 86% of intrauterine fetal survival rates after small rupture and 82% after a large rupture at 72 h |
| Lee, 2018 [53], South Korea | Micropig M-type, case–control study | 114 days | Amnion-analogous medical device (AMED), a biocompatible 3D–printed device containing amniotic membrane-derived gel, compared with AmnioGraftpatch and adhesive group or decellularized human membranes (DAM) group or nonsealing group | 1.2 mm | Short- and long-term evaluation AMED is easy, rapid, and is a better target to apply than an Amniopatch or DAM. Decellularized amniotic membrane gel heals wounds more than two times faster than collagen | AMED improved the preservation of the amniotic fluid, needs short surgical time for insertion, and is associated with better fetal survival and development |
| Engels, 2018 [54], Belgium | Case–control study | 31 days | Conventional collagen (Lyostypt, B. Braun Medical N.V., Melsungen, Germany), Lyostypt soaked in fibrinogen concentrate (Haemocomplettan, CSL Behring, Breda, The Netherlands), condensed collagen from the human amniotic membrane (CCHA), Tissuepatch (Tissuemed Ltd., Leeds, UK), and Duraseal (Integra LS N.V., Zaventem, Belgium) | 1.3 mm defect at 23 days of gestation | Evaluation at term CCHA and Tissuepatch had no effect on fetal survival when compared to unmanipulated control sacs (without sealant), also sealed sacs more efficiently with low fluid leakage but dissolved rapidly | Fetal survival rate is lower in the sealant groups, 72%, respectively, 78%, 77%, and 60%. |
| Zhao, 2022 [55], China | Mid-gestational New Zealand rabbit model, case–control study | 32 days | Ultrafast photoresponsive hydrogel (1.5 s) and a 7-axis bioprinting robot to perform subaqueous in situ bioprinting in a minimally invasive approach | 1.9 mm defect at 22 days of gestation | Evaluation at term 8 out of 10 patches show complete sealing. All patches were founded: 2 out of 10 patches were freely in the uterus. In 8 out of 10 cases no amniotic fluid leakage | Fetal survival rate was 72.7% in the sealing group and 81.3% in the native control group. After the rupture of membranes, no fetal weight gain |
| Avilla-Royo, 2023 [56], Switzerland | Swiss Alpine white Ewes model case–control | 145–155 days | Elastic Mussel glue of a copolymer of poly(propylene oxide) and flexible poly(ethylene) oxide applied by an umbrella-shaped device, followed by the closure of the uterine defect | 11 mm uterine and fetal defect at gestational age 56–69 days | Evaluation 10 days after introduction. Sutures and glue-induced adhesions were observed on 4 out of 8 horns. All implant sites were tightly sealed, and no fluid leakage, no amnion bands, or skin defects were observed in any of the fetuses | 10 survival from 11 cases. No reported maternal or fetal complications |
| Study *, Year, Country | Amniotic Membrane Tissue Model | Description of Membranes | Healing Mechanism or Material | Diameter of Membrane Defect | Possible Mechanism | Outcomes |
|---|---|---|---|---|---|---|
| Lee, 2020 [57], South Korea | Human amniotic membranes. Human amnion pore culture technique | 39–40 weeks | Spontaneous healing mechanism | 1, 2, and 3 mm | The human amnion might possibly retain pluripotent properties, such as promoting cell regeneration and natural healing membranes attributed to the amniotic epithelial stem cells (AESCs) | Cellular regrowth in the punched amniotic membrane tissue that covered the pore area within 10 days of incubation in all cases of membrane rupture by resealing small pores (<1 mm), but with no significant change in the size of the large pores (2 and 3 mm in diameter) |
| Kondoh, 2021 [58], Japan. | Ex vivo model of non-pregnant uterus | NS | Intracervical elastomeric sealant HydrofitR (Sanyo Chemical Industries, Ltd. Kyoto, Japan) compared with fibrin glue (0.3 mL of thrombin solution with 0.3 mL of fibrinogen solution, Bolheal (Teijin, Osaka, Japan) | NS | The sealant would have the potential to prevent the leakage of amniotic fluid in pregnancies with previable premature rupture of membranes | No amniotic fluid 15 min after application |
| Barrett, 2021 [59] UK | Human amniotic liquid (16–24 weeks). Human membranes | 39–40 weeks | Peptide amphiphiles (PAs) conjugated with ligands for cell-adhesion (RGDS), migratory (GHK), or regenerative (GHK/RGDS) peptides assembled with amniotic fluid. PAs are represented by PAK2, PAK3, PAK4, and PAH3. PAs were applied to the surface of the membrane defect and cultured for up to 5 days with amniotic fluid replaced every 48 h | 0.8 mm | PAK3 and amniotic fluid molecules form a solid membrane at the PAK3–AF interface; PAK2, PAK4, and PAH3 form a soft, liquid, or paste-like gel membrane that disintegrates after 6 h of culture | PAK3 forms a multi-layer nanofibrous network, a plug, that seals the membranes defect |
| Meuwese, 2022 [60], The Netherlands | Human fetal membranes 4 h–24 h after birth. A sac was formed and filled with water | NS | Crimped, froze, and crosslinked lyophilized type 1 collagen plug to obtain a highly purified collagen plug with shape memory | 3 mm | Crosslinking, expanding, shape recovery, freezing, lyophilization, and crimping. The plugs triple their diameter within a minute | No further rupture of the membranes caused by the expansion of the plug The plug expanded from 1.8 mm to more than 6 mm in 60 s, more than three times its diameter. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danciu, B.M.; Oţelea, M.R.; Marincaş, M.A.; Niţescu, M.; Simionescu, A.A. Is Spontaneous Preterm Prelabor of Membrane Rupture Irreversible? A Review of Potentially Curative Approaches. Biomedicines 2023, 11, 1900. https://doi.org/10.3390/biomedicines11071900
Danciu BM, Oţelea MR, Marincaş MA, Niţescu M, Simionescu AA. Is Spontaneous Preterm Prelabor of Membrane Rupture Irreversible? A Review of Potentially Curative Approaches. Biomedicines. 2023; 11(7):1900. https://doi.org/10.3390/biomedicines11071900
Chicago/Turabian StyleDanciu, Bianca Mihaela, Marina Ruxandra Oţelea, Marian Augustin Marincaş, Maria Niţescu, and Anca Angela Simionescu. 2023. "Is Spontaneous Preterm Prelabor of Membrane Rupture Irreversible? A Review of Potentially Curative Approaches" Biomedicines 11, no. 7: 1900. https://doi.org/10.3390/biomedicines11071900
APA StyleDanciu, B. M., Oţelea, M. R., Marincaş, M. A., Niţescu, M., & Simionescu, A. A. (2023). Is Spontaneous Preterm Prelabor of Membrane Rupture Irreversible? A Review of Potentially Curative Approaches. Biomedicines, 11(7), 1900. https://doi.org/10.3390/biomedicines11071900






