Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control
Abstract
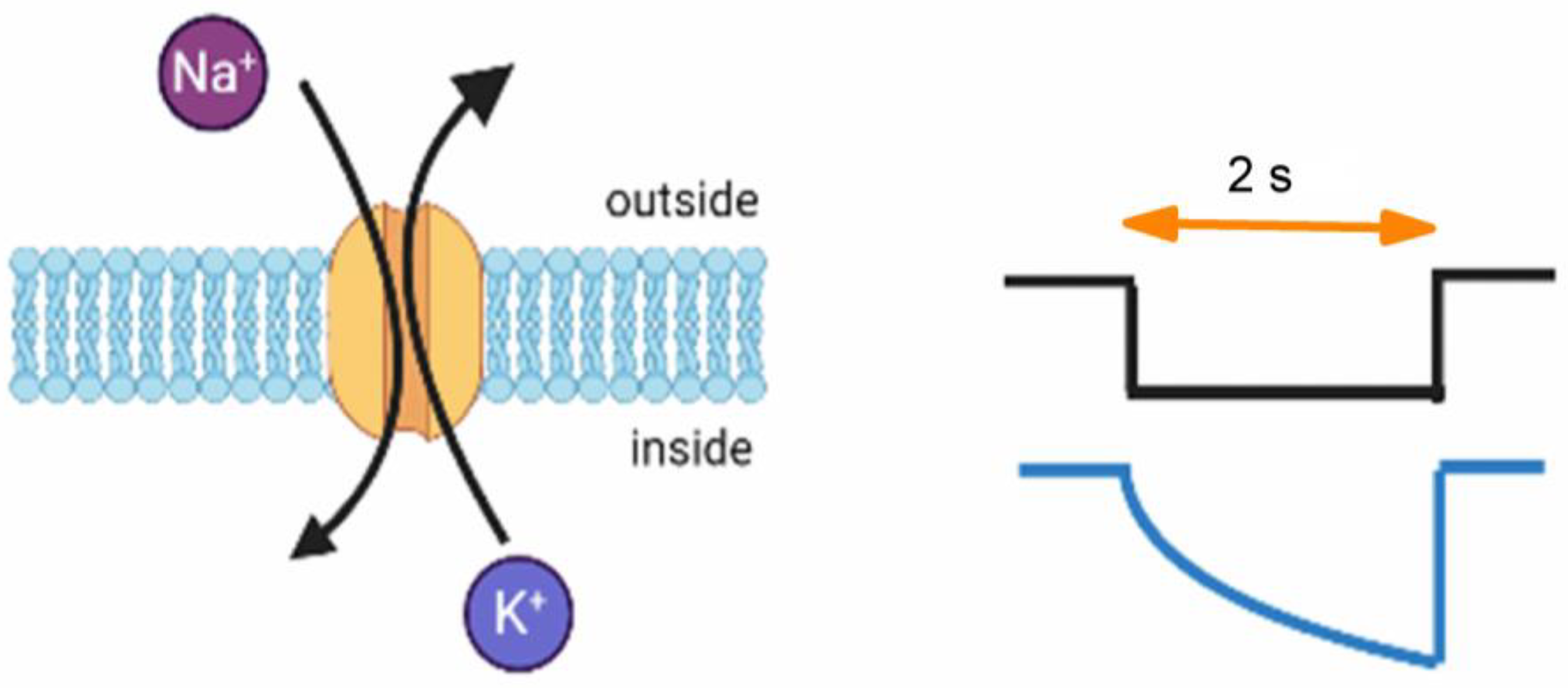
1. Introduction
2. Compounds That Are Known to Inhibit Ih
2.1. Brivaracetam
2.2. Carisbamate
2.3. Cilobradine
2.4. Dexmedetomidine
2.5. GAL-021tion
2.6. Lutein
2.7. Pirfenidone
2.8. Tramadol
3. Herbal Drugs That Are Known to Inhibit Ih
3.1. Ent-Kaurane-Type Diterpenoids (i.e., Croton-01, Croton-02, Croton-03) from Croton Tonkinensis
3.2. Ganoderma Triterpenoids (Active Constituents of Ganoderma Spores)
3.3. Honokiol
3.4. Pterostilbene
4. The Compound That Is Known to Stimulate Ih
Oxaliplatin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCN channel | hyperpolarization-activated cyclic nucleotide-gated channel |
| Hys(V) | voltage-dependent hysteresis |
| Vramp | ramp voltage |
| Ih | hyperpolarization-activated cation current |
References
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. 1997, 272 Pt 1, E405–E414. [Google Scholar] [CrossRef] [PubMed]
- Simasko, S.M.; Sankaranarayanan, S. Pacemaker mechanism of porcine sino-atrial node cells. J. Smooth Muscle Res. 2003, 39, 195–204. [Google Scholar]
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar] [CrossRef]
- Kretschmannova, K.; Gonzalez-Iglesias, A.E.; Tomic, M.; Stojilkovic, S.S. Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing GH3 cells. J. Neuroendocrinol. 2006, 18, 484–493. [Google Scholar] [CrossRef]
- Kretschmannova, K.; Kucka, M.; Gonzalez-Iglesias, A.E.; Stojilkovic, S.S. The expression and role of hyperpolarization-activated and cyclic nucleotide-gated channels in endocrine anterior pituitary cells. Mol. Endocrinol. 2012, 26, 153–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Gyulkhandanyan, A.V.; Xu, E.; Gaisano, H.Y.; Wheeler, M.B.; Wang, Q. Presence of functional hyperpolarisation-activated cyclic nucleotide-gated channels in clonal alpha cell lines and rat islet alpha cells. Diabetologia 2008, 51, 2290–2298. [Google Scholar] [CrossRef][Green Version]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- Kuo, P.C.; Kao, Z.H.; Lee, S.W.; Wu, S.N. Effects of Sesamin, the Major Furofuran Lignan of Sesame Oil, on the Amplitude and Gating of Voltage-Gated Na(+) and K(+) Currents. Molecules 2020, 25, 3062. [Google Scholar] [CrossRef]
- Wu, S.-N.; Fang, Y.-H.; Liu, P.-Y.; Liu, Y.-W. Characterization of Hyperpolarization-Induced Cation Current in Differentiated Human Embryonic Stem Cell-Derived Cardiomyocytes. J. Am. College Cardiol. 2020, 75 (Suppl. 1), 695. [Google Scholar] [CrossRef]
- Sartiani, L.; Mannaioni, G.; Masi, A.; Novella Romanelli, M.; Cerbai, E. The Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels: From Biophysics to Pharmacology of a Unique Family of Ion Channels. Pharmacol. Rev. 2017, 69, 354–395. [Google Scholar]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Mäki-Marttunen, T.; Mäki-Marttunen, V. Excitatory and inhibitory effects of HCN channel modulation on excitability of layer V pyramidal cells. PLoS Comput. Biol. 2022, 18, e1010506. [Google Scholar] [CrossRef]
- Männikkö, R.; Pandey, S.; Larsson, H.P.; Elinder, F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J. Gen. Physiol. 2005, 125, 305–326. [Google Scholar] [PubMed]
- Barthel, L.; Reetz, O.; Strauss, U. Use Dependent Attenuation of Rat HCN1-Mediated Ih in Intact HEK293 Cells. Cell. Physiol. Biochem. 2016, 38, 2079–2093. [Google Scholar] [CrossRef]
- Fürst, O.; D’avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef]
- Wu, P.M.; Lai, P.C.; ChO, H.Y.; Chuang, T.H.; Wu, S.N.; Tu, Y.F. Effective Perturbations by Phenobarbital on I(Na), I(K(erg)), I(K(M)) and I(K(DR)) during Pulse Train Stimulation in Neuroblastoma Neuro-2a Cells. Biomedicines 2022, 10, 1968. [Google Scholar] [CrossRef]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar] [CrossRef]
- Byczkowicz, N.; Eshra, A.; Montanaro, J.; Trevisiol, A.; Hirrlinger, J.; Kole, M.H.; Shigemoto, R.; Hallermann, S. HCN channel-mediated neuromodulation can control action potential velocity and fidelity in central axons. eLife 2019, 8, e42766. [Google Scholar] [CrossRef]
- Benedetti, B.; Bieler, L.; Erhardt-Kreutzer, C.; Jakubecova, D.; Benedetti, A.; Reisinger, M.; Dannehl, D.; Thome, C.; Engelhardt, M.; Couillard-Despres, S. Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity. Int. J. Mol. Sci. 2023, 24, 4715. [Google Scholar] [CrossRef]
- Dini, L.; Lungo, M.D.; Resta, F.; Melchiorre, M.; Spinelli, V.; Mannelli, L.D.C.; Ghelardini, C.; Laurino, A.; Sartiani, L.; Romanelli, M.N. Selective Blockade of HCN1/HCN2 Channels as a Potential Pharmacological Strategy Against Pain. Front. Pharmacol. 2018, 9, 1252. [Google Scholar] [CrossRef]
- Hung, T.-Y.; Wu, S.-N.; Huang, C.-W. The Integrated Effects of Brivaracetam, a Selective Analog of Levetiracetam, on Ionic Currents and Neuronal Excitability. Biomedicines 2021, 9, 369. [Google Scholar] [PubMed]
- Liu, Y.-C.; Wang, Y.-J.; Wu, P.-Y.; Wu, S.-N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-Y.; Wu, S.-N.; Huang, C.-W. Concerted suppressive effects of carisbamate, an anti-epileptic alkyl-carbamate drug, on voltage-gated Na+ and hyperpolarization-activated cation currents. Front. Cell. Neurosci. 2023, 17, 1159067. [Google Scholar]
- Kuo, P.-C.; Liu, Y.-C.; Lo, Y.-C.; Wu, S.-N. Characterization of Inhibitory Effectiveness in Hyperpolarization-Activated Cation Currents by a Group of ent-Kaurane-Type Diterpenoids from Croton tonkinensis. Int. J. Mol. Sci. 2020, 21, 1268. [Google Scholar]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Inhibitory Effective Perturbations of Cilobradine (DK-AH269), A Blocker of HCN Channels, on the Amplitude and Gating of Both Hyperpolarization-Activated Cation and Delayed-Rectifier Potassium Currents. Int. J. Mol. Sci. 2020, 21, 2416. [Google Scholar] [CrossRef]
- Chang, W.-T.; Gao, Z.-H.; Lo, Y.-C.; Wu, S.-N. Evidence for Effective Inhibitory Actions on Hyperpolarization-Activated Cation Current Caused by Ganoderma Triterpenoids, the Main Active Constituents of Ganoderma Spores. Molecules 2019, 24, 4256. [Google Scholar]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Effectiveness in Block by Dexmedetomidine of Hyperpolarization-Activated Cation Current, Independent of Its Agonistic Effect on α(2)-Adrenergic Receptors. Int. J. Mol. Sci. 2020, 21, 9110. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, H.H.; Lo, Y.C.; Wu, S.N. Effectiveness in the Block by Honokiol, a Dimerized Allylphenol from Magnolia Officinalis, of Hyperpolarization-Activated Cation Current and Delayed-Rectifier K(+) Current. Int. J. Mol. Sci. 2020, 21, 4260. [Google Scholar] [CrossRef]
- Lu, T.L.; Gao, Z.H.; Li, S.W.; Wu, S.N. High Efficacy by GAL-021: A Known Intravenous Peripheral Chemoreceptor Modulator that Suppresses BK(Ca)-Channel Activity and Inhibits I(K(M)) or I(h). Biomolecules 2020, 10, 188. [Google Scholar]
- Tan, K.; Chen, P.; Li, S.; Ke, T.; Lin, S.; Yang, C. Characterization of Effectiveness in Concerted I(h) Inhibition and I(K(Ca)) Stimulation by Pterostilbene (Trans-3,5-dimethoxy-4′-hydroxystilbene), a Stilbenoid. Int. J. Mol. Sci. 2020, 21, 357. [Google Scholar]
- Chuang, C.W.; Chang, K.P.; Cho, H.Y.; Chuang, T.H.; Yu, M.C.; Wu, C.L.; Wu, S.N. Characterization of Inhibitory Capability on Hyperpolarization-Activated Cation Current Caused by Lutein (β,ε-Carotene-3,3′-Diol), a Dietary Xanthophyll Carotenoid. Int. J. Mol. Sci. 2022, 23, 7186. [Google Scholar] [PubMed]
- Chang, W.-T.; Gao, Z.-H.; Li, S.-W.; Liu, P.-Y.; Lo, Y.-C.; Wu, S.-N. Characterization in Dual Activation by Oxaliplatin, a Platinum-Based Chemotherapeutic Agent of Hyperpolarization-Activated Cation and Electroporation-Induced Currents. Int. J. Mol. Sci. 2020, 21, 396. [Google Scholar] [CrossRef]
- Chang, W.-T.; Ragazzi, E.; Liu, P.-Y.; Wu, S.-N. Effective block by pirfenidone, an antifibrotic pyridone compound (5-methyl-1-phenylpyridin-2[H-1]-one), on hyperpolarization-activated cation current: An additional but distinctive target. Eur. J. Pharmacol. 2020, 882, 173237. [Google Scholar]
- Huang, M.H.; Huang, Y.M.; Wu, S.N. The Inhibition by Oxaliplatin, a Platinum-Based Anti-Neoplastic Agent, of the Activity of Intermediate-Conductance Ca²⁺-Activated K⁺ Channels in Human Glioma Cells. Cell Physiol. Biochem. 2015, 37, 1390–1406. [Google Scholar]
- Malawska, B.; Kulig, K. Brivaracetam: A new drug in development for epilepsy and neuropathic pain. Expert Opin. Investig. Drugs 2008, 17, 361–369. [Google Scholar]
- Strzelczyk, A.; Klein, K.M.; Willems, L.M.; Rosenow, F.; Bauer, S. Brivaracetam in the treatment of focal and idiopathic generalized epilepsies and of status epilepticus. Expert. Rev. Clin. Pharmacol. 2016, 9, 637–645. [Google Scholar]
- Tsymbalyuk, S.; Smith, M.; Gore, C.; Tsymbalyuk, O.; Ivanova, S.; Sansur, C.; Gerzanich, V.; Simard, J.M. Brivaracetam attenuates pain behaviors in a murine model of neuropathic pain. Mol. Pain. 2019, 15, 1744806919886503. [Google Scholar] [CrossRef]
- Nicolas, J.; Hannestad, J.; Holden, D.; Kervyn, S.; Nabulsi, N.; Tytgat, D.; Huang, Y.; Chanteux, H.; Staelens, L.; Matagne, A.; et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 2016, 57, 201–209. [Google Scholar]
- da Silva Fernandes, M.J.; Marques-Carneiro, J.E.; Amorim, R.P.; Araujo MG, L.; Nehlig, A. Neuroprotective agents and modulation of temporal lobe epilepsy. Front Biosci. Elite 2015, 7, 79–93. [Google Scholar]
- Strzelczyk, A.; Schubert-Bast, S. Expanding the Treatment Landscape for Lennox-Gastaut Syndrome: Current and Future Strategies. CNS Drugs 2021, 35, 61–83. [Google Scholar] [PubMed]
- Elkommos, S.; Mula, M. Current and future pharmacotherapy options for drug-resistant epilepsy. Expert. Opin. Pharmacother. 2022, 23, 2023–2034. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Nagarkatti, N.; Ziobro, J.M.; Sombati, S.; DeLorenzo, R.J. Carisbamate prevents the development and expression of spontaneous recurrent epileptiform discharges and is neuroprotective in cultured hippocampal neurons. Epilepsia 2008, 49, 1795–1802. [Google Scholar] [CrossRef]
- Deshpande, L.S.; DeLorenzo, R.J. Novel therapeutics for treating organophosphate-induced status epilepticus co-morbidities, based on changes in calcium homeostasis. Neurobiol. Dis. 2020, 133, 104418. [Google Scholar]
- Stieber, J.; Wieland, K.; Stöckl, G.; Ludwig, A.; Hofmann, F. Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol. Pharmacol. 2006, 69, 1328–1337. [Google Scholar] [CrossRef]
- El-Kholy, W.; Patrick, E.M.; Jocelyn-Manning, F.; Alpana, B.; Xue, T.; Xiaodong, G.; Yi, Z.; Juliane, S.; Ronald, A.L.; Robert, G.T.; et al. Hyperpolarization-activated cyclic nucleotide-gated channels in pancreatic beta-cells. Mol. Endocrinol. 2007, 21, 753–764. [Google Scholar]
- Horwitz, G.C.; Risner-Janiczek, J.R.; Jones, S.M.; Holt, J.R. HCN channels expressed in the inner ear are necessary for normal balance function. J. Neurosci. 2011, 31, 16814–16825. [Google Scholar]
- Chambers, A.R.; Pilati, N.; Balaram, P.; Large, C.H.; Kaczmarek, L.K.; Polley, D.B. Pharmacological modulation of Kv3.1 mitigates auditory midbrain temporal processing deficits following auditory nerve damage. Sci. Rep. 2017, 7, 17496. [Google Scholar]
- Martin, E.; Ramsay, G.; Mantz, J.; Sum-Ping, S.T.J. The role of the alpha2-adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. J. Intensive Care Med. 2003, 18, 29–41. [Google Scholar] [CrossRef]
- Lehto, J.; Scheinin, A.; Johansson, J.; Marjamäki, P.; Arponen, E.; Scheinin, H.; Scheinin, M. Detecting a dexmedetomidine-evoked reduction of noradrenaline release in the human brain with the alpha2C-adrenoceptor PET ligand [11C]ORM-13070. Synapse 2016, 70, 57–65. [Google Scholar] [CrossRef]
- Lehto, J.; Scheinin, A.; Johansson, J.; Marjamäki, P.; Arponen, E.; Scheinin, H.; Scheinin, M. Dexmedetomidine Exerts a Negative Chronotropic Action on Sinoatrial Node Cells Through the Activation of Imidazoline Receptors. J. Cardiovasc. Pharmacol. 2021, 78, 826–838. [Google Scholar]
- Shehabi, Y.; Howe, B.D.; Bellomo, R.; Arabi, Y.M.; Bailey, M.; Bass, F.E.; Bin Kadiman, S.; McArthur, C.J.; Murray, L.; Reade, M.C.; et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. N. Engl. J. Med. 2019, 380, 2506–2517. [Google Scholar] [CrossRef] [PubMed]
- Schwerin, S.; Westphal, C.; Klug, C.; Schneider, G.; Kreuzer, M.; Haseneder, R.; Kratzer, S. Sedative Properties of Dexmedetomidine Are Mediated Independently from Native Thalamic Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Function at Clinically Relevant Concentrations. Int. J. Mol. Sci. 2022, 24, 519. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Feng, C.; Jin, Y.; Zhao, X. Dexmedetomidine as an Adjuvant in Peripheral Nerve Block. Drug Des. Devel Ther. 2023, 17, 1463–1484. [Google Scholar] [CrossRef]
- Shirasaka, T.; Kannan, H.; Takasaki, M. Activation of a G protein-coupled inwardly rectifying K+ current and suppression of Ih contribute to dexmedetomidine-induced inhibition of rat hypothalamic paraventricular nucleus neurons. Anesthesiology 2007, 107, 605–615. [Google Scholar] [CrossRef]
- Chen, B.-S.; Peng, H.; Wu, S.-N. Dexmedetomidine, an alpha2-adrenergic agonist, inhibits neuronal delayed-rectifier potassium current and sodium current. Br. J. Anaesth. 2009, 103, 244–254. [Google Scholar] [CrossRef]
- Kosugi, T.; Mizuta, K.; Fujita, T.; Nakashima, M.; Kumamoto, E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha(2) adrenoceptor activation. Br. J. Pharmacol. 2010, 160, 1662–1676. [Google Scholar] [CrossRef]
- Chiu, K.-M.; Lin, T.-Y.; Lee, M.-Y.; Lu, C.-W.; Wang, M.-J.; Wang, S.-J. Dexmedetomidine protects neurons from kainic acid-induced excitotoxicity by activating BDNF signaling. Neurochem. Int. 2019, 129, 104493. [Google Scholar] [CrossRef]
- Golder, F.J.; Dax, S.; Baby, S.M.; Gruber, R.; Hoshi, T.; Ideo, C.; Kennedy, A.; Peng, S.; Puskovic, V.; Ritchie, D.; et al. Identification and Characterization of GAL-021 as a Novel Breathing Control Modulator. Anesthesiology 2015, 123, 1093–1104. [Google Scholar] [CrossRef]
- Dallas, M.L.; Peers, C.; Golder, F.J.; Baby, S.; Gruber, R.; MacIntyre, D.E. GAL-021 and GAL-160 are Efficacious in Rat Models of Obstructive and Central Sleep Apnea and Inhibit BKCa in Isolated Rat Carotid Body Glomus Cells. Adv. Exp. Med. Biol. 2015, 860, 361–370. [Google Scholar]
- Hawkins, V.E.; Hawryluk, J.M.; Takakura, A.C.; Tzingounis, A.V.; Moreira, T.S.; Mulkey, D.K.; Zhuang, J.; Zang, N.; Ye, C.; Xu, F.; et al. HCN channels contribute to serotonergic modulation of ventral surface chemosensitive neurons and respiratory activity. J. Neurophysiol. 2015, 113, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Bin Emran, T.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef]
- Bemme, S.; Weick, M.; Gollisch, T. Differential Effects of HCN Channel Block on On and Off Pathways in the Retina as a Potential Cause for Medication-Induced Phosphene Perception. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4754–4767. [Google Scholar] [CrossRef]
- Barravecchia, I.; Demontis, G.C. HCN1 channels: A versatile tool for signal processing by primary sensory neurons. Prog. Biophys. Mol. Biol. 2021, 166, 133–146. [Google Scholar] [CrossRef]
- Gerhardt, M.J.; Priglinger, S.G.; Biel, M.; Michalakis, S. Biology, Pathobiology and Gene Therapy of CNG Channel-Related Retinopathies. Biomedicines 2023, 11, 269. [Google Scholar] [CrossRef]
- Kitano, Y.; Wakimoto, S.; Tamura, S.; Kubota, K.; Domon, Y.; Arakawa, N.; Saito, M.; Sava, B.; Buisson, B. Effects of mirogabalin, a novel ligand for the α₂δ subunit of voltage-gated calcium channels, on N-type calcium channel currents of rat dorsal root ganglion culture neurons. Pharmazie 2019, 74, 147–149. [Google Scholar]
- Krämer, M.; Markart, P.; Drakopanagiotakis, F.; Mamazhakypov, A.; Schaefer, L.; Didiasova, M.; Wygrecka, M. Pirfenidone inhibits motility of NSCLC cells by interfering with the urokinase system. Cell Signal 2020, 65, 109432. [Google Scholar] [CrossRef]
- Gillen, C.; Haurand, M.; Kobelt, D.J.; Wnendt, S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human mu-opioid receptor. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 116–121. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.-S.; Li, B.-M.; Zhang, X.-H. Presynaptic HCN channels constrain GABAergic synaptic transmission in pyramidal cells of the medial prefrontal cortex. Biol. Open 2022, 11, bio058840. [Google Scholar] [CrossRef]
- Giang, P.M.; Son, P.T.; Lee, J.J.; Otsuka, H. Four ent-kaurane-type diterpenoids from Croton tonkinensis Gagnep. Chem. Pharm. Bull. 2004, 52, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; E Machen, T.; Widdicombe, J.H.; Carlson, T.J.; King, S.R.; Chow, J.W.; Illek, B. A novel extract SB-300 from the stem bark latex of Croton lechleri inhibits CFTR-mediated chloride secretion in human colonic epithelial cells. J. Ethnopharmacol. 2004, 93, 351–357. [Google Scholar] [CrossRef]
- Shiao, M.S. Natural products of the medicinal fungus Ganoderma lucidum: Occurrence, biological activities, and pharmacological functions. Chem. Rec. 2003, 3, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-F.; Fu, H.-Y.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.-K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Fujita, M.; Itokawa, H.; Sashida, Y. [Studies on the components of Magnolia obovata Thunb. 3. Occurrence of magnolol and hõnokiol in M. obovata and other allied plants]. Yakugaku Zasshi 1973, 93, 429–434. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chen, S.-H.; Chang, Y.-S.; Liu, Y.-W.; Wu, J.-Y.; Lim, Y.-P.; Yu, H.-I.; Lee, Y.-R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef]
- Tachikawa, E.; Takahashi, M.; Kashimoto, T. Effects of extract and ingredients isolated from Magnolia obovata thunberg on catecholamine secretion from bovine adrenal chromaffin cells. Biochem. Pharmacol. 2000, 60, 433–440. [Google Scholar] [CrossRef]
- Zhai, H.; Nakade, K.; Mitsumoto, Y.; Fukuyama, Y. Honokiol and magnolol induce Ca2+ mobilization in rat cortical neurons and human neuroblastoma SH-SY5Y cells. Eur. J. Pharmacol. 2003, 474, 199–204. [Google Scholar] [CrossRef]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016, 24, 974–990. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Mi, Y.; Li, T.; Gong, Y.; Ouyang, H.; Hou, Y. Long Non-coding RNAs Contribute to the Inhibition of Proliferation and EMT by Pterostilbene in Human Breast Cancer. Front. Oncol. 2018, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Chen, P.; Li, S.; Ke, T.; Lin, S.; Yang, C. Pterostilbene inhibits lung squamous cell carcinoma growth in vitro and in vivo by inducing S phase arrest and apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef]
- Li, H.-F.; Chen, S.-A.; Wu, S.-N. Evidence for the stimulatory effect of resveratrol on Ca(2+)-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef]
- Graham, J.; Mushin, M.; Kirkpatrick, P. Oxaliplatin. Nat. Rev. Drug Discov. 2004, 3, 11–12. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, L.-T.; Tsou, T.-C.; Pan, W.-Y.; Kuo, C.-C.; Liu, J.-F.; Yeh, S.-C.; Tsai, F.-Y.; Hsieh, H.-P.; Chang, J.-Y. Combined modalities of resistance in an oxaliplatin-resistant human gastric cancer cell line with enhanced sensitivity to 5-fluorouracil. Br. J. Cancer 2007, 97, 334–344. [Google Scholar] [CrossRef][Green Version]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef]
- Jerremalm, E.; Wallin, I.; Ehrsson, H. New insights into the biotransformation and pharmacokinetics of oxaliplatin. J. Pharm. Sci. 2009, 98, 3879–3885. [Google Scholar] [CrossRef]
- Ta, L.E.; Espeset, L.; Podratz, J.; Windebank, A.J. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology 2006, 27, 992–1002. [Google Scholar] [CrossRef]
- Chuang, T.H.; Cho, H.Y.; Wu, S.N. The Evidence for Sparsentan-Mediated Inhibition of I(Na) and I(K(erg)): Possibly Unlinked to Its Antagonism of Angiotensin II or Endothelin Type a Receptor. Biomedicines 2021, 10, 86. [Google Scholar] [CrossRef]
- Wu, S.-N.; Wu, C.-L.; Cho, H.-Y.; Chiang, C.-W. Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents. Int. J. Mol. Sci. 2022, 23, 9453. [Google Scholar] [CrossRef]
- Del Lungo, M.; Melchiorre, M.; Guandalini, L.; Sartiani, L.; Mugelli, A.; Koncz, I.; Szel, T.; Varro, A.; Romanelli, M.N.; Cerbai, E. Novel blockers of hyperpolarization-activated current with isoform selectivity in recombinant cells and native tissue. Br. J. Pharmacol. 2012, 166, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Knaus, A.; Zong, X.; Beetz, N.; Jahns, R.; Lohse, M.J.; Biel, M.; Hein, L. Direct inhibition of cardiac hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by clonidine. Circulation 2007, 115, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Oniani, T.; Vinnenberg, L.; Chaudhary, R.; Schreiber, J.A.; Riske, K.; Williams, B.; Pape, H.-C.; White, J.A.; Junker, A.; Seebohm, G.; et al. Effects of axonal demyelination, inflammatory cytokines and divalent cation chelators on thalamic HCN channels and oscillatory bursting. Int. J. Mol. Sci. 2022, 23, 6285. [Google Scholar] [CrossRef]


|
|
|
|
|
|
| Compound | IC50 | Refs. | Compound | IC50 | Refs. |
|---|---|---|---|---|---|
| Brivaracetam | greater than 25 μM | [22] | Tramadol | 13.6 μM | [23] |
| Carisbamate | 38 μM | [24] | Croton-01 *, croton-02 *, and croton-03 * | 2.89, 6.25, and 2.84 μM | [25] |
| Cilobradine | 3.38 μM | [26] | Ganoderma triterpenoids | 11.7 μg/ml | [27] |
| Dexmedetomidine | 1.21 μM | [28] | Honokiol | 2.1 μM | [29] |
| GAL-021 | greater than 30 μM | [30] | Pterostilbene | 0.84 μM | [31] |
| Lutein | 4.1 μM | [32] | Oxaliplatin | 1.3 μM ** | [33] |
| Pirfenidone | 3.65 μM | [34] |
| Compound | Chemical Structure |
|---|---|
| Brivaracetam |  |
| Carisbamate |  |
| Cilobradine |  |
| Dexmedetomidine |  |
| GAL-021 |  |
| Lutein |  |
| Pirfenidone |  |
| Tramadol |  |
| Croton-01, croton-02, and croton-03 |  |
| Honokiol |  |
| Pterostilbene |  |
| Oxaliplatin |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-S.; So, E.C.; Wu, S.-N. Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control. Biomedicines 2023, 11, 2177. https://doi.org/10.3390/biomedicines11082177
Chen C-S, So EC, Wu S-N. Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control. Biomedicines. 2023; 11(8):2177. https://doi.org/10.3390/biomedicines11082177
Chicago/Turabian StyleChen, Cheng-Shih, Edmund Cheung So, and Sheng-Nan Wu. 2023. "Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control" Biomedicines 11, no. 8: 2177. https://doi.org/10.3390/biomedicines11082177
APA StyleChen, C.-S., So, E. C., & Wu, S.-N. (2023). Modulating Hyperpolarization-Activated Cation Currents through Small Molecule Perturbations: Magnitude and Gating Control. Biomedicines, 11(8), 2177. https://doi.org/10.3390/biomedicines11082177






