The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM)
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
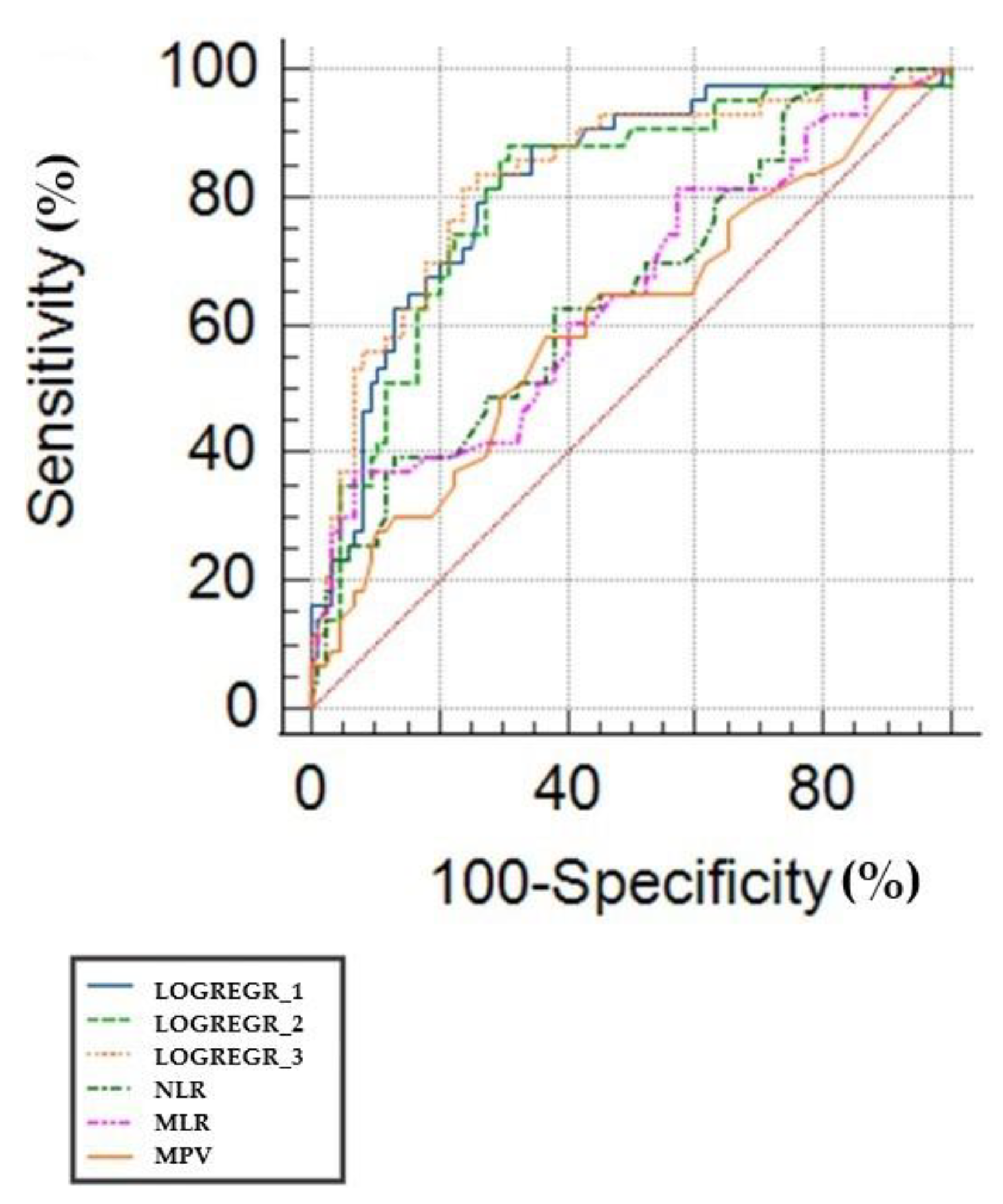
3.1. ROC Curves and Predicting the Value of White Cell Inflammatory Biomarkers
3.2. Logistic Regression Approach
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Kempler, P.; Raz, I.; Rizvi, A.A.; Rizzo, M.; Tudor, C.; Tudosie, M.; Tanasescu, D.; et al. Significance of Neutrophil to Lymphocyte Ratio (NLR) and Platelet Lymphocyte Ratio (PLR) in Diabetic Foot Ulcer and Potential New Therapeutic Targets. Int. J. Low. Extrem. Wounds 2021, 18, 15347346211057742. [Google Scholar] [CrossRef] [PubMed]
- GBD Collaborator Group. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, A.F.; Temachu, Y.Z.; Mekonnen, C.K. Prevalence and factors associated with diabetes retinopathy among type 2 diabetic patients at Northwest Amhara Comprehensive Specialized Hospitals, Northwest Ethiopia 2021. BMC Ophthalmol. 2023, 23, 9. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [Green Version]
- Curran, K.; Piyasena, P.; Congdon, N.; Duke, L.; Malanda, B.; Peto, T. Inclusion of diabetic retinopathy screening strategies in national-level diabetes care planning in low- and middle-income countries: A scoping review. Health Res. Policy Syst. 2023, 21, 2. [Google Scholar] [CrossRef]
- Rübsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Busnatu, S.-S.; Salmen, T.; Pana, M.-A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Sharma, Y.; Saxena, S.; Mishra, A.; Saxena, A.; Natu, S.M. Advanced glycation end products and diabetic retinopathy. J. Ocul. Biol. Dis. Inform. 2013, 5, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, S.; Zhang, J.; Wu, J.; Teng, W.; Liu, L.; Chen, L. Use of the monocyte-to-lymphocyte ratio to predict diabetic retinopathy. Int. J. Environ. Res. Public Health 2015, 12, 10009–10019. [Google Scholar] [CrossRef]
- Benhar, I.; Reemst, K.; Kalchenko, V.; Schwartz, M. The retinal pigment epithelium as a gateway for monocyte trafficking into the eye. EMBO J. 2016, 35, 1219–1235. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.Y.J.; Murphy, R.; Rupenthal, I.D.; Mugisho, O.O. Correlation between the progression of diabetic retinopathy and inflammasome biomarkers in vitreous and serum—A systematic review. BMC Ophthalmol. 2022, 22, 238. [Google Scholar] [CrossRef]
- Gomułka, K.; Ruta, M. The Role of Inflammation and Therapeutic Concepts in Diabetic Retinopathy—A Short Review. Int. J. Mol. Sci. 2023, 24, 1024. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef] [Green Version]
- Yumnamcha, T.; Guerra, M.; Singh, L.P.; Ibrahim, A.S. Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy. Antioxidants 2020, 9, 1244. [Google Scholar] [CrossRef]
- Aldosari, D.I.; Malik, A.; Alhomida, A.S.; Ola, M.S. Implications of Diabetes-Induced Altered Metabolites on Retinal Neurodegeneration. Front. Neurosci. 2022, 16, 938029. [Google Scholar] [CrossRef] [PubMed]
- Chittawar, S.; Dutta, D.; Qureshi, Z.; Surana, V.; Khandare, S.; Dubey, T.N. Neutrophil-lymphocyte Ratio is a Novel Reliable Predictor of Nephropathy, Retinopathy, and Coronary Artery Disease in Indians with Type-2 Diabetes. Indian J. Endocrinol. Metab. 2017, 21, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Branescu, C.; Serban, D.; Dascalu, A.M.; Oprescu, S.M.; Savlovschi, C. Interleukin 6 and lipopolysaccharide binding protein—Markers of inflammation in acute appendicitis. Chirurgia 2013, 108, 206–214. [Google Scholar] [PubMed]
- Quevedo- Martínez, J.U.; Garfias, Y.; Jimenez, J.; Garcia, O.; Venegas, D.; de Lucio, V.M.B. Pro-inflammatory cytokine profile is present in the serum of Mexican patients with different stages of diabetic retinopathy secondary to type 2 diabetes. BMJ Open Ophthalmol. 2021, 6, e000717. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Liu, M.L. Pathogenic roles of microvesicles in diabetic retinopathy. Acta Pharmacol. Sin. 2018, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Buch, A.; Kaur, S.; Nair, R.; Jain, A. Platelet volume indices as predictive biomarkers for diabetic complications in Type 2 diabetic patients. J. Lab. Physicians 2017, 9, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Fawwad, A.; Rizvi, H.A.; Alvi, F. Role of platelet indices, glycemic control and hs-CRP in pathogenesis of vascular complications in type-2 diabetic patients. Pak. J. Med. Sci. 2013, 29, 152–156. [Google Scholar] [PubMed]
- Guan, Y.; Zuo, W.; Jia, K.; Yu, C.; Liu, F.; Lv, Z.; Wang, D.; Shi, F.D.; Wang, X. Association of Red Blood Cell Distribution Width with Stroke Prognosis among Patients with Small Artery Occlusion: A Hospital-Based Prospective Follow-Up Study. Int. J. Gen. Med. 2022, 15, 7449–7457. [Google Scholar] [CrossRef]
- Li, C.; Tian, W.; Zhao, F.; Li, M.; Ye, Q.; Wei, Y.; Li, T.; Xie, K. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget 2018, 9, 35293–35299. [Google Scholar] [CrossRef] [Green Version]
- Mercan, R.; Bitik, B.; Tufan, A.; Bozbulut, U.B.; Atas, N.; Ozturk, M.A.; Haznedaroglu, S.; Goker, B. The Association Between Neutrophil/Lymphocyte Ratio and Disease Activity in Rheumatoid Arthritis and Ankylosing Spondylitis. J. Clin. Lab. Anal. 2016, 30, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Khandare, S.A.; Chittawar, S.; Nahar, N.; Dubey, T.N.; Qureshi, Z. Study of Neutrophil-lymphocyte Ratio as Novel Marker for Diabetic Nephropathy in Type 2 Diabetes. Indian J. Endocrinol. Metab. 2017, 21, 387–392. [Google Scholar]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.K.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, C.P.; Ferris, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Global Diabetic Retinopathy Project Group: Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Liu, Y.; Chen, L.; Wei, G.; Yang, J.; Zheng, P.; He, F.; Wang, W.; Ming, L. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: A posteriori and big-data-based. J. Clin. Lab. Anal. 2018, 32, e22228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Berdine, G. The receiver operating characteristic (ROC) curve. Southwest Respir. Crit. Care Chron. 2017, 5, 34–36. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, M.; Feng, Q.; Wan, H.; Wang, J.; Yang, F.; Cao, H. The Platelet-to-Lymphocyte Ratio Predicts Diabetic Retinopathy in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2022, 15, 3617–3626. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Wang, M.; Zhang, D.; Song, Y. Correlation between ultrawide-field fluorescence contrast results and white blood cell indexes in diabetic retinopathy. BMC Ophthalmol. 2022, 22, 231. [Google Scholar] [CrossRef]
- Luo, W.J.; Zhang, W.F. The relationship of blood cell-associated inflammatory indices and diabetic retinopathy: A Meta-analysis and systematic review. Int. J. Ophthalmol. 2019, 12, 312–323. [Google Scholar]
- Wan, H.; Cai, Y.; Wang, Y.; Fang, S.; Chen, C.; Chen, Y.; Xia, F.; Wang, N.; Guo, M.; Lu, Y. The unique association between the level of peripheral blood monocytes and the prevalence of diabetic retinopathy: A cross-sectional study. J. Transl. Med. 2020, 18, 248. [Google Scholar] [CrossRef]
- Gawiński, C.; Michalski, W.; Mróz, A.; Wyrwicz, L. Correlation between Lymphocyte-to-Monocyte Ratio (LMR), Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Tumor-Infiltrating Lymphocytes (TILs) in Left-Sided Colorectal Cancer Patients. Biology 2022, 11, 385. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Luo, R.; Li, H.; Yang, Z.; Qin, X.; Mo, Z. Predictive Values of the Selected Inflammatory Indexes in Colon Cancer. Cancer Control J. Moffitt Cancer Cent. 2022, 29, 10732748221091333. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, H.; Wo, M.; Ma, J.; Song, Y.; Fei, X. Clinical and predictive significance of Plasma Fibrinogen Concentrations combined Monocyte-lymphocyte ratio in patients with Diabetic Retinopathy. Int. J. Med. Sci. 2021, 18, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, Z.; Xu, Y. Association of monocyte-lymphocyte ratio and proliferative diabetic retinopathy in the US population with type 2 diabetes. J. Transl. Med. 2022, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.P.; Latinne, D.; Van Pel, M.C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zou, S.; Wang, C.; Tan, X.; Yu, M. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in Chinese Han population from Chaoshan region in South China. BMC Cardiovasc. Disord. 2019, 19, 125. [Google Scholar] [CrossRef] [Green Version]
- Moosazadeh, M.; Maleki, I.; Alizadeh-Navaei, R.; Kheradmand, M.; Hedayatizadeh-Omran, A.; Shamshirian, A.; Barzegar, A. Normal values of neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio among Iranian population: Results of Tabari cohort. Casp. J. Intern. Med. 2019, 10, 320–325. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; Ikram, M.A.; Voortman, T.; van Eijck, C.H.J.; Stricker, B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018, 8, 10566. [Google Scholar] [CrossRef] [Green Version]
- Mathur, K.; Kurbanova, N.; Qayyum, R. Platelet-lymphocyte ratio (PLR) and all-cause mortality in general population: Insights from national health and nutrition education survey. Platelets 2019, 30, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Şahan, D.; Yıldırım, M.S.; Demir, M.; Çoban, N.F.; Şahan, L. Can neutrophil-to-lymphocyte ratio be used as a diagnostic tool in diabetic retinopathy? Curr. Eye Res. 2020, 45, 295–300. [Google Scholar]
- Man, M.-A.; Davidescu, L.; Motoc, N.-S.; Rajnoveanu, R.-M.; Bondor, C.-I.; Pop, C.-M.; Toma, C. Diagnostic Value of the Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) in Various Respiratory Diseases: A Retrospective Analysis. Diagnostics 2022, 12, 81. [Google Scholar] [CrossRef]
- Ilhan, C.; Citirik, M.; Uzel, M.M.; Tekin, K. The Optimal Cut-off Value of Neutrophil/Lymphocyte Ratio for Severe Grades of Diabetic Retinopathy. Beyoglu Eye J. 2019, 4, 76–81. [Google Scholar]
- Hu, Y.; Cheng, Y.; Xu, X.; Yang, B.; Mei, F.; Zhou, Q.; Yan, L.; Wang, J.; Wu, X. Pretreatment neutrophil-to-lymphocyte ratio predicts prognosis in patients with diabetic macular edema treated with ranibizumab. BMC Ophthalmol. 2019, 19, 194. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.R.; Chen, Z.; Yang, K.; Yang, H.J.; Tao, W.Y.; Li, Y.P.; Jiang, Z.J.; Bai, C.F.; Yin, Y.C.; Duan, J.M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Qi, S.; Zhang, X.; Pan, J. The relationship between the neutrophil-to-lymphocyte ratio and diabetic retinopathy in adults from the United States: Results from the National Health and nutrition examination survey. BMC Ophthalmol. 2022, 22, 346. [Google Scholar] [CrossRef]
- Chen, W.; Chen, K.; Xu, Z.; Hu, Y.; Liu, Y.; Liu, W.; Hu, X.; Ye, T.; Hong, J.; Zhu, H.; et al. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Predict Mortality in Patients with Diabetic Foot Ulcers Undergoing Amputations. Diabetes Metab. Syndr. Obes. 2021, 14, 821–829. [Google Scholar] [CrossRef]
- Li, L.; Shen, Q.; Rao, S. Association of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio with Diabetic Kidney Disease in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Ther. Clin. Risk Manag. 2022, 18, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Cho, S.I. Treatment variation related to comorbidity and complications in type 2 diabetes: A real world analysis. Medicine 2018, 97, e12435. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, L.; Degirmenci, H.; Aktaş, A.H.; Ozturk, C. Relationship between neutrophil-lymphocyte ratio and diabetic retinopathy. Ann. Ophthalmol. 2017, 49, 190–194. [Google Scholar]
- Wang, X.; Li, W.; Zhang, Y.; Wan, X. Association of neutrophil-to-lymphocyte ratio and diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis. Ther. Adv. Endocrinol. Metab. 2010, 11, 2042018820977801. [Google Scholar]
- Bai, Y.Y.; Xi, Y.; Yin, B.B.; Zhang, J.H.; Chen, F.; Zhu, B. Reference intervals of systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio during normal pregnancy in China. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1033–1044. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Zhang, G.; Yu, J.; Chen, Y.; Yin, H.; Goyal, H.; Zhang, G.M.; Xiao, Y.; Gu, C.; et al. Normal Reference Intervals of Neutrophil-To-Lymphocyte Ratio, Platelet-To-Lymphocyte Ratio, Lymphocyte-To-Monocyte Ratio, and Systemic Immune Inflammation Index in Healthy Adults: A Large Multi-Center Study from Western China. Clin. Lab. 2019, 65, 255–265. [Google Scholar] [CrossRef]
- Feng, J.F.; Chen, S.; Yang, X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine 2017, 96, e5886. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Fujii, Y.; Kaji, S.; Kawabata, Y.; Hyakudomi, R.; Yamamoto, T.; Taniura, T.; Tajima, Y. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget 2020, 11, 2625–2635. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, C.; Wu, L.; Li, Z.; Li, H.; Zhang, J. Systemic Immune Inflammation Index (SII), System Inflammation Response Index (SIRI) and Risk of All-Cause Mortality and Cardiovascular Mortality: A 20-Year Follow-Up Cohort Study of 42,875 US Adults. J. Clin. Med. 2023, 12, 1128. [Google Scholar] [CrossRef]
- Guo, W.; Song, Y.; Sun, Y.; Du, H.; Cai, Y.; You, Q.; Fu, H.; Shao, L. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front. Endocrinol. 2022, 13, 1071465. [Google Scholar] [CrossRef]
- Elbeyli, A.; Kurtul, B.E.; Ozcan, S.C.; Ozarslan Ozcan, D. The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin. Exp. Optom. 2022, 105, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Muhamad, I.I.; Niazmand, R.; Dikshit, P.K.; Kim, B.S. Recent progress in polymeric non-invasive insulin delivery. Int. J. Biol. Macromol. 2022, 203, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Dincer, N.; Yildirim, A.; Yesil, N.; Sezer, S. The relationship between neutrophil-to-lymphocyte ratio and diabetic retinopathy: A systematic review and meta-analysis. J. Clin. Lab. Anal. 2021, 35, e23613. [Google Scholar]
| Total | NDR | NDPR | PDR | p-Value | |
|---|---|---|---|---|---|
| N | 129 | 36 | 49 | 44 | |
| Age (mean ± SD) | 65.6 ± 8.9 | 67 ± 6.6 | 65.2 ± 7.1 | 63 ± 9.4 | 0.078 * |
| Males (n, %) | 67 (51%) | 16 (44.4%) | 23 (46.9%) | 26 (59%) | 0.393 ** |
| Duration of diabetes (yrs.) | 8.9 ± 3.8 | 5.3 ± 2.4 | 9.36 ± 3.3 | 11 ± 3.1 | <0.001 * |
| Associated DM complications: | |||||
| 13 (10%) | 3 (8.3%) | 2 (4%) | 8 (18.1%) | 0.072 ** |
| 11 (8.5%) | 2 (5.5%) | 3 (6.1%) | 6 (13.6%) | 0.325 ** |
| 4 (3.1%) | 1 (2.7%) | 1 (2%) | 2 (4.5%) | 0.778 ** |
| Associated diseases (n, %): | |||||
| 47 | 20 | 8 | 19 | 0.518 ** |
| 4 | 2 | 1 | 1 | 0.563 ** |
| 5 | 1 | 2 | 2 | 0.916 ** |
| FBG (mean ± SD) | 164.6 ± 60.3 | 145.5 ± 41.4 | 173.6 ± 78.6 | 170.4 ± 46 | 0.078 * |
| HbA1C (mean ± SD) | 7.6 ± 1.6 | 7.2 ± 1.1 | 7.5 ± 1.8 | 8.2 ± 1.8 | 0.113 * |
| Hb (mean ± SD) | 13.5 ± 1.6 | 13.6 ± 1.3 | 13.5 ± 1.6 | 13.4 ± 1.2 | 0.646 * |
| RDW (mean ± SD) | 13.9 ± 1.3 | 14 ±1.1 | 13.7 ± 1.4 | 14.2 ± 1.3 | 0.031 * |
| Neutrophils (mean ± SD) | 4.9 ± 1.4 | 4.8 ± 1.4 | 4.6 ± 1.2 | 5.4 ± 1.5 | 0.007 * |
| Lymphocytes (mean ± SD) | 2.1 ± 2.8 | 2.1 ± 0.7 | 2.2 ± 0.8 | 1.9 ± 0.7 | 0.285 |
| Monocytes (mean ± SD) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.066 * |
| Platelets (mean ± SD) | 237.4 ± 53 | 224.3 ± 42.6 | 248.7 ± 46.3 | 235.3 ± 64.9 | 0.106 * |
| MPV mean ± SD) | 9 ± 1.1 | 8.9 ± 1.1 | 8.7 ± 0.8 | 9.3 ± 1.2 | 0.02 * |
| TG (mean ± SD) | 148.3 ± 78.2 | 136.3 ± 63.4 | 149 ± 64.2 | 152.9 ± 92.4 | 0.076 * |
| Cholesterol (mean ± SD) | 163.19 ± 73.2 | 158.39 ± 61.7 | 162.14 ± 53.2 | 165.9 ± 70.5 | 0.341 * |
| Serum urea (mean ± SD) | 51.8 ± 28.7 | 48.4 ± 31.2 | 49.1 ± 25.8 | 57.6 ± 29.3 | 0.264 * |
| Serum Creatinine | 1.1 ± 0.6 | 0.9 ± 0.3 | 1 ± 0.5 | 1.3 ± 0.8 | 0.024 * |
| Creatinine > 1.2mg/dL (n, %): | 29 (22.4%) | 6 (16.6%) | 6 (12.2%) | 16 (36.3) | 0.013 ** |
| Urea > 60 mg/dL (n, %) | 30 (23.2%) | 7 (19.4%) | 8 (16.3%) | 15 (34%) | 0.035 ** |
| Total | NDR | NDPR | PDR | p-Value | |
|---|---|---|---|---|---|
| NLR (mean ± SD) | 2.6 ± 1.3 | 2.4 ± 0.9 | 2.4 ± 1.1 | 3.2 ± 1.6 | 0.005 * |
| PLR (mean ± SD) × 109 cells/L | 126 ± 54.1 | 115.4 ± 38.9 | 122.1 ± 35.4 | 138.9 ± 76.1 | 0.127 * |
| MLR (mean ± SD) | 0.308 ± 0.157 | 0.269 ± 0.083 | 0.275 ± 0.111 | 0.376 ± 0.216 | 0.001 * |
| SII (mean ± SD) × 109 cells/L | 624 ± 365.5 | 551.5 ± 215.1 | 560.3 ± 248.6 | 754.4 ± 514.4 | 0.013 * |
| PDR Sensitivity (%) | PDR Specificity (5) | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 40.0 | 86.9 | >3.18 | 0.662 | 0.001 |
| MLR | 35.6 | 92.9 | >0.364 | 0.643 | 0.006 |
| SII | 35.6 | 85.7 | >763.8 (×109 cells/L) | 0.627 | 0.015 |
| MPV | 55.6 | 63.1 | >9.24 | 0.593 | 0.084 |
| PLR | 26.7 | 91.7 | >168.8 (×109 cells/L) | 0.536 | 0.518 |
| Risk | Estimated Co-Efficient | Standard Error | Wald | Degrees of Freedom | p-Value | OR | Lower | Upper |
|---|---|---|---|---|---|---|---|---|
| Duration of diabetes | 0.263 | 0.061 | 18.55 | 1 | <0.0001 | 1.301 | 1.154 | 1.467 |
| MPV | 0.348 | 0.174 | 3.984 | 1 | 0.045 | 1.41 | 1.006 | 1.994 |
| NLR | 0.498 | 0.165 | 9.062 | 1 | 0.002 | 1.645 | 1.189 | 2.275 |
| MLR × 10 | 0.508 | 0.162 | 9.82 | 1 | 0.0017 | 1.662 | 1.209 | 2.284 |
| SII | 0.001 | 0.000 | 7.23 | 1 | 0.007 | 1.001 | 1 | 1.003 |
| creatinine | 0.936 | 0.414 | 5.11 | 1 | 0.02 | 2.551 | 1.132 | 5.746 |
| Model No. | Variable | Coefficient | Std.Error | Wald | p | OR | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|---|
| LOGREG_1 | NLR | 0.46364 | 0.17296 | 7.1857 | 0.0073 | 1.632 | 1.156 | 2.304 |
| Duration DM | 0.28342 | 0.067991 | 17.3759 | <0.0001 | 1.314 | 1.154 | 1.498 | |
| Constant | −4.59859 | 0.89211 | 26.5713 | <0.0001 | ||||
| LOGREGR_2 | MPV | 0.46284 | 0.21874 | 4.4771 | 0.0344 | 1.5886 | 1.0347 | 2.439 |
| PLR | 0.011012 | 0.0048070 | 5.2481 | 0.0220 | 1.0111 | 1.0016 | 1.0206 | |
| creatinine | 0.87851 | 0.42178 | 4.3384 | 0.0373 | 2.4073 | 1.0532 | 5.5025 | |
| Duration of DM | 0.30659 | 0.071052 | 18.6195 | <0.0001 | 1.3588 | 1.1821 | 1.5618 | |
| Constant | −10.14928 | 2.51771 | 16.2502 | 0.0001 | ||||
| LOGREGR_3 | MLR | 0.5234 | 0.1747 | 8.9763 | 0.0027 | 1.6879 | 1.1984 | 2.3772 |
| Duration DM | 0.2853 | 0.0706 | 16.3062 | <0.0001 | 1.3302 | 1.1582 | 1.5278 | |
| Constant | −4.97103 | 0.9542 | 27.1372 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dascalu, A.M.; Serban, D.; Tanasescu, D.; Vancea, G.; Cristea, B.M.; Stana, D.; Nicolae, V.A.; Serboiu, C.; Tribus, L.C.; Tudor, C.; et al. The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM). Biomedicines 2023, 11, 2106. https://doi.org/10.3390/biomedicines11082106
Dascalu AM, Serban D, Tanasescu D, Vancea G, Cristea BM, Stana D, Nicolae VA, Serboiu C, Tribus LC, Tudor C, et al. The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM). Biomedicines. 2023; 11(8):2106. https://doi.org/10.3390/biomedicines11082106
Chicago/Turabian StyleDascalu, Ana Maria, Dragos Serban, Denisa Tanasescu, Geta Vancea, Bogdan Mihai Cristea, Daniela Stana, Vanessa Andrada Nicolae, Crenguta Serboiu, Laura Carina Tribus, Corneliu Tudor, and et al. 2023. "The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM)" Biomedicines 11, no. 8: 2106. https://doi.org/10.3390/biomedicines11082106






