Immunohistological Analysis of Lichen Sclerosus of the Foreskin in Pediatric Age: Could It Be Considered a Premalignant Lesion?
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
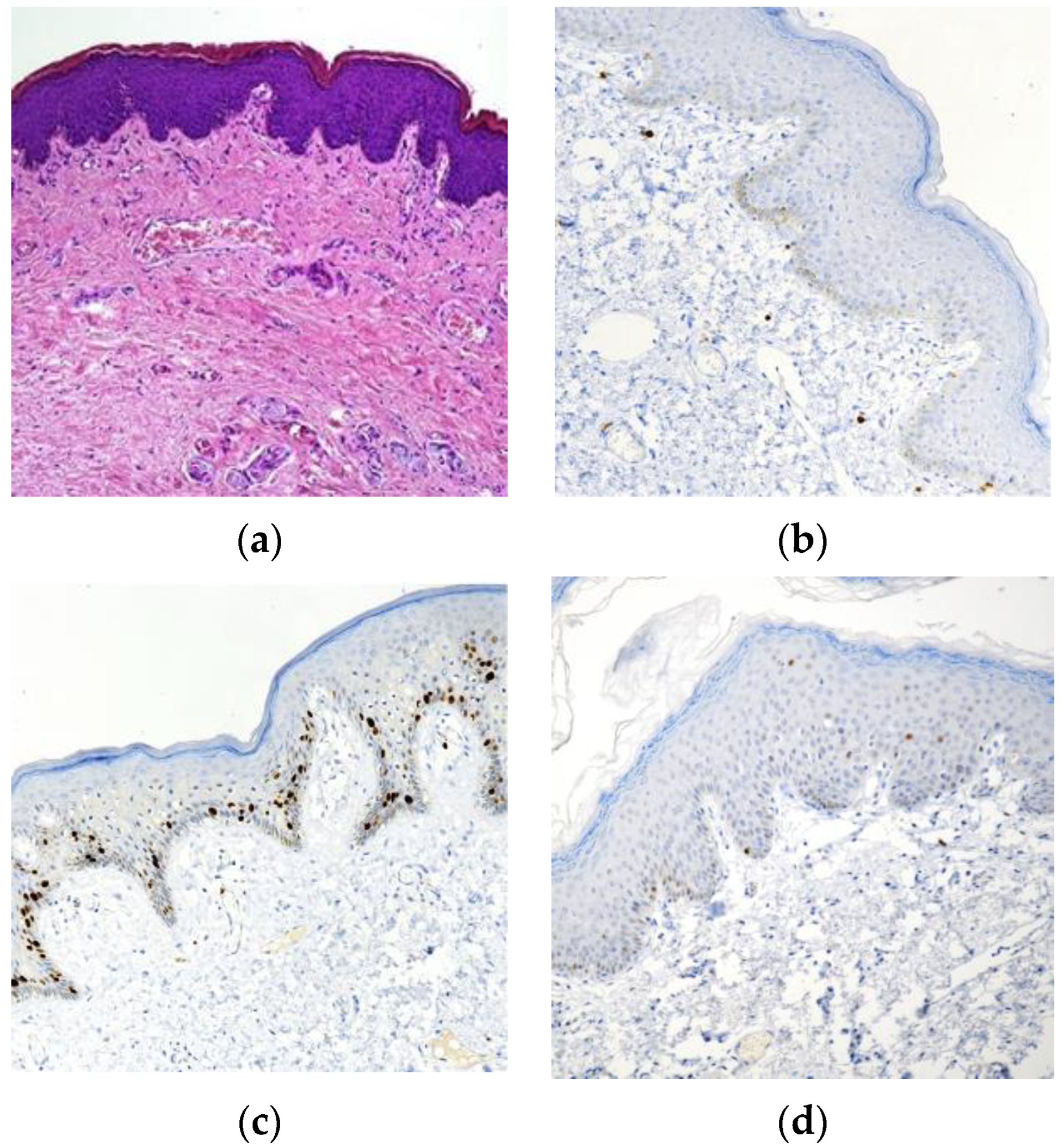
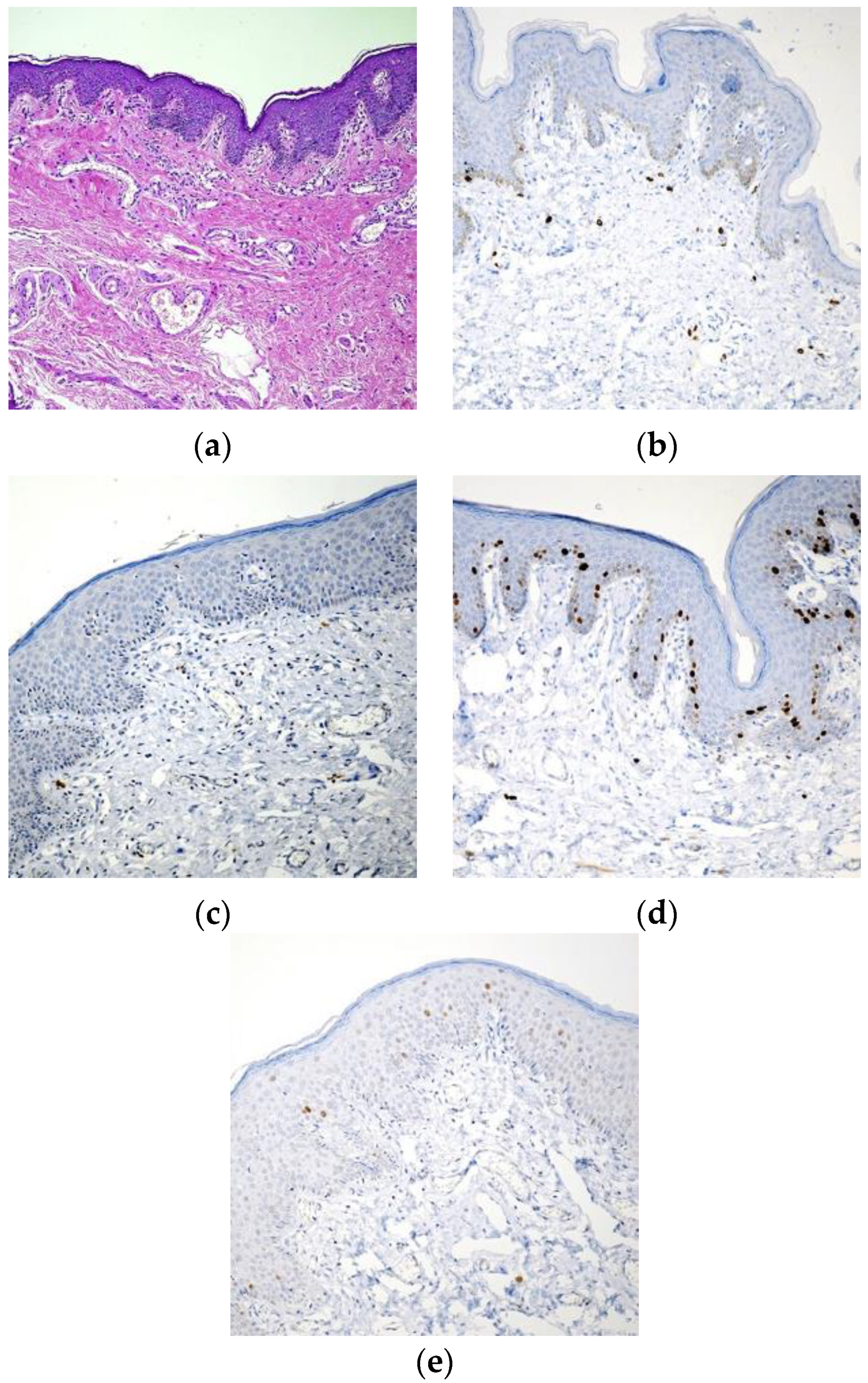
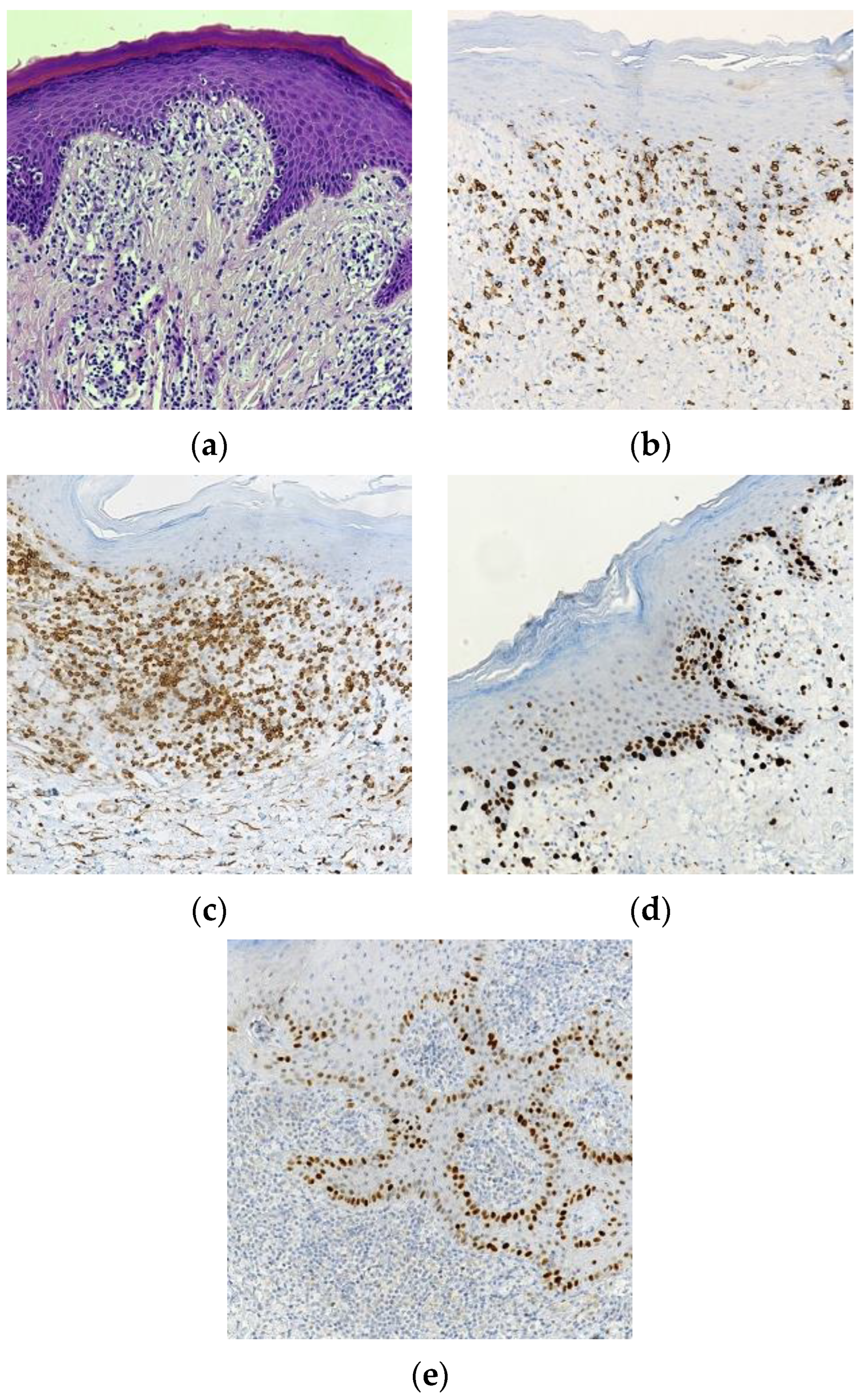
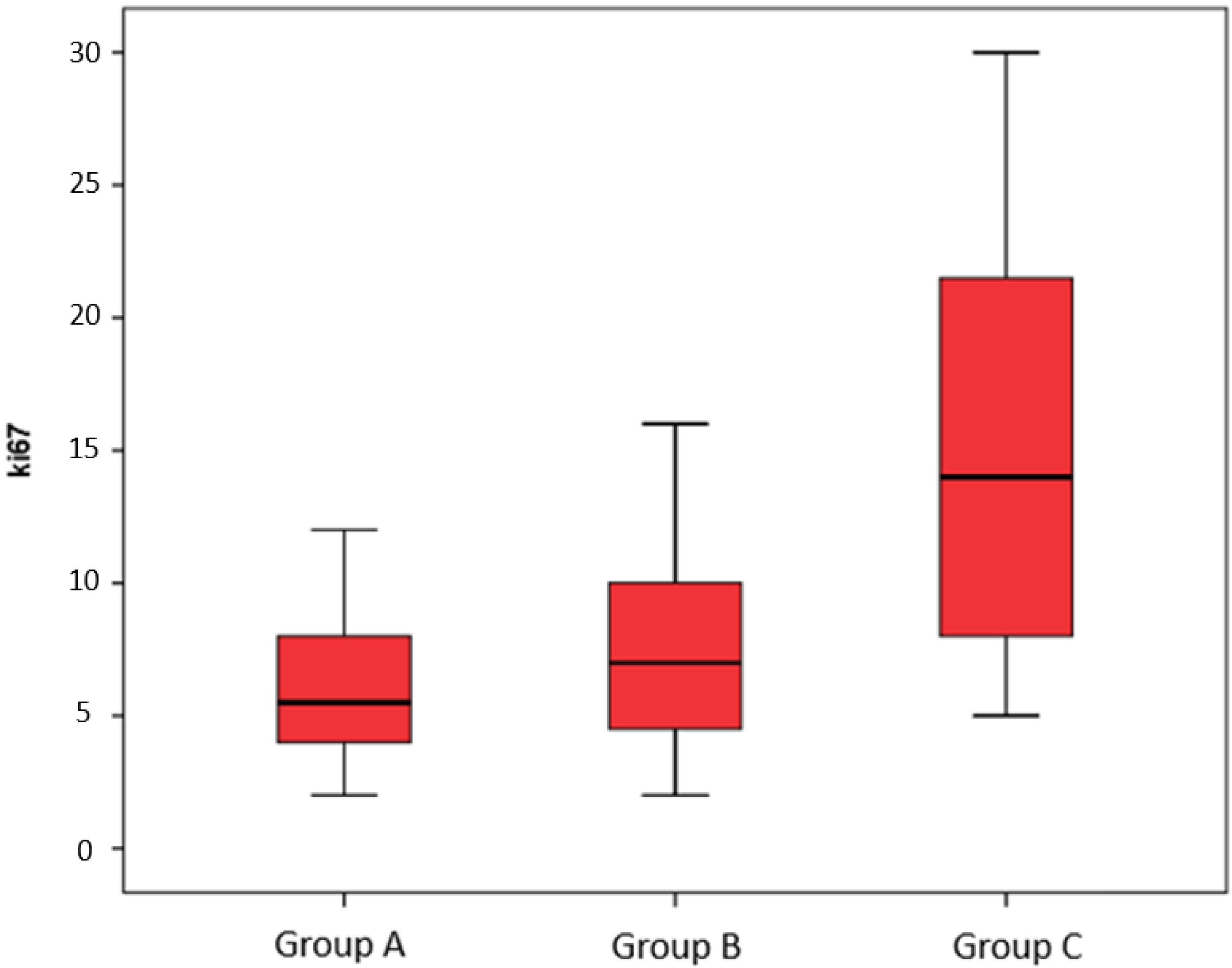
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallopeau, H. Du lichen plan et particulièrement de sa forme atrophique: Lichen plan scléreux. Ann. Dermatol. Syphiligr. 1887, 8, 790–791. [Google Scholar]
- Kreuter, A.; Kryvosheyeva, Y.; Terras, S.; Moritz, R.; Möllenhoff, K.; Altmeyer, P.; Scola, N.; Gambichler, T. Association of Autoimmune Diseases with Lichen Sclerosus in 532 Male and Female Patients. Acta Derm. -Venereol. 2013, 93, 238–241. [Google Scholar] [CrossRef]
- Fistarol, S.K.; Itin, P.H. Diagnosis and Treatment of Lichen Sclerosus: An update. Am. J. Clin. Dermatol. 2013, 14, 27–47. [Google Scholar] [CrossRef]
- Christman, M.S.; Chen, J.T.; Holmes, N.M. Obstructive complications of lichen sclerosus. J. Pediatr. Urol. 2009, 5, 165–169. [Google Scholar] [CrossRef]
- Dinh, H.; Purcell, S.M.; Chung, C.; Zaenglein, A.L. Pediatric Lichen Sclerosus: A Review of the Literature and Management Recommendations. J. Clin. Aesthetic Dermatol. 2016, 9, 49–54. [Google Scholar]
- Arena, S.; Russo, T.; Impellizzeri, P.; Parisi, S.; Perrone, P.; Romeo, C. Utility of uroflowmetry during the follow-up of children affected by balanitis xerotica obliterans (BXO). Arch. Ital. Urol. Androl. 2018, 90, 123–126. [Google Scholar] [CrossRef]
- McGregor, T.B.; Pike, J.G.; Leonard, M.P. Pathologic and physiologic phimosis: Approach to the phimotic foreskin. Can. Fam. Physician 2007, 53, 445–448. [Google Scholar] [PubMed]
- Ghidini, F.; Virgone, C.; Pulvirenti, R.; Trovalusci, E.; Gamba, P. Could a careful clinical examination distinguish physiologic phimosis from balanitis xerotica obliterans in children? Eur. J. Pediatr. 2021, 180, 591–595. [Google Scholar] [CrossRef]
- Bochove-Overgaauw, D.M.; Gelders, W.; De Vylder, A.M. Routine biopsies in pediatric circumcision: (Non) sense? J. Pediatr. Urol. 2009, 5, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Azurdia, R.; Luzzi, G.; Byren, I.; Welsh, K.; Wojnarowska, F.; Marren, P.; Edwards, A. Lichen sclerosus in adult men: A study of HLA associations and susceptibility to autoimmune disease. Br. J. Dermatol. 1999, 140, 79–83. [Google Scholar] [CrossRef]
- Bjekić, M.; Šipetić, S.; Marinković, J. Risk factors for genital lichen sclerosus in men. Br. J. Dermatol. 2011, 164, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Sherman, V.; McPherson, T.; Baldo, M.; Salim, A.; Gao, X.; Wojnarowska, F. The high rate of familial lichen sclerosus suggests a genetic contribution: An observational cohort study. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1031–1034. [Google Scholar] [CrossRef]
- Fergus, K.; Lee, A.W.; Baradaran, N.; Cohen, A.J.; Stohr, B.A.; Erickson, B.; Mmonu, N.A.; Breyer, B.N. Pathophysiology, Clinical Manifestations, and Treatment of Lichen Sclerosus: A Systematic Review. Urology 2020, 135, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.; Kaur, M.; Rai, C.B.; Singh, A.; Ramesh, V. Histopathological spectrum of lichen sclerosus Et atrophicus. Indian J. Dermatopathol. Diagn. Dermatol. 2017, 4, 8. [Google Scholar] [CrossRef]
- Nguyen, A.T.M.; Holland, A.J.A. Balanitis xerotica obliterans: An update for clinicians. Eur. J. Pediatr. 2020, 179, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Paulis, G.; Berardesca, E. Lichen sclerosus: The role of oxidative stress in the pathogenesis of the disease and its possible transformation into carcinoma. Res. Rep. Urol. 2019, 11, 223–232. [Google Scholar] [CrossRef]
- Carlson, B.C.; Hofer, M.D.; Ballek, N.; Yang, X.J.; Meeks, J.J.; Gonzalez, C.M. Protein Markers of Malignant Potential in Penile and Vulvar Lichen Sclerosus. J. Urol. 2013, 190, 399–406. [Google Scholar] [CrossRef]
- Carlson, J.A.; Amin, S.; Malfetano, J.; Tien, A.T.; Selkin, B.; Hou, J.; Goncharuk, V.; Wilson, V.L.; Rohwedder, A.; Ambros, R.; et al. Concordant p53 and mdm-2 Protein Expression in Vulvar Squamous Cell Carcinoma and Adjacent Lichen Sclerosus. Appl. Immunohistochem. Mol. Morphol. 2001, 9, 150–163. [Google Scholar] [CrossRef]
- De Luca, D.A.; Papara, C.; Vorobyev, A.; Staiger, H.; Bieber, K.; Thaçi, D.; Ludwig, R.J. Lichen sclerosus: The 2023 update. Front. Med. 2023, 10, 1106318. [Google Scholar] [CrossRef]
- Li, L.T.; Jiang, G.; Chen, Q.; Zheng, J.N. Ki67 is a promising molecular target in the diagnosis of cancer (Review). Mol. Med. Rep. 2015, 11, 1566–1572. [Google Scholar] [CrossRef]
- Stühmer, A. Balanitis xerotica obliterans (post operationem) und ihre Beziehungen zur “Kraurosis glandis et praeputii penis”. Arch. Dermatol. Res. 1928, 156, 613–623. [Google Scholar] [CrossRef]
- Charlton, O.A.; Smith, S.D. Balanitis xerotica obliterans: A review of diagnosis and management. Int. J. Dermatol. 2019, 58, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Kravvas, G.; Muneer, A.; Watchorn, R.E.; Castiglione, F.; Haider, A.; Freeman, A.; Hadway, P.; Alnajjar, H.; Lynch, M.; Bunker, C.B. Male genital lichen sclerosus, microincontinence and occlusion: Mapping the disease across the prepuce. Clin. Exp. Dermatol. 2022, 47, 1124–1130. [Google Scholar] [CrossRef]
- Russo, T.; Currò, M.; Barbera, A.; Caccamo, D.; Antonuccio, P.; Arena, S.; Montalto, A.S.; Parisi, S.; Marseglia, L.; Gitto, E.; et al. Expression of Transglutaminase in Foreskin of Children with Balanitis Xerotica Obliterans. Int. J. Mol. Sci. 2016, 17, 1551. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, J.M.; Morey, A.F.; Peterson, A.C. Lichen Sclerosus: Review of the Literature and Current Recommendations for Management. J. Urol. 2007, 178, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Currò, M.; Russo, T.; Ferlazzo, N.; Caccamo, D.; Antonuccio, P.; Arena, S.; Parisi, S.; Perrone, P.; Ientile, R.; Romeo, C.; et al. Anti-Inflammatory and Tissue Regenerative Effects of Topical Treatment with Ozonated Olive Oil/Vitamin E Acetate in Balanitis Xerotica Obliterans. Molecules 2018, 23, 645. [Google Scholar] [CrossRef]
- Dunsmuir, W.; Gordon, E. The history of circumcision. BJU Int. 1999, 83 (Suppl. 1), 1–12. [Google Scholar] [CrossRef]
- Edmonds, E.; Hunt, S.; Hawkins, D.; Dinneen, M.; Francis, N.; Bunker, C. Clinical parameters in male genital lichen sclerosus: A case series of 329 patients: Clinical parameters in male genital lichen sclerosus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 730–737. [Google Scholar] [CrossRef]
- Singh, V.; Gautam, M.M.; Nadkarni, N.J.; Patil, S.P. Anogenital lichen sclerosus. Indian J. Sex. Transm. Dis. AIDS 2020, 41, 1–9. [Google Scholar] [CrossRef]
- Edmonds, E.; Barton, G.; Buisson, S.; Francis, N.; Gotch, F.; Game, L.; Haddad, M.; Dinneen, M.; Bunker, C. Gene expression profiling in male genital lichen sclerosus. Int. J. Exp. Pathol. 2011, 92, 320–325. [Google Scholar] [CrossRef]
- Liu, X.; Peng, G. Mitochondria orchestrate T cell fate and function. Nat. Immunol. 2021, 22, 276–278. [Google Scholar] [CrossRef]
- Bao, Y.; Li, Z.; Liu, W.; Fu, Y.; Lv, J.; Sun, K.; Chang, J. Study of Langerhans cells and T lymphocytes in vulvar lichen sclerosus lesions. Australas. J. Dermatol. 2021, 62, e217–e222. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Thomson, J.; Harwood, C.; Leigh, I. The Role of the Immune System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 20, 2009. [Google Scholar] [CrossRef]
- Lopez-Vergès, S.; Milush, J.M.; Pandey, S.; York, V.A.; Arakawa-Hoyt, J.; Pircher, H.; Norris, P.J.; Nixon, D.F.; Lanier, L.L. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood 2010, 116, 3865–3874. [Google Scholar] [CrossRef]
- Bottomley, M.J.; Harden, P.N.; Wood, K.J. CD8+ Immunosenescence Predicts Post-Transplant Cutaneous Squamous Cell Carcinoma in High-Risk Patients. J. Am. Soc. Nephrol. 2016, 27, 1505–1515. [Google Scholar] [CrossRef]
- Tran, D.A.; Tan, X.; Macri, C.J.; Goldstein, A.T.; Fu, S.W. Lichen Sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int. J. Biol. Sci. 2019, 15, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Hantschmann, P.; Sterzer, S.; Jeschke, U.; Friese, E.K. P53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer Res. 2005, 25, 1739–1745. [Google Scholar]
- Liegl, B.; Regauer, S. p53 immunostaining in lichen sclerosus is related to ischaemic stress and is not a marker of differentiated vulvar intraepithelial neoplasia (d-VIN). Histopathology 2006, 48, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.; Ali, I.; Dean, D.; Thiele, J.; Wojnarowska, F. Oxidative stress is implicated in the pathogenesis of lichen sclerosus. Br. J. Dermatol. 2004, 151, 627–635. [Google Scholar] [CrossRef]
- Sander, C.; Hamm, F.; Elsner, P.; Thiele, J. Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br. J. Dermatol. 2003, 148, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, M.; Irrera, N.; Cutroneo, G.; Rizzo, G.; Vaccaro, F.; Anastasi, G.P.; Borgia, F.; Cannavò, S.P.; Altavilla, D.; Squadrito, F. Differential Expression of Nitric Oxide Synthase Isoforms nNOS and iNOS in Patients with Non-Segmental Generalized Vitiligo. Int. J. Mol. Sci. 2017, 18, 2533. [Google Scholar] [CrossRef]
- Bagnato, G.L.; Irrera, N.; Pizzino, G.; Santoro, D.; Roberts, W.N.; Bagnato, G.; Pallio, G.; Vaccaro, M.; Squadrito, F.; Saitta, A.; et al. Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin. Sci. 2018, 132, 231–242. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Zamarron, B.F.; Chen, W. Dual Roles of Immune Cells and Their Factors in Cancer Development and Progression. Int. J. Biol. Sci. 2011, 7, 651–658. [Google Scholar] [CrossRef]
- Morrel, B.; van Eersel, R.; Burger, C.W.; Bramer, W.M.; Kate-Booij, M.J.T.; van der Avoort, I.A.; Pasmans, S.G. The long-term clinical consequences of juvenile vulvar lichen sclerosus: A systematic review. J. Am. Acad. Dermatol. 2020, 82, 469–477. [Google Scholar] [CrossRef]
- Kiss, A.; Kiraly, L.; Kutasy, B.; Merksz, M. High Incidence of Balanitis Xerotica Obliterans in Boys with Phimosis: Prospective 10-Year Study. Pediatr. Dermatol. 2005, 22, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Celis, S.; Reed, F.; Murphy, F.; Adams, S.; Gillick, J.; Abdelhafeez, A.H.; Lopez, P.-J. Balanitis xerotica obliterans in children and adolescents: A literature review and clinical series. J. Pediatr. Urol. 2014, 10, 34–39. [Google Scholar] [CrossRef]
- EAU. EAU Guidelines on Paediatric Urology; EAU: Arnhem, The Netherlands, 2022. [Google Scholar]
- Tong, L.X.; Sun, G.S.; Teng, J.M. Pediatric Lichen Sclerosus: A Review of the Epidemiology and Treatment Options. Pediatr. Dermatol. 2015, 32, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Dahlman-Ghozlan, K.; Hedblad, M.-A.; von Krogh, G. Penile lichen sclerosus et atrophicus treated with clobetasol dipropionate 0.05% cream: A retrospective clinical and histopathologic study. J. Am. Acad. Dermatol. 1999, 40, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.V.; MacKinnon, E. The response of clinical balanitis xerotica obliterans to the application of topical steroid-based creams. J. Pediatr. Surg. 2005, 40, 709–712. [Google Scholar] [CrossRef]
- Ghysel, C.; Eeckt, K.V.; Bogaert, G.A. Long-Term Efficiency of Skin Stretching and a Topical Corticoid Cream Application for Unretractable Foreskin and Phimosis in Prepubertal Boys. Urol. Int. 2009, 82, 81–88. [Google Scholar] [CrossRef]
- Russo, T.; Currò, M.; Ferlazzo, N.; Caccamo, D.; Perrone, P.; Arena, S.; Antonelli, E.; Antonuccio, P.; Ientile, R.; Romeo, C.; et al. Stable Ozonides with Vitamin E Acetate versus Corticosteroid in the Treatment of Lichen Sclerosus in Foreskin: Evaluation of Effects on Inflammation. Urol. Int. 2019, 103, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-C.; Kirtschig, G.; Baldo, M.; Brackenbury, F.; Lewis, F.; Wojnarowska, F. Topical interventions for genital lichen sclerosus. Cochrane Database Syst. Rev. 2011, 2011, CD008240. [Google Scholar] [CrossRef]
- Boms, S.; Gambichler, T.; Freitag, M.; Altmeyer, P.; Kreuter, A. Pimecrolimus 1% cream for anogenital lichen sclerosus in childhood. BMC Dermatol. 2004, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Guven, E.S.G.; Guven, S.; Sakinci, M.; Dogan, N.U.; Kucukali, T. Testosterone versus clobetasol for maintenance of vulvar lichen sclerosus associated with variable degrees of squamous cell hyperplasia. Acta Obstet. Gynecol. Scand. 2007, 86, 715–719. [Google Scholar] [CrossRef]
- Parker, L.U.; Bergfeld, W.F. Virilization secondary to topical testosterone. Clevel. Clin. J. Med. 1991, 58, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Zeisler, H.; Banchertodesca, D.; Sator, M.O.; Schneider, B.; Gitsch, G. Short-term effects of topical testosterone in vulvar lichen sclerosus. Obstet. Gynecol. 1997, 89, 297–299. [Google Scholar] [CrossRef]
- Zawislak, A.A.; McCluggage, W.G.; Donnelly, R.F.; Maxwell, P.; Price, J.H.; Dobbs, S.P.; McClelland, H.R.; Woolfson, A.D.; Mccarron, P.A. Response of vulval lichen sclerosus and squamous hyperplasia to photodynamic treatment using sustained topical delivery of aminolevulinic acid from a novel bioadhesive patch system. Photodermatol. Photoimmunol. Photomed. 2009, 25, 111–113. [Google Scholar] [CrossRef]
- Sotiriou, E.; Apalla, Z.; Patsatsi, A.; Panagiotidou, D. Recalcitrant vulvar lichen sclerosis treated with aminolevulinic acid-photodynamic therapy: A report of five cases. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1398–1399. [Google Scholar] [CrossRef] [PubMed]

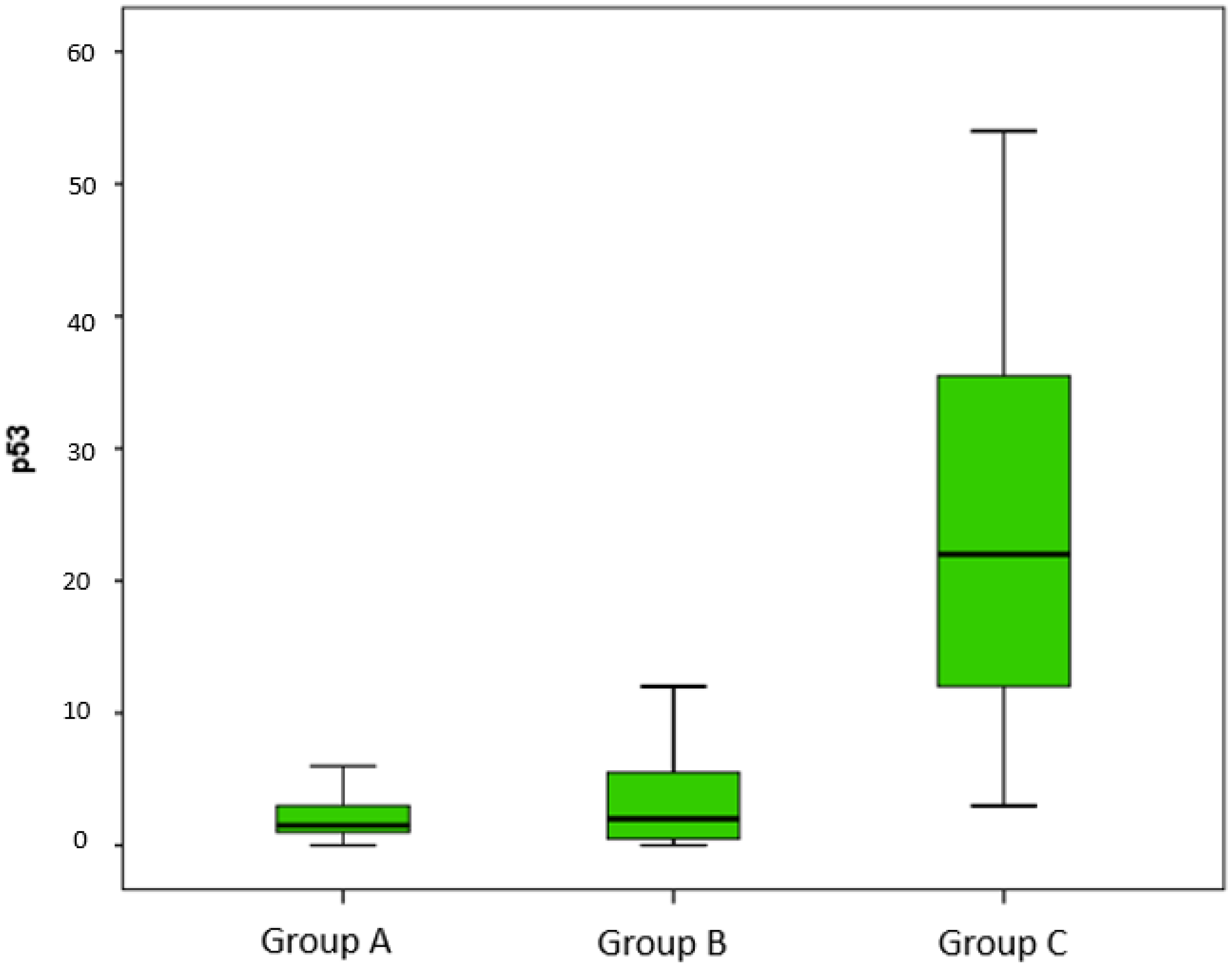
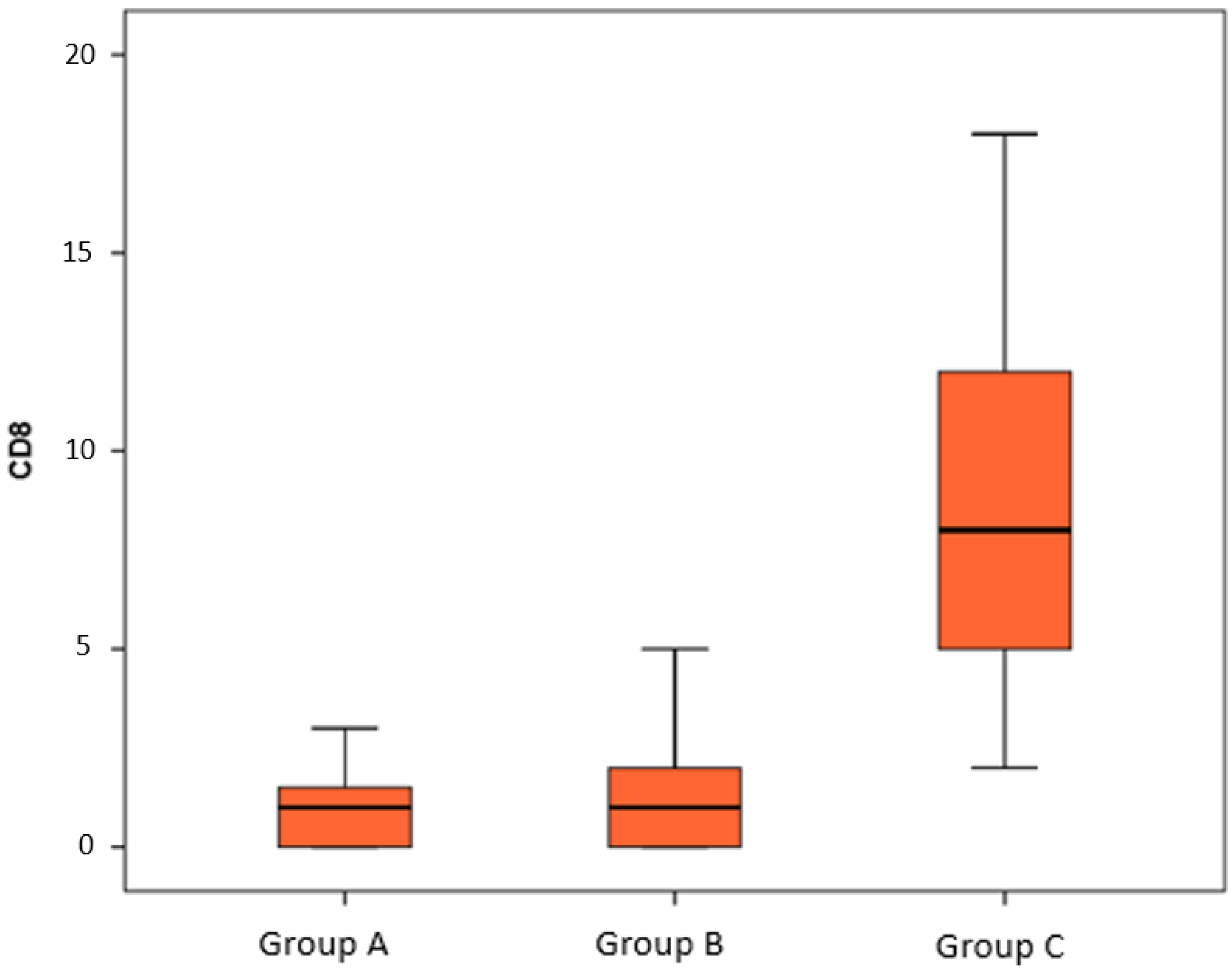
| CD8 | CD57 | Ki67 | p53 | |
|---|---|---|---|---|
| χ2 | 77.597 | 85.822 | 45.495 | 77.666 |
| p | 0.000 | 0.000 | 0.000 | 0.000 |
| CD8 | CD57 | Ki67 | p53 | |
|---|---|---|---|---|
| U di Mann-Whitney | 484.000 | 466.500 | 448.500 | 477.000 |
| W di Wilcoxon | 784.000 | 766.500 | 748.500 | 777.000 |
| Z | −1.143 | −1.380 | −1.535 | −1.199 |
| p | 0.253 | 0.167 | 0.125 | 0.231 |
| CD8 | CD57 | Ki67 | p53 | |
|---|---|---|---|---|
| U di Mann-Whitney | 4.500 | 8.500 | 114.000 | 8.500 |
| W di Wilcoxon | 304.500 | 308.500 | 414.000 | 308.500 |
| Z | −6.862 | −6.821 | −5.540 | −6.789 |
| p | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| CD8 | CD57 | Ki67 | p53 | |
|---|---|---|---|---|
| U di Mann-Whitney | 103.000 | 17.000 | 383.500 | 88.000 |
| W di Wilcoxon | 1279.000 | 1193.000 | 1559.500 | 1264.000 |
| Z | −7.732 | 8.372 | −5.652 | −7.813 |
| p | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, S.; Ieni, A.; Currò, M.; Vaccaro, M.; Di Fabrizio, D.; Cassaro, F.; Bonfiglio, R.; Montalto, A.S.; Tuccari, G.; Alibrandi, A.; et al. Immunohistological Analysis of Lichen Sclerosus of the Foreskin in Pediatric Age: Could It Be Considered a Premalignant Lesion? Biomedicines 2023, 11, 1986. https://doi.org/10.3390/biomedicines11071986
Arena S, Ieni A, Currò M, Vaccaro M, Di Fabrizio D, Cassaro F, Bonfiglio R, Montalto AS, Tuccari G, Alibrandi A, et al. Immunohistological Analysis of Lichen Sclerosus of the Foreskin in Pediatric Age: Could It Be Considered a Premalignant Lesion? Biomedicines. 2023; 11(7):1986. https://doi.org/10.3390/biomedicines11071986
Chicago/Turabian StyleArena, Salvatore, Antonio Ieni, Monica Currò, Mario Vaccaro, Donatella Di Fabrizio, Fabiola Cassaro, Roberta Bonfiglio, Angela Simona Montalto, Giovanni Tuccari, Angela Alibrandi, and et al. 2023. "Immunohistological Analysis of Lichen Sclerosus of the Foreskin in Pediatric Age: Could It Be Considered a Premalignant Lesion?" Biomedicines 11, no. 7: 1986. https://doi.org/10.3390/biomedicines11071986
APA StyleArena, S., Ieni, A., Currò, M., Vaccaro, M., Di Fabrizio, D., Cassaro, F., Bonfiglio, R., Montalto, A. S., Tuccari, G., Alibrandi, A., Impellizzeri, P., & Romeo, C. (2023). Immunohistological Analysis of Lichen Sclerosus of the Foreskin in Pediatric Age: Could It Be Considered a Premalignant Lesion? Biomedicines, 11(7), 1986. https://doi.org/10.3390/biomedicines11071986








