Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs and Treatment
2.3. In Vivo Experiments
2.4. Quantitative Real-Time PCR
2.5. Western Blot Analysis
2.6. In Vitro Experiments
2.6.1. Cell Culture
2.6.2. Cell Differentiation and Viability
2.7. Statistical Analysis
3. Results
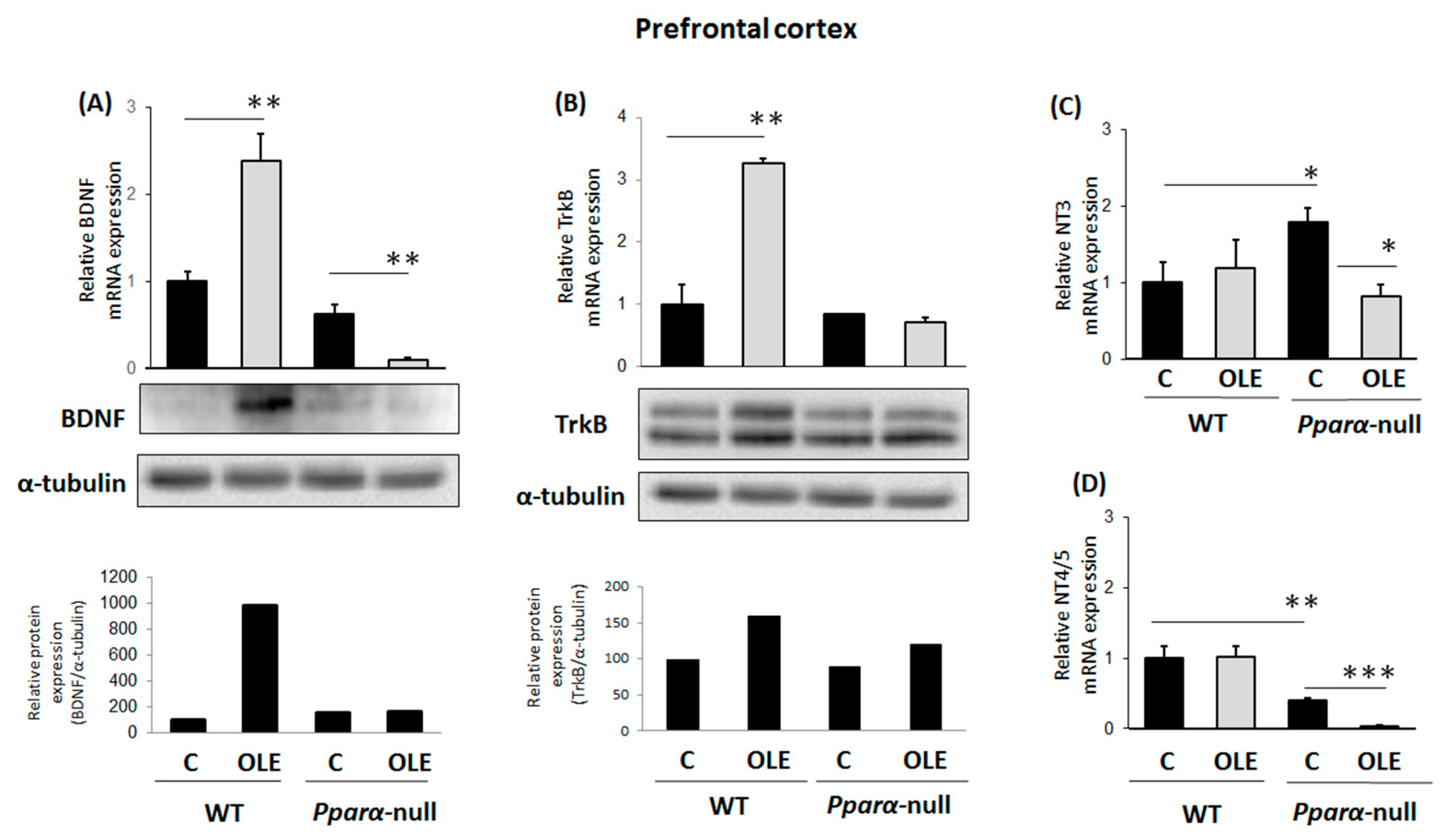
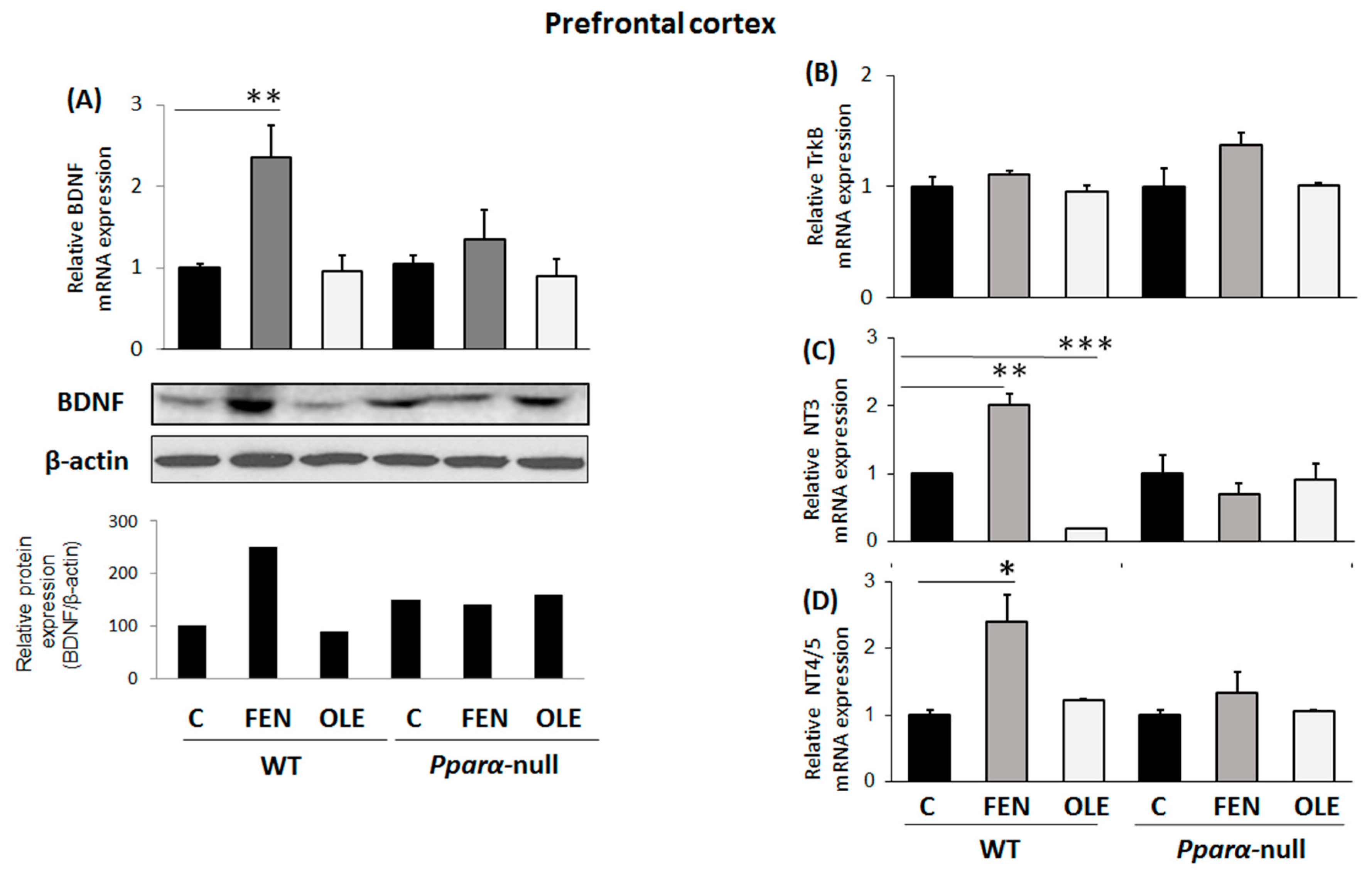
3.1. Assessment of Chronic OLE Treatment in Neural Plasticity Indices in PFC
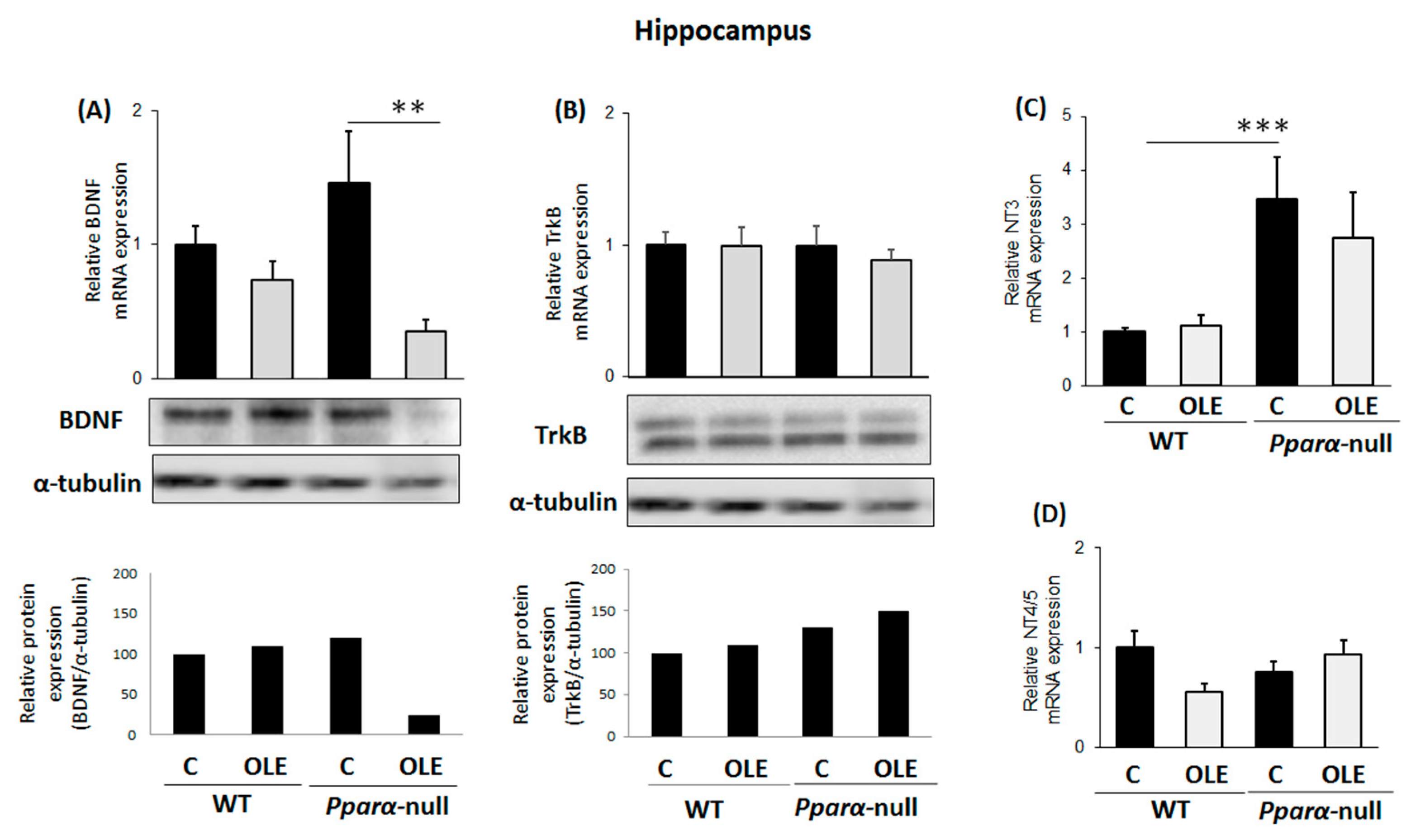
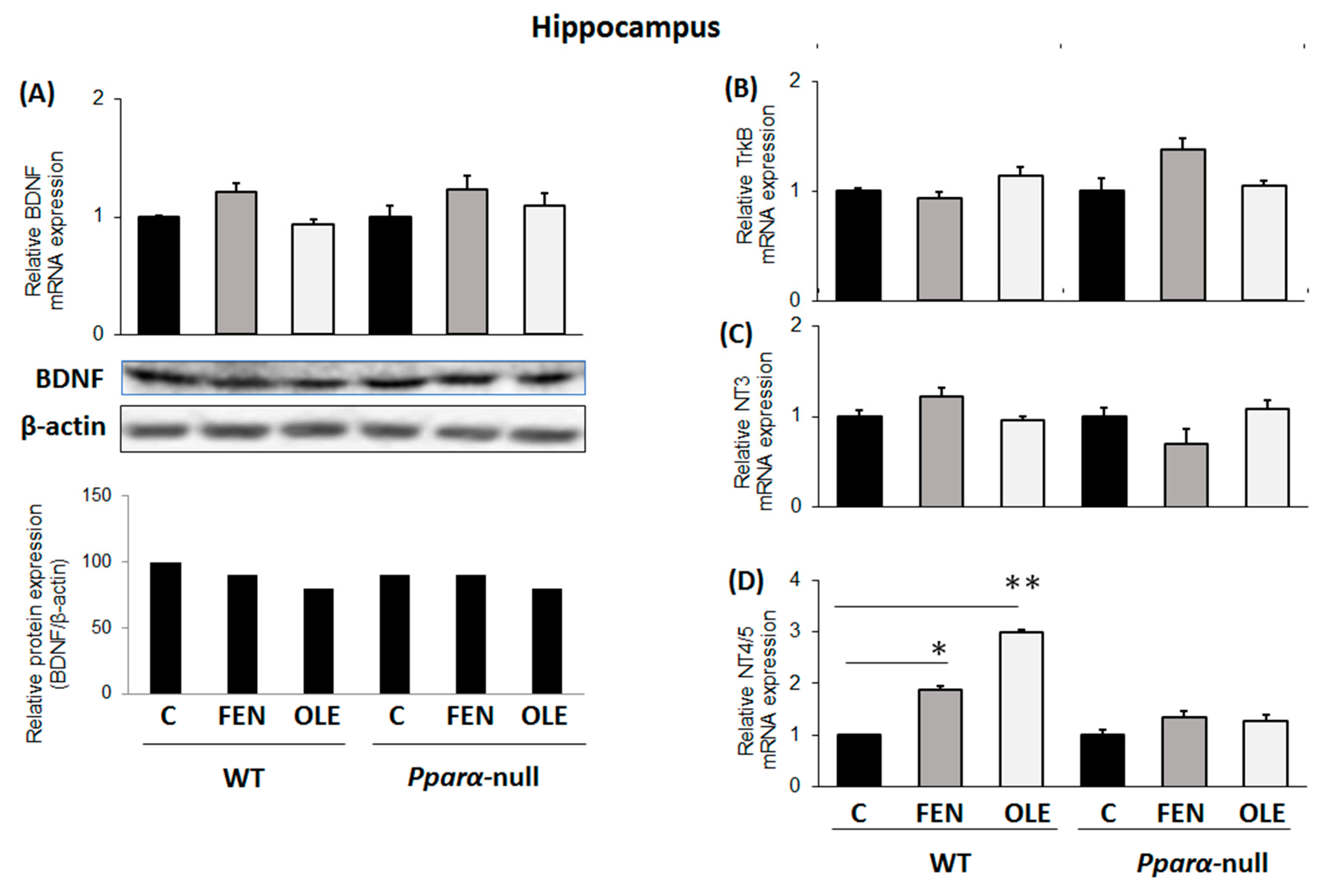
3.2. Assessment of Chronic OLE Treatment on Neural Plasticity Indices in the Hippocampus
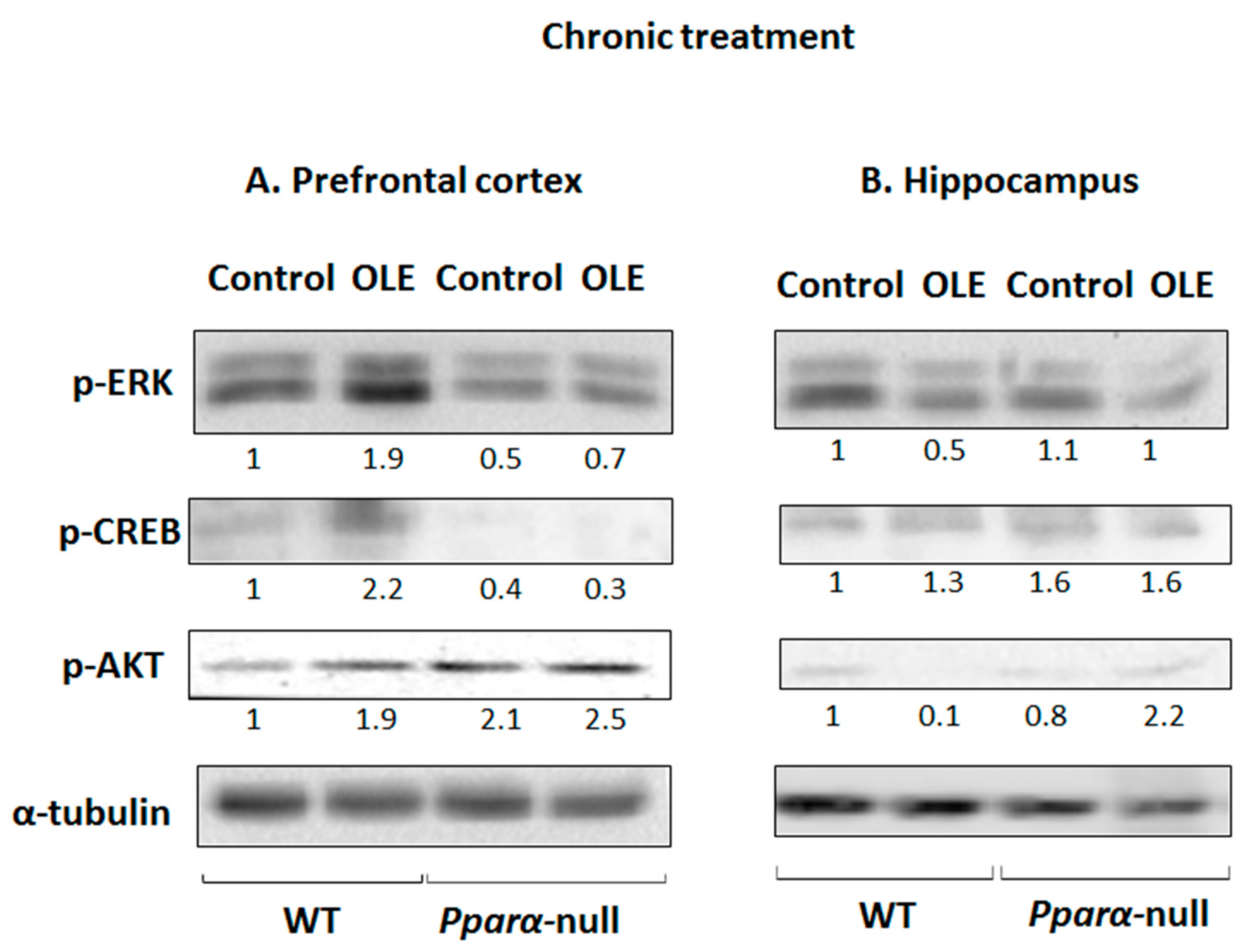
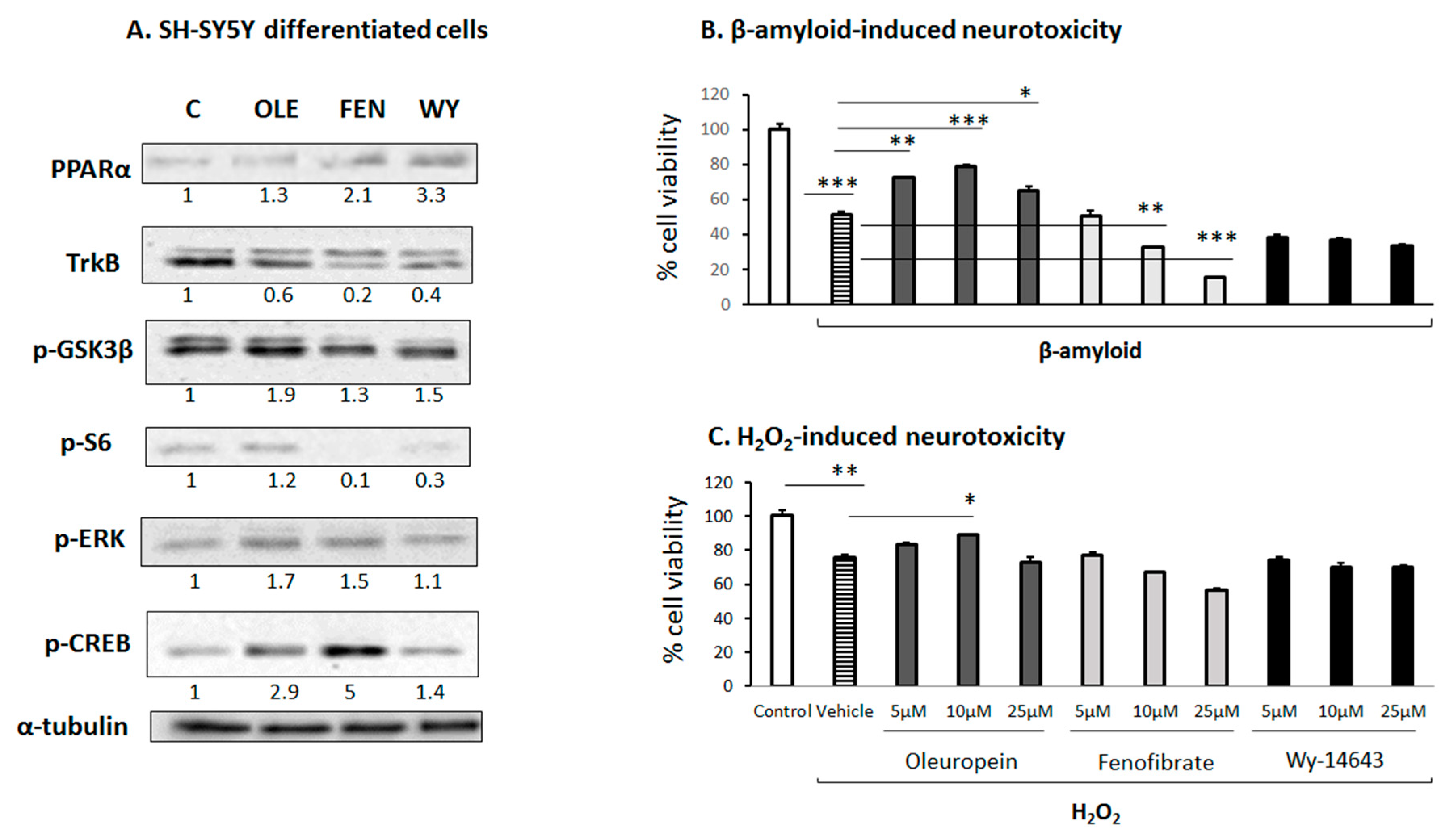
3.3. OLE-Induced ERK, AKT and PKA/CREB Activation
3.4. Assessment of Subacute OLE Treatment in Neural Plasticity Indices in PFC
3.5. Assessment of Subacute OLE Treatment in Neural Plasticity Indices in Hippocampus
3.6. Effect of OLE on Differentiated Human SH-SY5Y Neuroblastoma Cells
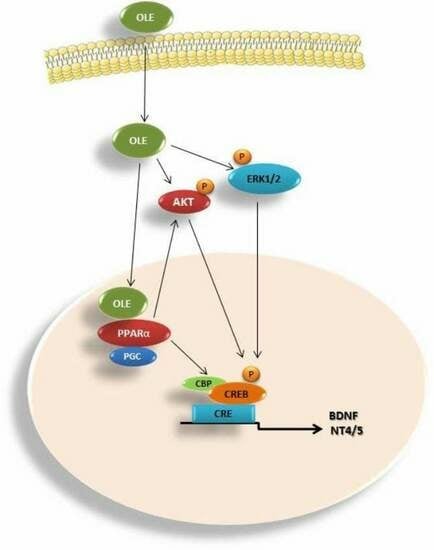
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPARα | peroxisome proliferator-activated receptor α |
| BDNF | Brain-derived neurotrophic factor |
| TrkB | tyrosine kinase receptor B |
| NT3 | neurotrophic factor 3 |
| NT4/5 | neurotrophic factor 4/5 |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator-1 alpha |
| OLE | Oleuropein |
| FEN | Fenofibrate |
| WY | Wy-14643 |
| PFC | prefrontal cortex |
| AD | Alzheimer’s disease |
| WT | wild type |
References
- Mateos-Aparicio, P.; Rodríguez-Moreno, A. The Impact of Studying Brain Plasticity. Front. Cell. Neurosci. 2019, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Bastings, E.P. Mapping clinically relevant plasticity after stroke. Neuropharmacology 2000, 39, 842–851. [Google Scholar] [CrossRef]
- Chen, R.; Cohen, L.G.; Hallett, M. Nervous system reorganization following injury. Neuroscience 2002, 111, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Bessis, A.; Béchade, C.; Bernard, D.; Roumier, A. Microglial control of neuronal death and synaptic properties. Glia 2007, 55, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhu, Y.-L.; Su, Z.; Lv, B.; Huang, Z.; Mu, L.; He, C. Olfactory ensheathing cells promote migration of Schwann cells by secreted nerve growth factor. Glia 2007, 55, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kondo, E.; Dai, Y.; Hashimoto, N.; Noguchi, K. Brain-Derived Neurotrophic Factor Increases in the Uninjured Dorsal Root Ganglion Neurons in Selective Spinal Nerve Ligation Model. J. Neurosci. 2001, 21, 4891–4900. [Google Scholar] [CrossRef]
- Bagayogo, I.P.; Dreyfus, C.F. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN Neuro 2009, 1, AN20090006. [Google Scholar] [CrossRef]
- Lessmann, V.; Gottmann, K.; Malcangio, M. Neurotrophin secretion: Current facts and future prospects. Prog. Neurobiol. 2003, 69, 341–374. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, I.; Meyer, M.; Thoenen, H. Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: Comparison of Schwann cells with other cell types. J. Neurosci. 1991, 11, 3165–3177. [Google Scholar] [CrossRef]
- Ohta, K.; Kuno, S.; Inoue, S.; Ikeda, E.; Fujinami, A.; Ohta, M. The effect of dopamine agonists: The expression of GDNF, NGF, and BDNF in cultured mouse astrocytes. J. Neurol. Sci. 2010, 291, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef]
- Verderio, C.; Bianco, F.; Blanchard, M.P.; Bergami, M.; Canossa, M.; Scarfone, E.; Matteoli, M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biol. 2006, 35, 187–201. [Google Scholar] [CrossRef]
- Yune, T.Y.; Lee, J.Y.; Jung, G.Y.; Kim, S.J.; Jiang, M.H.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline Alleviates Death of Oligodendrocytes by Inhibiting Pro-Nerve Growth Factor Production in Microglia after Spinal Cord Injury. J. Neurosci. 2007, 27, 7751–7761. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Soler, R.M.; Yuste, V.J.; Giné, E.; Sanz-Rodríguez, C.; Egea, J.; Martín-Zanca, D.; Comella, J.X. Development of survival responsiveness to brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5, but not to nerve growth factor, in cultured motoneurons from chick embryo spinal cord. J. Neurosci. 1998, 18, 7903–7911. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New Insights in the Biology of BDNF Synthesis and Release: Implications in CNS Function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef]
- Prior, M.; Dargusch, R.; Ehren, J.L.; Chiruta, C.; Schubert, D. The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer’s disease mice. Alzheimers Res. Ther. 2013, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Lian, Y.; Chen, Y.; Wu, T.; Zheng, Y.; Zong, H.; Sun, L.; Zhang, R.; Wang, Z.; et al. Brain-Derived Neurotrophic Factor Ameliorates Learning Deficits in a Rat Model of Alzheimer’s Disease Induced by Aβ1-42. PLoS ONE 2015, 10, e0122415. [Google Scholar] [CrossRef] [PubMed]
- Middlemas, D. Neurotrophin 3. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–3. [Google Scholar]
- Warner, S.C.; Valdes, A.M. The Genetics of Osteoarthritis: A Review. J. Funct. Morphol. Kinesiol. 2016, 1, 140–153. [Google Scholar] [CrossRef]
- Kang, H.; Schuman, E.M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 1995, 267, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Vicario-Abejón, C.; Owens, D.; McKay, R.; Segal, M. Role of neurotrophins in central synapse formation and stabilization. Nat. Rev. Neurosci. 2002, 3, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ramasamy, V.S.; Kang, H.K.; Jo, J. Oleuropein promotes hippocampal LTP via intracellular calcium mobilization and Ca2+-permeable AMPA receptor surface recruitment. Neuropharmacology 2020, 176, 108196. [Google Scholar] [CrossRef]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly Stabilized Curcumin Nanoparticles Tested in an In Vitro Blood–Brain Barrier Model and in Alzheimer’s Disease Tg2576 Mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Maher, P.; Akaishi, T.; Schubert, D.; Abe, K. A pyrazole derivative of curcumin enhances memory. Neurobiol. Aging 2010, 31, 706–709. [Google Scholar] [CrossRef]
- Praag, H.V.; Lucero, M.J.; Yeo, G.W.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (−)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878. [Google Scholar] [CrossRef] [PubMed]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Oleuropein protects low density lipoprotein from oxidation. Life Sci. 1994, 55, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Briante, R.; La Cara, F.; Tonziello, M.P.; Febbraio, F.; Nucci, R. Antioxidant activity of the main bioactive derivatives from oleuropein hydrolysis by hyperthermophilic beta-glycosidase. J. Agric. Food Chem. 2001, 49, 3198–3203. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F.; Watanabe, K.; Senbongi, R.; Iwai, K. Oleuropein, a phenolic compound in extra virgin olive oil, increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J. Nutr. Sci. Vitaminol. 2008, 54, 363–370. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Galli, G.; Caruso, D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002, 104, 677–684. [Google Scholar] [CrossRef]
- Malliou, F.; Andreadou, I.; Gonzalez, F.J.; Lazou, A.; Xepapadaki, E.; Vallianou, I.; Lambrinidis, G.; Mikros, E.; Marselos, M.; Skaltsounis, A.-L.; et al. The olive constituent oleuropein, as a PPARα agonist, markedly reduces serum triglycerides. J. Nutr. Biochem. 2018, 59, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Constantinou, M.; Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Kamber, E.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J. Nutr. 2006, 136, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Qabaha, K.; Al-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2017, 21, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and Antioxidant Effects of Hydroxytyrosol and Oleuropein from Olive Leaves in Alloxan-Diabetic Rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Dezfoulian, O.; Sookhtehzari, A.; Asadian, P.; Khoshdel, Z. Antioxidant effects of oleuropein versus oxidative stress induced by ethanol in the rat intestine. Comp. Clin. Pathol. 2014, 23, 1359–1365. [Google Scholar] [CrossRef]
- Luccarini, I.; Grossi, C.; Rigacci, S.; Coppi, E.; Pugliese, A.M.; Pantano, D.; la Marca, G.; Ed Dami, T.; Berti, A.; Stefani, M.; et al. Oleuropein aglycone protects against pyroglutamylated-3 amyloid-ß toxicity: Biochemical, epigenetic and functional correlates. Neurobiol. Aging 2015, 36, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet Supplementation with Hydroxytyrosol Ameliorates Brain Pathology and Restores Cognitive Functions in a Mouse Model of Amyloid-β Deposition. J. Alzheimer’s Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Ed Dami, T.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The Polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß Plaque Pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef] [PubMed]
- Pourkhodadad, S.; Alirezaei, M.; Moghaddasi, M.; Ahmadvand, H.; Karami, M.; Delfan, B.; Khanipour, Z. Neuroprotective effects of oleuropein against cognitive dysfunction induced by colchicine in hippocampal CA1 area in rats. J. Physiol. Sci. 2016, 66, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Pitozzi, V.; Jacomelli, M.; Catelan, D.; Servili, M.; Taticchi, A.; Biggeri, A.; Dolara, P.; Giovannelli, L.; Dal-Pan, A.; Dudonné, S.; et al. Long-Term Dietary Extra-Virgin Olive Oil Rich in Polyphenols Reverses Age-Related Dysfunctions in Motor Coordination and Contextual Memory in Mice: Role of Oxidative Stress. Rejuvenation Res. 2012, 15, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Price, T.O.; Dominguez, L.J.; Motisi, A.; Saiano, F.; Niehoff, M.L.; Morley, J.E.; Banks, W.A.; Ercal, N.; Barbagallo, M. Extra Virgin Olive Oil Improves Learning and Memory in SAMP8 Mice. J. Alzheimer’s Dis. 2012, 28, 81–92. [Google Scholar] [CrossRef]
- Rizk, F.H.; Soliman, N.A.; Heabah, N.A.; Abdel Ghafar, M.T.; El-Attar, S.H.; Elsaadany, A. Fenofibrate Improves Cognitive Impairment Induced by High-Fat High-Fructose Diet: A Possible Role of Irisin and Heat Shock Proteins. ACS Chem. Neurosci. 2022, 13, 1782–1789. [Google Scholar] [CrossRef]
- Akiyama, T.E.; Nicol, C.J.; Fievet, C.; Staels, B.; Ward, J.M.; Auwerx, J.; Lee, S.S.; Gonzalez, F.J.; Peters, J.M. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: Studies with congenic mouse lines. J. Biol. Chem. 2001, 276, 39088–39093. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Pineau, T.; Drago, J.; Lee, E.J.; Owens, J.W.; Kroetz, D.L.; Fernandez-Salguero, P.M.; Westphal, H.; Gonzalez, F.J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell Biol. 1995, 15, 3012–3022. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Perri, E.; Britti, D.; et al. The effects of a polyphenol present in olive oil, oleuropein aglycone, in an experimental model of spinal cord injury in mice. Biochem. Pharmacol. 2012, 83, 1413–1426. [Google Scholar] [CrossRef]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an antioxidant polyphenol from olive oil, is poorly absorbed from isolated perfused rat intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, J.R.; Reiss, K.Z. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J. Membr. Biol. 1987, 100, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Del Boccio, P.; Di Deo, A.; De Curtis, A.; Celli, N.; Iacoviello, L.; Rotilio, D. Liquid chromatography-tandem mass spectrometry analysis of oleuropein and its metabolite hydroxytyrosol in rat plasma and urine after oral administration. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 785, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Podlisny, M.B.; Ostaszewski, B.L.; Squazzo, S.L.; Koo, E.H.; Rydell, R.E.; Teplow, D.B.; Selkoe, D.J. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J. Biol. Chem. 1995, 270, 9564–9570. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.N.; Nie, F. Brain-derived neurotrophic factor enhances the therapeutic effect of oleuropein in the lipopolysaccharide-induced models of depression. Folia Neuropathol. 2021, 59, 249–262. [Google Scholar] [CrossRef]
- Impey, S.; Obrietan, K.; Wong, S.T.; Poser, S.; Yano, S.; Wayman, G.; Deloulme, J.C.; Chan, G.; Storm, D.R. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron 1998, 21, 869–883. [Google Scholar] [CrossRef]
- Cañón, E.; Cosgaya, J.M.; Scsucova, S.; Aranda, A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell 2004, 15, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Bonni, A.; Brunet, A.; West, A.E.; Datta, S.R.; Takasu, M.A.; Greenberg, M.E. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999, 286, 1358–1362. [Google Scholar] [CrossRef]
- Nakagawa, S.; Kim, J.E.; Lee, R.; Chen, J.; Fujioka, T.; Malberg, J.; Tsuji, S.; Duman, R.S. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J. Neurosci. 2002, 22, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, A.J.; Greenberg, M.E. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 1999, 68, 821–861. [Google Scholar] [CrossRef]
- Cooke, S.F.; Bliss, T.V. Plasticity in the human central nervous system. Brain 2006, 129 Pt 7, 1659–1673. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019, 169, 185–199. [Google Scholar] [CrossRef]
- Balint, L.B.; Nagy, L. Selective Modulators of PPAR Activity as New Therapeutic Tools in Metabolic Diseases. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.F.; Yin, S.; Chen, H.Y.; Dai, Z.; Qian, T.X.; Wang, C.C.; Xu, H.; Wang, X.Y. Oleuropein Improves Long Term Potentiation at Perforant Path-dentate Gyrus Synapses in vivo. Chin. Herb. Med. 2015, 7, 255–260. [Google Scholar] [CrossRef]
- Gelé, P.; Vingtdeux, V.; Potey, C.; Drobecq, H.; Ghestem, A.; Melnyk, P.; Buée, L.; Sergeant, N.; Bordet, R. Recovery of brain biomarkers following peroxisome proliferator-activated receptor agonist neuroprotective treatment before ischemic stroke. Proteome Sci. 2014, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tariq, U.; Haq, I.U.; Naz, A.; Rizwan, M. Neuroprotective effects of oleuropein: Recent developments and contemporary research. J. Food Biochem. 2021, 45, e13967. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequences of Primers | |
|---|---|---|
| BDNF | F | 5′-TGAGTCTCCAGGACAGCAAA-3′ |
| R | 5′-GACGTTTACTTCTTTCATGGGC-3′ | |
| TrkB | F | 5′-TGATGTTGCTCCTGCTCAAG-3′ |
| R | 5′-CCCAGCCTTTGTCTTTCCTT-3′ | |
| NT3 | F | 5′-CGGATGCCATGGTTACTTCT-3′ |
| R | 5′-AGTCTTCCGGCAAACTCCTT-3′ | |
| NT4/5 | F | 5′-AGCCGGGGAGCAGAGAAG-3′ |
| R | 5′-CACCTCCTCACTCTGGGACT-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malliou, F.; Andriopoulou, C.E.; Kofinas, A.; Katsogridaki, A.; Leondaritis, G.; Gonzalez, F.J.; Michaelidis, T.M.; Darsinou, M.; Skaltsounis, L.A.; Konstandi, M. Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways. Biomedicines 2023, 11, 2250. https://doi.org/10.3390/biomedicines11082250
Malliou F, Andriopoulou CE, Kofinas A, Katsogridaki A, Leondaritis G, Gonzalez FJ, Michaelidis TM, Darsinou M, Skaltsounis LA, Konstandi M. Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways. Biomedicines. 2023; 11(8):2250. https://doi.org/10.3390/biomedicines11082250
Chicago/Turabian StyleMalliou, Foteini, Christina E. Andriopoulou, Aristeidis Kofinas, Allena Katsogridaki, George Leondaritis, Frank J. Gonzalez, Theologos M. Michaelidis, Marousa Darsinou, Leandros A. Skaltsounis, and Maria Konstandi. 2023. "Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways" Biomedicines 11, no. 8: 2250. https://doi.org/10.3390/biomedicines11082250
APA StyleMalliou, F., Andriopoulou, C. E., Kofinas, A., Katsogridaki, A., Leondaritis, G., Gonzalez, F. J., Michaelidis, T. M., Darsinou, M., Skaltsounis, L. A., & Konstandi, M. (2023). Oleuropein Promotes Neural Plasticity and Neuroprotection via PPARα-Dependent and Independent Pathways. Biomedicines, 11(8), 2250. https://doi.org/10.3390/biomedicines11082250