Functional State of the Motor Centers of the Lumbar Spine after Contusion (Th8-Th9) with Application of Methylprednisolone-Copolymer at the Site of Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Object of Study and Bioethical Standards
2.2. Spinal Cord Injury Model
2.3. Application of the Gel
2.4. Methylprednisolone Administration
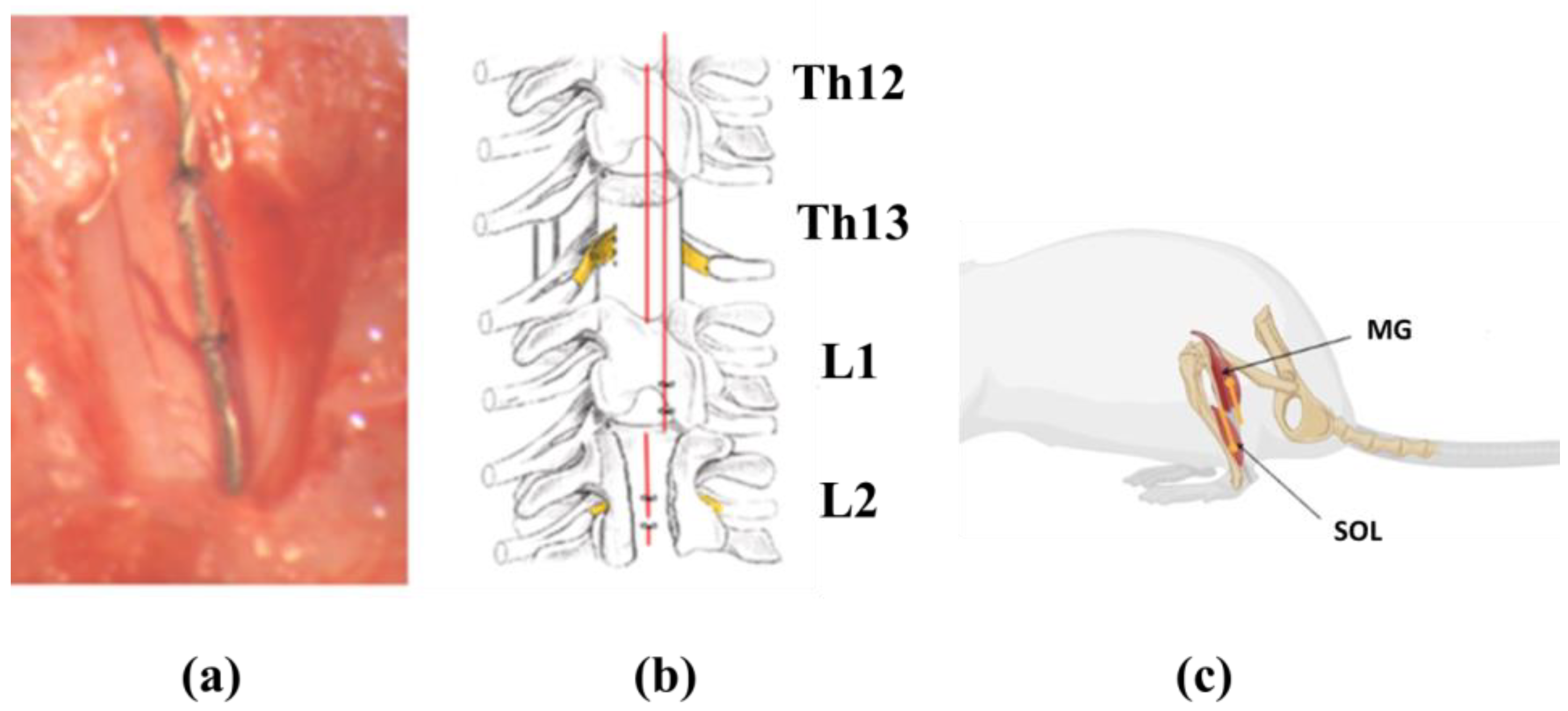
2.5. Electrode Implantation
2.6. Motor Function Analysis by Vicon Video Capture System
2.7. Electrophysiological Testing
2.8. Organization of the Experiment
- (1)
- Control group (Con; n = 7): intact animals.
- (2)
- Untreated spinal cord injury (SCI; n = 7) group: animals underwent laminectomy and spinal cord injury.
- (3)
- Methylprednisolone sodium succinate (MPS; n = 8) group: animals received a single high dose of MPS (30 mg/kg) intravenously immediately after injury.
- (4)
- MPS in combination with a copolymer group (MPS + TBC; n = 8)—a complex of MPS and the polymer was applied to the dura of the injured part of the spinal cord for 6 h, after 6 h polymer was removed.
- (5)
- Tri-block copolymer (TBC; n = 8) group: TBS was applied to the dura mater of the injured part of the spinal cord for 6 h, and after 6 h polymer was removed.
2.9. Data Analyses
3. Results
3.1. Changes in the Maximum Amplitude of the Evoked Motor Response of the Leg Muscles in Rats during Epidural Stimulation of the Spinal Cord
3.2. Motion Estimation Using Vicon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, S.; Jacquemin, G.; Fournier, C.; Lamarre, Y.; Rossignol, S. Spontaneous motor rhythms of the back and legs in a patient with a complete spinal cord transection. Neurorehabilit. Neural Repair 2010, 24, 377–383. [Google Scholar] [CrossRef]
- Shepard, C.T.; Pocratsky, A.M.; Brown, B.L.; Van Rijswijck, M.A.; Zalla, R.M.; Burke, D.A.; Morehouse, J.R.; Riegler, A.S.; Whittemore, S.R.; Magnuson, D.S. Silencing long ascending propriospinal neurons after spinal cord injury improves hindlimb stepping in the adult rat. eLife 2021, 10, e70058. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Streeter, K.A.; Sunshine, M.D.; Patel, S.R.; Gonzalez-Rothi, E.J.; Reier, P.J.; Baekey, D.M.; Fuller, D.D. Mid-cervical interneuron networks following high cervical spinal cord injury. Respir. Physiol. Neurobiol. 2020, 271, 103305. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Delivet-Mongrain, H.; Rossignol, S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J. Neurophysiol. 2013, 109, 2909–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakuta, Y.; Adachi, A.; Yokohama, M.; Horii, T.; Mieda, T.; Iizuka, Y.; Takagishi, K.; Chikuda, H.; Iizuka, H.; Nakamura, K. Spontaneous functional full recovery from motor and sensory deficits in adult mice after mild spinal cord injury. Heliyon 2019, 5, e01847. [Google Scholar] [CrossRef] [Green Version]
- Stolbkov, Y.K.; Gerasimenko, Y.P. Plastic Changes Induced by Motor Activity in Spinal Cord Injury. Neurosci. Behav. Physiol. 2023, 53, 399–408. [Google Scholar] [CrossRef]
- Brown, A.R.; Martinez, M. From cortex to cord: Motor circuit plasticity after spinal cord injury. Neural Regen. Res. 2019, 14, 2054–2062. [Google Scholar] [CrossRef]
- Costăchescu, B.; Niculescu, A.G.; Dabija, M.G.; Teleanu, R.I.; Grumezescu, A.M.; Eva, L. Novel Strategies for Spinal Cord Regeneration. Int. J. Mol. Sci. 2022, 23, 4552. [Google Scholar] [CrossRef]
- Baptiste, D.C.; Fehlings, M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma 2006, 23, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Bracken, M.B.; Shepard, M.J.; Holford, T.R.; Leo-Summers, L.; Aldrich, E.F.; Fazl, M.; Fehlings, M.; Herr, D.L.; Hitchon, P.W.; Marshall, L.F.; et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. Natl. Acute Spinal Cord Inj. Study JAMA 1997, 277, 1597–1604. [Google Scholar] [CrossRef]
- Druschel, C.; Schaser, K.D.; Schwab, J.M. Current practice of methylprednisolone administration for acute spinal cord injury in Germany: A national survey. Spine 2013, 38, E669–E677. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, P.; Zhou, W.; Zhong, Z.; Qu, D. Alkaline-phosphatase triggered self-assemblies enhances the anti-inflammatory property of methylprednisolone in spinal cord injury. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020978505. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.J.; Guo, S.W.; Zhu, L.; Xu, X.; Liu, J.B. Methylprednisolone Induces Neuro-Protective Effects via the Inhibition of A1 Astrocyte Activation in Traumatic Spinal Cord Injury Mouse Models. Front. Neurosci. 2021, 15, 628917. [Google Scholar] [CrossRef]
- Chen, H.C.; Fong, T.H.; Lee, A.W.; Chiu, W.T. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine 2012, 37, 470–475. [Google Scholar] [CrossRef]
- Xu, J.; Chen, S.; Chen, H.; Xiao, Q.; Hsu, C.Y.; Michael, D.; Bao, J. STAT5 mediates antiapoptotic effects of methylprednisolone on oligodendrocytes. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2022–2026. [Google Scholar] [CrossRef] [Green Version]
- Hurlbert, R.J.; Hadley, M.N.; Walters, B.C.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Rozzelle, C.J.; Ryken, T.C.; Theodore, N. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2015, 76 (Suppl. 1), S71–S83. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wilson, J.R.; Tetreault, L.A.; Aarabi, B.; Anderson, P.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Chiba, K.; Dettori, J.R.; et al. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Glob. Spine J. 2017, 7 (Suppl. 3), 203S–211S. [Google Scholar] [CrossRef]
- Karabey-Akyurek, Y.; Gurcay, A.G.; Gurcan, O.; Turkoglu, O.F.; Yabanoglu-Ciftci, S.; Eroglu, H.; Sargon, M.F.; Bilensoy, E.; Oner, L. Localized delivery of methylprednisolone sodium succinate with polymeric nanoparticles in experimental injured spinal cord model. Pharm. Dev. Technol. 2017, 22, 972–981. [Google Scholar] [CrossRef]
- Hazra, A.; Pyszczynski, N.; DuBois, D.C.; Almon, R.R.; Jusko, W.J. Pharmacokinetics of methylprednisolone after intravenous and intramuscular administration in rats. Biopharm. Drug Dispos. 2007, 28, 263–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koszdin, K.L.; Shen, D.D.; Bernards, C.M. Spinal Cord Bioavailability of Methylprednisolone after Intravenous and Intrathecal Administration. Anesthesiology 2000, 92, 156. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, C.; Li, J.; Deng, R.; Huang, J.; Zhang, Q.; Lyu, J.; Hao, N.; Zhong, Z. NEP1-40-modified human serum albumin nanoparticles enhance the therapeutic effect of methylprednisolone against spinal cord injury. J. Nanobiotechnology 2019, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhayay, A.; Singh, D.; Sharma, K.P. Neat Ionic liquid and α-Chymotrypsin-Polymer Surfactant Conjugate-Based Biocatalytic Solvent. Biomacromolecules 2020, 21, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Dong, C.; Zhang, T.; Zhang, S. Hydrogels in Spinal Cord Injury Repair: A Review. Front. Bioeng. Biotechnol. 2022, 10, 931800. [Google Scholar] [CrossRef]
- Kamalov, M.I.; Lavrov, I.A.; Yergeshov, A.A.; Siraeva, Z.Y.; Baltin, M.E.; Rizvanov, A.A.; Kuznetcova, S.V.; Petrova, N.V.; Savina, I.N.; Abdullin, T.I. Non-invasive topical drug delivery to spinal cord with carboxyl-modified trifunctional copolymer of ethylene oxide and propylene oxide. Colloids Surf. B Biointerfaces 2016, 140, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Lu, J.; Huang, Y.; Li, Q.-A. Zonisamide-loaded triblock copolymer nanomicelle as a controlled drug release platform for the treatment of oxidative stress-induced spinal cord neuronal damage. J. Mol. Liq. 2021, 326, 115233. [Google Scholar] [CrossRef]
- Aziz, Z.A.B.A.; Ahmad, A.; Mohd-Setapar, S.H.; Hassan, H.; Lokhat, D.; Kamal, M.A.; Ashraf, G.M. Recent Advances in Drug Delivery of Polymeric Nano-Micelles. Curr. Drug Metab. 2017, 18, 16–29. [Google Scholar] [CrossRef]
- Kamalov, M.I.; Đặng, T.; Petrova, N.V.; Laikov, A.V.; Luong, D.; Akhmadishina, R.A.; Lukashkin, A.N.; Abdullin, T.I. Self-assembled nanoformulation of methylprednisolone succinate with carboxylated block copolymer for local glucocorticoid therapy. Colloids Surf. B Biointerfaces 2018, 164, 78–88. [Google Scholar] [CrossRef]
- Amirmahani, N.; Mahmoodi, N.O.; Galangash, M.M.; Ghavidast, A. Advances in nanomi-celles for sustained drug delivery. J. Ind. Eng. Chem. 2017, 55, 21–34. [Google Scholar] [CrossRef]
- Baltin, M.E.; Sabirova, D.E.; Kiseleva, E.I.; Kamalov, M.I.; Abdullin, T.I.; Petrova, N.V.; Ahmetov, N.F.; Sachenkov, O.A.; Baltina, T.V.; Lavrov, I.A. Comparison of systemic and localized carrier-mediated delivery of methylprednisolone succinate for treatment of acute spinal cord injury. Exp. Brain Res. 2021, 239, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of The European Parliament and of The Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 53, 33–73. [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.R. Surgery of experimental lesion of spinal cord equivalent to crush injury of fractured is location of spinal column. JAMA 1911, 57, 878–880. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhou, J.; Zhang, J.; Wang, D.; Ma, J. Construction of rat spinal cord injury model based on Allen’s animal model. Saudi J. Biol. Sci. 2019, 26, 2122–2126. [Google Scholar] [CrossRef]
- Roy, F.D.; Gibson, G.; Stein, R.B. Effect of percutaneous stimulation at different spinal levels on the activation of sensory and motor roots. Exp. Brain Res. 2012, 223, 281–289. [Google Scholar] [CrossRef]
- Smirnova, V.; Khamatnurova, R.; Kharin, N.; Yaikova, E.; Baltina, T.; Sachenkov, O. The Automatization of the Gait Analysis by the Vicon Video System: A Pilot Study. Sensors 2022, 22, 7178. [Google Scholar] [CrossRef]
- Smirnova, V.; Yaikova, E.; Baltin, M.; Kharin, N.; Baltina, T.; Sachenkov, O. Movement estimation methods based on the motion capture system. In Proceedings of the 2022 Fourth International Conference Neurotechnologies and Neurointerfaces (CNN), Kaliningrad, Russia, 14–16 September 2022; pp. 158–161. [Google Scholar] [CrossRef]
- Fedianin, A.; Zaytceva, T.; Baltin, M.; Bikeeva, S.; Smirnova, V.; Sachenkov, O.; Baltina, T.; Eremeev, A. Motor reorganization during simulation of gravitational unloading. In Proceedings of the 2022 Fourth International Conference Neurotechnologies and Neurointerfaces (CNN), Kaliningrad, Russia, 14–16 September 2022; pp. 25–28. [Google Scholar] [CrossRef]
- Zimmermann, R.; Vieira Alves, Y.; Sperling, L.E.; Pranke, P. Nanotechnology for the Treatment of Spinal Cord Injury. Tissue Eng. Part B Rev. 2021, 27, 353–365. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Fauzi Daud, M.; Idris, J.; Ng, A.M.H.; Selvi Naicker, A.; Ismail, O.H.R.; Athi Kumar, R.K.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef]
- Huang, H.; Young, W.; Skaper, S.; Chen, L.; Moviglia, G.; Saberi, H.; Al-Zoubi, Z.; Sharma, H.S.; Muresanu, D.; Sharma, A.; et al. Clinical Neurorestorative Therapeutic Guidelines for Spinal Cord Injury (IANR/CANR version 2019). J. Orthop. Transl. 2019, 20, 14–24. [Google Scholar] [CrossRef]
- Bowers, C.A.; Kundu, B.; Hawryluk, G.W. Methylprednisolone for acute spinal cord injury: An increasingly philosophical debate. Neural Regen. Res. 2016, 11, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Lamba, N.; Liew, A.; Doung, P.; Tewarie, I.; Amamoo, J.J.; Gannu, L.; Chawla, S.; Doucette, J.; Cerecedo-Lopez, C.D.; et al. The safety and efficacy of steroid treatment for acute spinal cord injury: A Systematic Review and meta-analysis. Heliyon 2020, 6, e03414. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Jeong, J.H. Review: Steroid Use in Patients With Acute Spinal Cord Injury and Guideline Update. Korean J. Neurotrauma 2022, 18, 22–30. [Google Scholar] [CrossRef]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, A.A.; Xiao, S.N.; Zhong, N.S.; Tong, W.L.; Wang, S.J.; Liu, J.M.; Liu, Z.L. A Bibliometric Analysis of Publications on Spinal Cord Injury Treatment With Glucocorticoids Using VOSviewer. Front. Public Health 2022, 10, 907372. [Google Scholar] [CrossRef]
- Ilik, K.; Keskin, F.; Erdi, M.F.; Kaya, B.; Karataş, Y.; Kalkan, E. The effects of steroids in traumatic thoracolumbar junction patients on neurological outcome. Travmatik torakolomber bileşke yaralanmalı hastalarda steroidin nörolojik sonuçlar üzerine etkisi. Ulus. Travma Ve Acil Cerrahi Derg. Turk. J. Trauma Emerg. Surg. TJTES 2019, 25, 484–488. [Google Scholar] [CrossRef]
- Choi, S.H.; Sung, C.H.; Heo, D.R.; Jeong, S.Y.; Kang, C.N. Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: A national population-based study in South Korea. Spinal Cord 2020, 58, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Schröter, A.; Lustenberger, R.M.; Obermair, F.J.; Thallmair, M. High-dose corticosteroids after spinal cord injury reduce neural progenitor cell proliferation. Neuroscience 2009, 161, 753–763. [Google Scholar] [CrossRef]
- Pereira, J.E.; Costa, L.M.; Cabrita, A.M.; Couto, P.A.; Filipe, V.M.; Magalhães, L.G.; Fornaro, M.; Di Scipio, F.; Geuna, S.; Maurício, A.C.; et al. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp. Neurol. 2009, 220, 71–81. [Google Scholar] [CrossRef]
- Bowers, C.A.; Kundu, B.; Rosenbluth, J.; Hawryluk, G.W. Patients with Spinal Cord Injuries Favor Administration of Methylprednisolone. PLoS ONE 2016, 11, e0145991. [Google Scholar] [CrossRef]
- Heo, J.Y.; Noh, J.H.; Park, S.H.; Ji, Y.B.; Ju, H.J.; Kim, D.Y.; Lee, B.; Kim, M.S. An Injectable Click-Crosslinked Hydrogel that Prolongs Dexamethasone Release from Dexamethasone-Loaded Microspheres. Pharmaceutics 2019, 11, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lv, H.Q.; Chao, X.; Xu, W.X.; Liu, Y.; Ling, G.X.; Zhang, P. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Liaw, J.; Lin, Y. Evaluation of poly (ethylene oxide)-poly (propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) gels as a release vehicle for percutaneous fentanyl. J. Control. Release Off. J. Control. Release Soc. 2000, 68, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Lemieux, P.; Vinogradov, S.; Alakhov, V. Pluronic block copolymers: Novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 2002, 54, 223–233. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2003, 304, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Silant’eva, D.I.; Deryabina, I.B.; Baltin, M.E.; Kamalov, M.I.; Moiseeva, M.V.; Andrianov, V.V.; Batlina, T.V.; Gainutdinov, K.L. The Effects of Repeated Administration of the Micellar Complex of Methylprednisolone on the Locomotor Activity of a Terrestrial Snails. Bull. Exp. Biol. Med. 2020, 170, 5–9. [Google Scholar] [CrossRef]
- Baltin, M.E.; Sabirova, D.E.; Chernova, O.N.; Baltina, T.V.; Sachenkov, O.A. Morphofunctional Changes in the Spinal Cord of Rats after Contusion Injury with Local Delivery of Methylprednisolone in Combination with a Copolymer. Bull. Exp. Biol. Med. 2023, 174, 810–815. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Metz, G.A.; Merkler, D.; Dietz, V.; Schwab, M.E.; Fouad, K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000, 883, 165–177. [Google Scholar] [CrossRef]
- Baltina, T.V.; Silantyeva, D.I.; Loban, E.Y.; Raimova, M.V.; Lavrov, I.A. Effects of local hypothermia on spinal cord injury in rats. Uchenye Zapiski Kazanskogo Universiteta. Seriya Estestv. Nauk. 2018, 160, 630–644. [Google Scholar]
- Islam, R.; Cuellar, C.A.; Felmlee, B.; Riccelli, T.; Silvernail, J.; Boschen, S.L.; Grahn, P.; Lavrov, I. Multifactorial motor behavior assessment for real-time evaluation of emerging therapeutics to treat neurologic impairments. Sci. Rep. 2019, 9, 16503. [Google Scholar] [CrossRef] [Green Version]
- Kozuka, Y.; Kawamata, M.; Furue, H.; Ishida, T.; Tanaka, S.; Namiki, A.; Yamakage, M. Changes in synaptic transmission of substantia gelatinosa neurons after spinal cord hemisection revealed by analysis using in vivo patch-clamp recording. Mol. Pain 2016, 12, 1744806916665827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, D. The lesioned spinal cord is a “new” spinal cord: Evidence from functional changes after spinal injury in Lamprey. Front. Neural Circuits 2017, 11, 84. [Google Scholar] [CrossRef] [Green Version]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef]
- Gazula, V.R.; Roberts, M.; Luzzio, C.; Jawad, A.F.; Kalb, R.G. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J. Comp. Neurol. 2004, 476, 130–145. [Google Scholar] [CrossRef]
- Bose, P.; Parmer, R.; Reier, P.J.; Thompson, F.J. Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp. Neurol. 2005, 191, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Schwab, J.M. Determinants of Axon Growth, Plasticity, and Regeneration in the Context of Spinal Cord Injury. Am. J. Pathol. 2018, 188, 53–62. [Google Scholar] [CrossRef] [Green Version]
- London, M.; Häusser, M. Dendritic computation. Annu. Rev. Neurosci. 2005, 28, 503–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynskey, J.V.; Belanger, A.; Jung, R. Activity-dependent plasticity in spinal cord injury. J. Rehabil. Res. Dev. 2008, 45, 229–240. [Google Scholar] [CrossRef]
- Beaumont, E.; Houlé, J.D.; Peterson, C.A.; Gardiner, P.F. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve 2004, 29, 234–242. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Yu, B.; Zhang, Z.; Brommer, B.; Williams, P.R.; Liu, Y.; Hegarty, S.V.; Zhou, S.; Zhu, J.; et al. Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations. Cell 2018, 174, 521–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muir, G.D.; Whishaw, I.Q. Ground reaction forces in locomoting hemi-parkinsonian rats: A definitive test for impairments and compensations. Exp. Brain Res. 1999, 126, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Okajima, K.; Uchiba, M.; Johno, M. Methylprednisolone reduces spinal cord injury in rats without affecting tumor necrosis factor-alpha production. J. Neurotrauma 2001, 18, 533–543. [Google Scholar] [CrossRef]
- Jiang, S.; Khan, M.I.; Middlemiss, P.J.; Lu, Y.; Werstiuk, E.S.; Crocker, C.E.; Ciccarelli, R.; Caciagli, F.; Rathbone, M.P. AIT-082 and methylprednisolone singly, but not in combina-tion, enhance functional and histological improvement after acute spinal cord injury in rats. Int. J. Immunopathol. Pharmacol. 2004, 17, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.H.; Lee, K.H.; Yoon, D.H.; Kim, U.J.; Hwang, Y.S.; Park, S.K.; Choi, J.U.; Park, Y.G. Effects of methylprednisolone on the neural conduction of the motor evoked potentials in spinal cord injured rats. J. Korean Med. Sci. 2005, 20, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Liu-Snyder, P.; Logan, M.P.; Shi, R.; Smith, D.T.; Borgens, R.B. Neuroprotection from secondary injury by polyethylene glycol requires its internalization. J. Exp. Biol. 2007, 210 Pt 8, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- White-Schenk, D.; Shi, R.; Leary, J.F. Nanomedicine strategies for treatment of secondary spinal cord injury. Int. J. Nanomed. 2015, 10, 923–938. [Google Scholar] [CrossRef] [Green Version]
- Zavvarian, M.M.; Hong, J.; Fehlings, M.G. The Functional Role of Spinal Interneurons Following Traumatic Spinal Cord In-jury. Front. Cell. Neurosci. 2020, 14, 127. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Hamilton, L.; Franklin, R.J.; Jeffery, N.D. Quantification of deficits in lateral paw positioning after spinal cord injury in dogs. BMC Vet. Res. 2008, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Hurd, C.; Weishaupt, N.; Fouad, K. Anatomical correlates of recovery in single pellet reaching in spinal cord injured rats. Exp. Neurol. 2013, 247, 605–614. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baltin, M.; Smirnova, V.; Khamatnurova, R.; Sabirova, D.; Samigullin, B.; Sachenkov, O.; Baltina, T. Functional State of the Motor Centers of the Lumbar Spine after Contusion (Th8-Th9) with Application of Methylprednisolone-Copolymer at the Site of Injury. Biomedicines 2023, 11, 2026. https://doi.org/10.3390/biomedicines11072026
Baltin M, Smirnova V, Khamatnurova R, Sabirova D, Samigullin B, Sachenkov O, Baltina T. Functional State of the Motor Centers of the Lumbar Spine after Contusion (Th8-Th9) with Application of Methylprednisolone-Copolymer at the Site of Injury. Biomedicines. 2023; 11(7):2026. https://doi.org/10.3390/biomedicines11072026
Chicago/Turabian StyleBaltin, Maxim, Victoriya Smirnova, Regina Khamatnurova, Diana Sabirova, Bulat Samigullin, Oskar Sachenkov, and Tatyana Baltina. 2023. "Functional State of the Motor Centers of the Lumbar Spine after Contusion (Th8-Th9) with Application of Methylprednisolone-Copolymer at the Site of Injury" Biomedicines 11, no. 7: 2026. https://doi.org/10.3390/biomedicines11072026
APA StyleBaltin, M., Smirnova, V., Khamatnurova, R., Sabirova, D., Samigullin, B., Sachenkov, O., & Baltina, T. (2023). Functional State of the Motor Centers of the Lumbar Spine after Contusion (Th8-Th9) with Application of Methylprednisolone-Copolymer at the Site of Injury. Biomedicines, 11(7), 2026. https://doi.org/10.3390/biomedicines11072026







