Mast Cell Deficiency in Mice Attenuates Insulin Phenolic Preservative-Induced Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Air Pouch Generation
2.2. Assessment of IPP-Induced Inflammation in MC-Deficient Mouse Strains
2.3. Flow Cytometry Analysis
2.4. Total Protein Analysis of Air Pouch Lavage Fluid
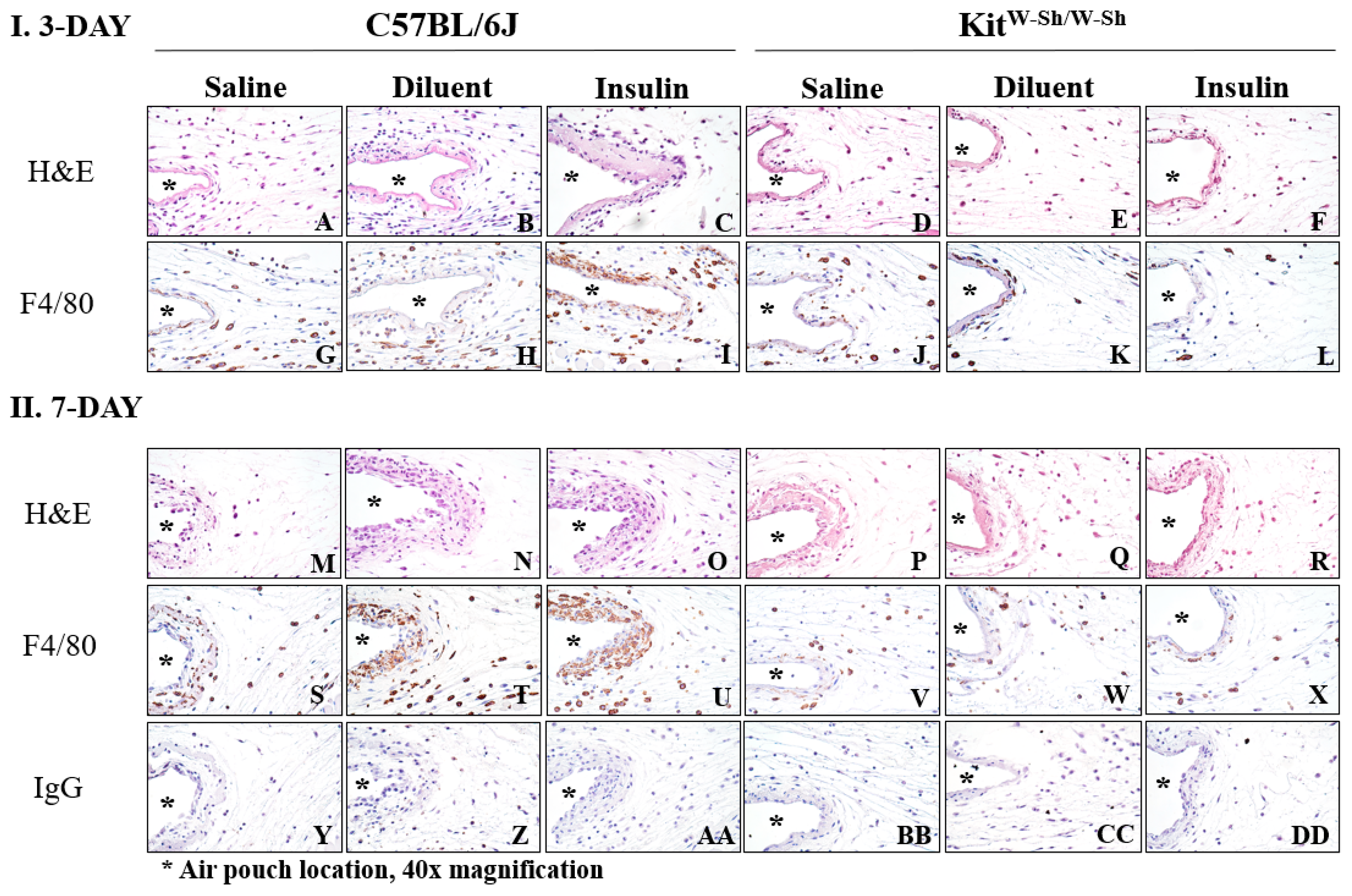
2.5. Immunohistochemistry
2.6. Statistical Analysis
3. Results
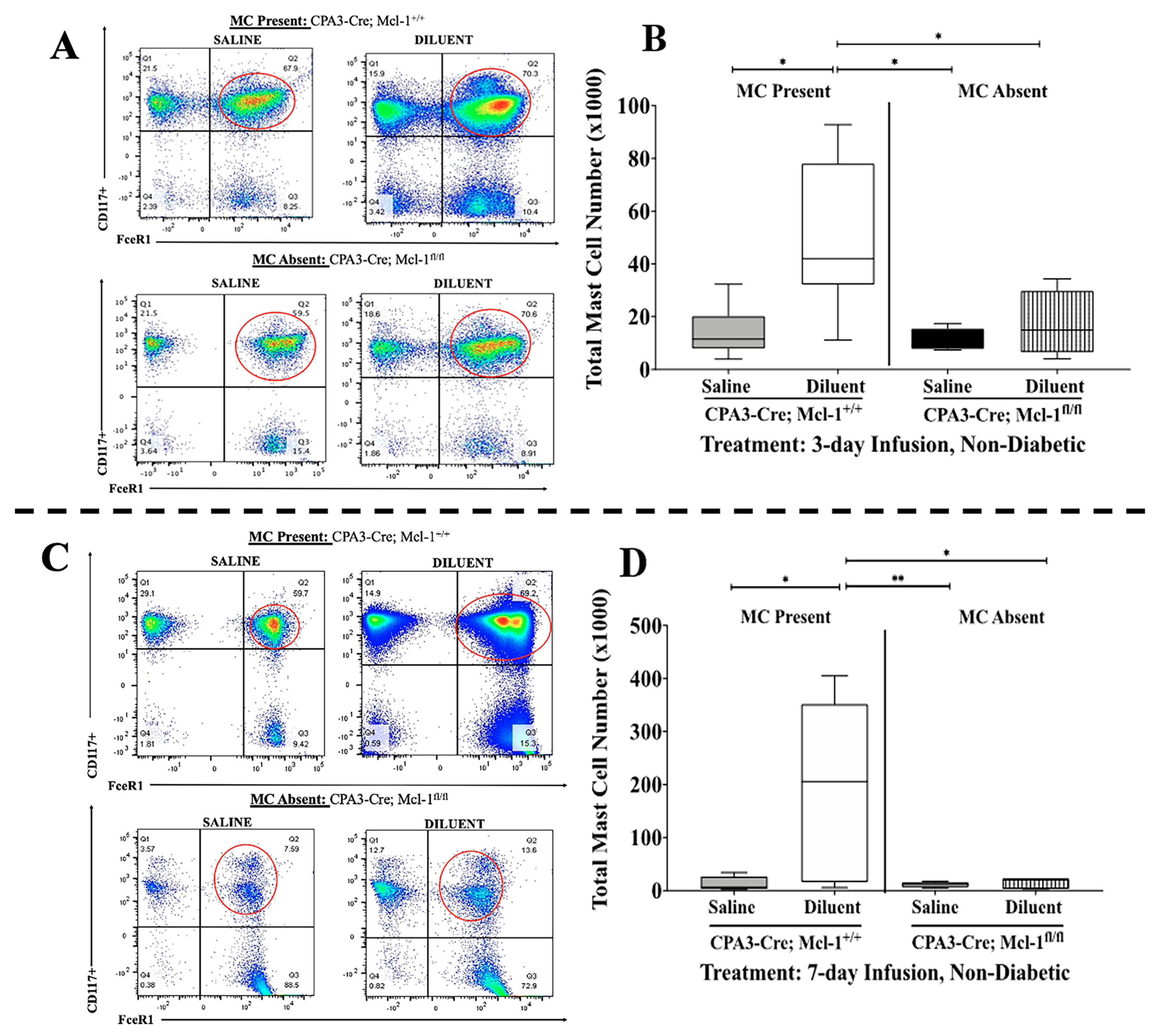
3.1. Cpa3-Cre; Mcl-1fl/fl Mice Exhibit a Marked Reduction in MCs in the Air Pouch Lavage
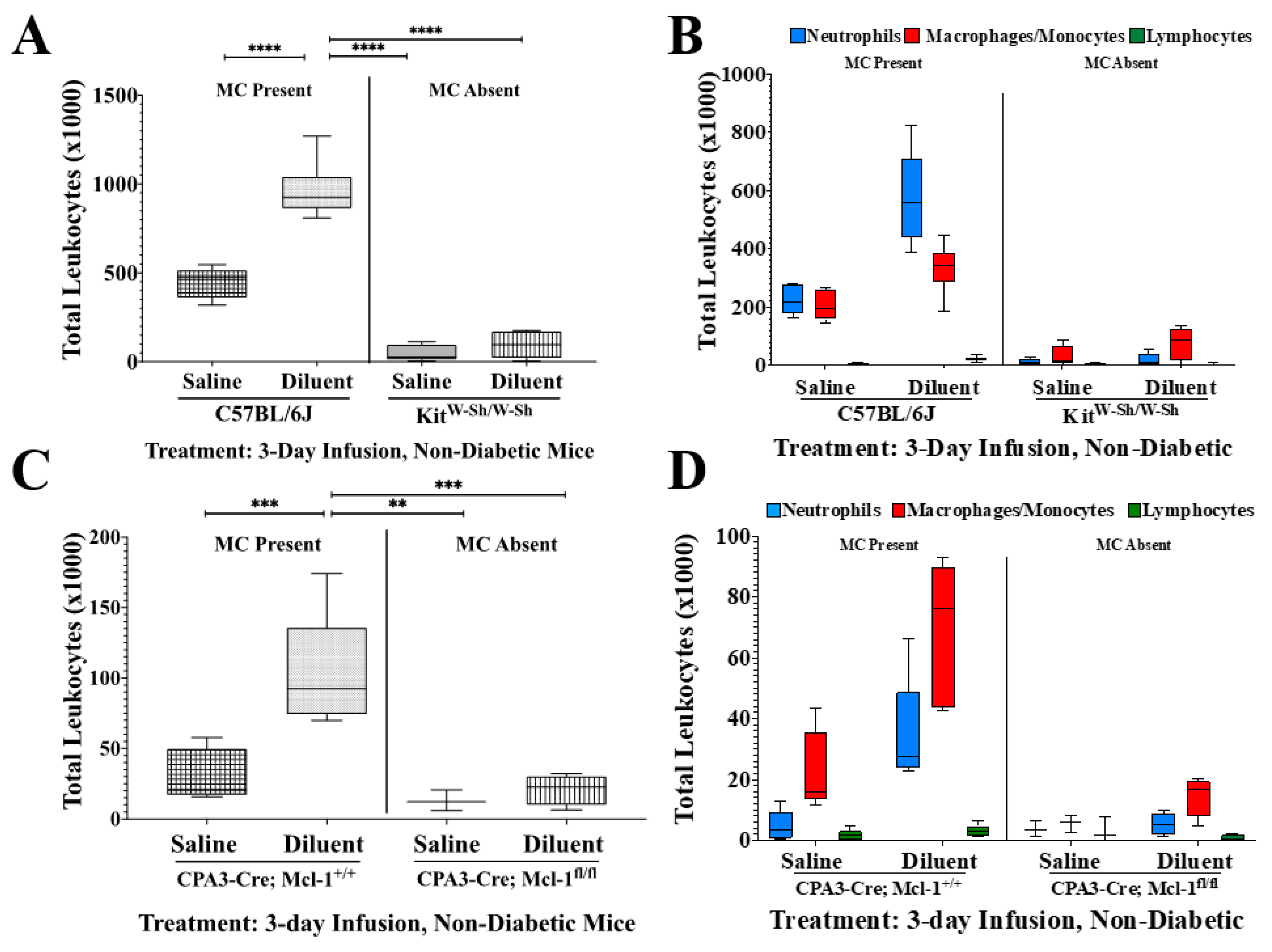
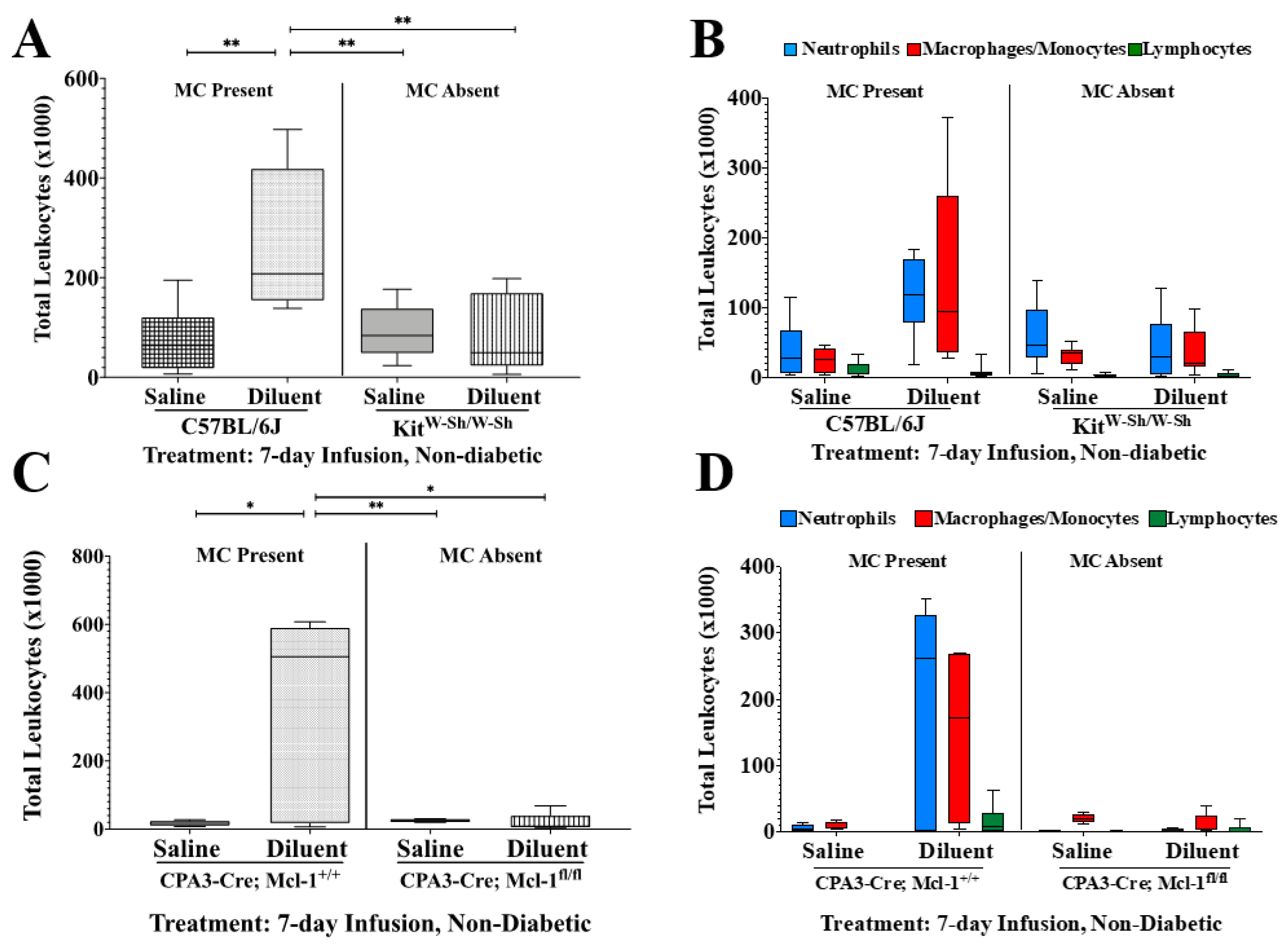
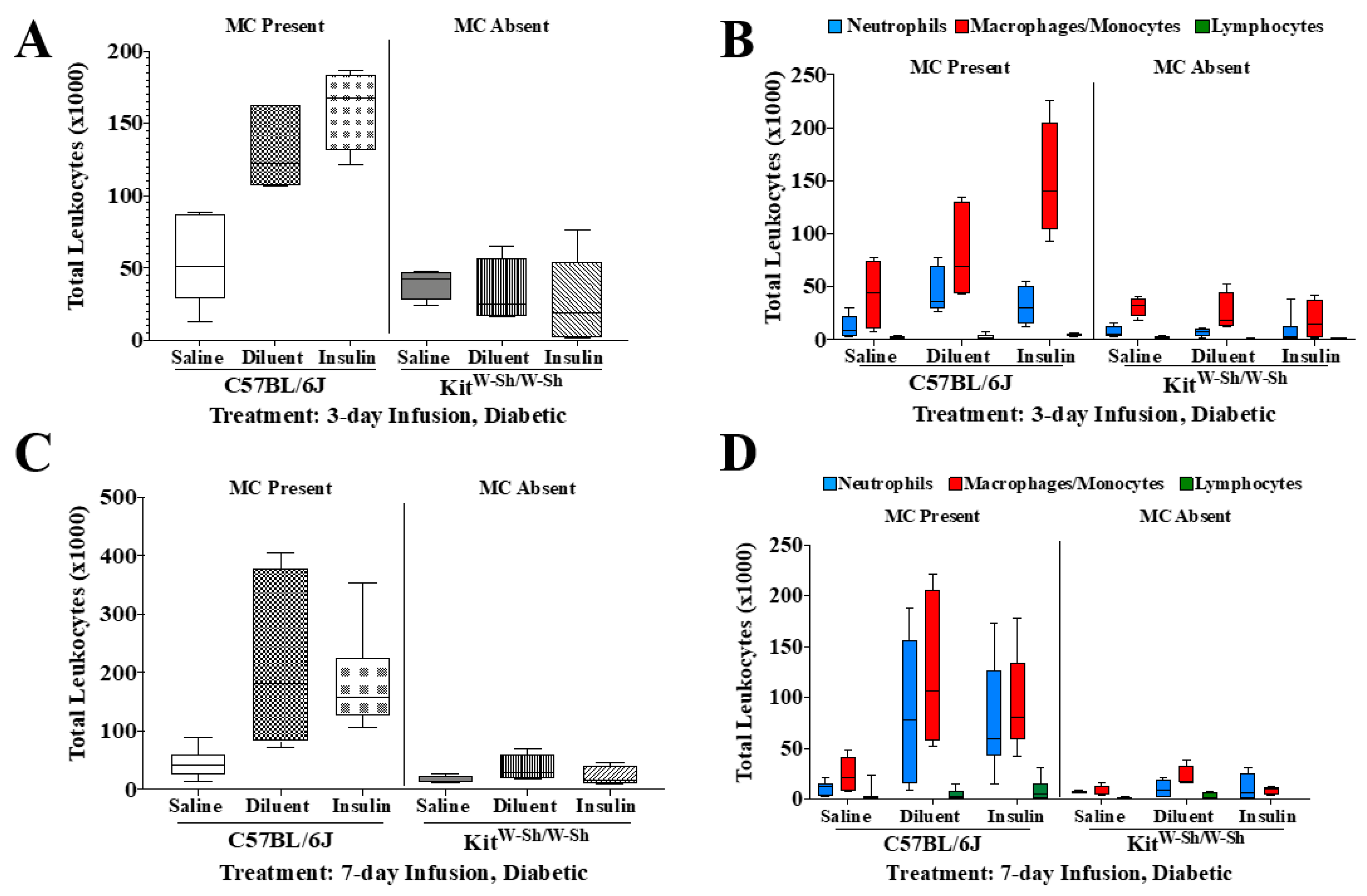
3.2. MC-Deficient Mice Exhibit Evidently Reduced Leukocyte Recruitment in the Air Pouch
3.3. Total Protein Analysis of Lavage Fluid following Saline and Diluent Infusion
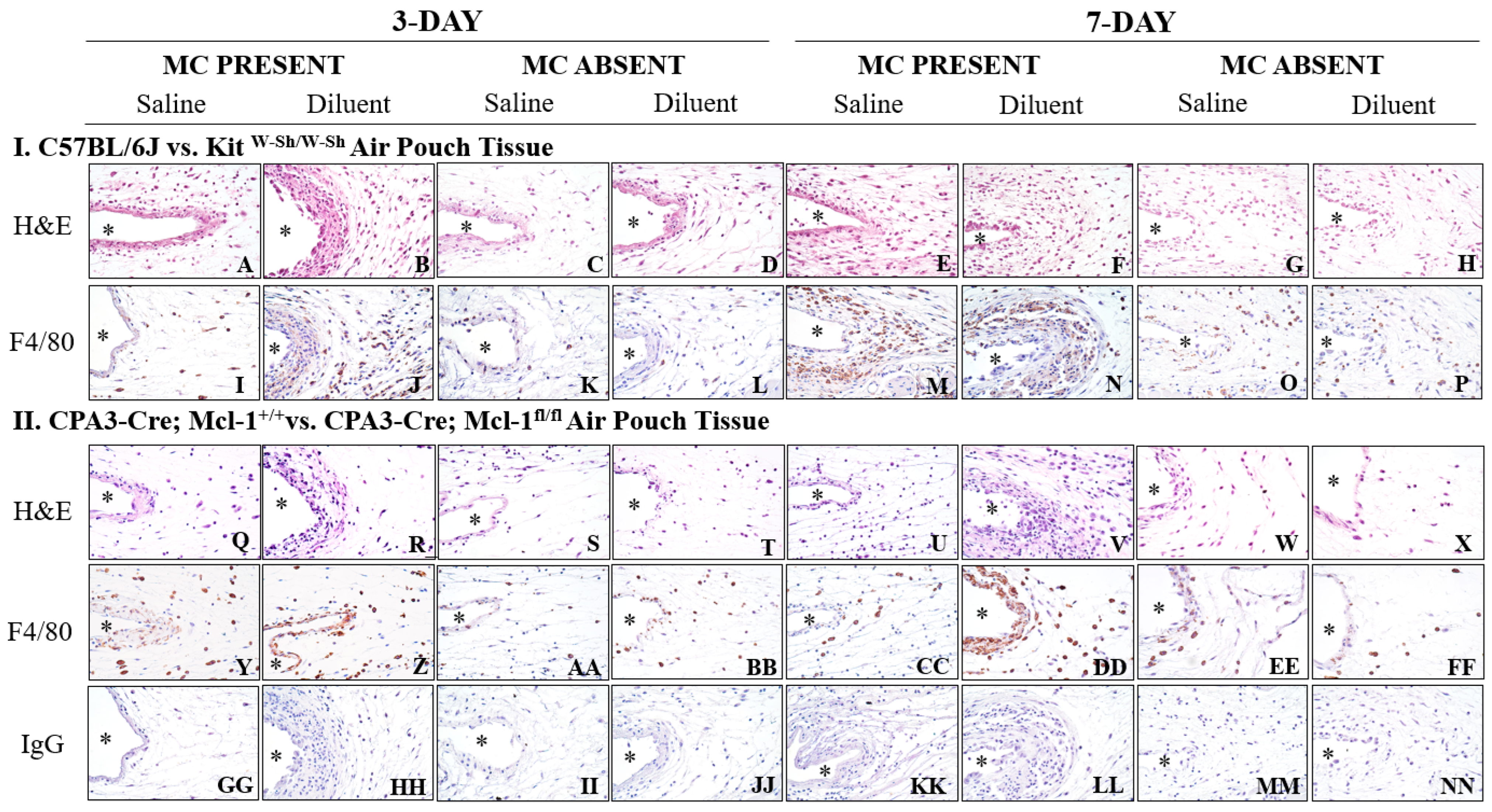
3.4. KitW-Sh and Cpa3-Cre; Mcl-1fl/fl MC-Deficient Mice Exhibit Reduced Tissue Reactions following Phenolic Preservative Infusion
3.5. Insulin Infusion into Air Pouch Does Not Augment Leukocyte Influx
3.6. Insulin Infusion Does Not Intensify Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forlenza, G.P.; Lal, R.A. Current Status and Emerging Options for Automated Insulin Delivery Systems. Diabetes Technol. Ther. 2022, 24, 362–371. [Google Scholar]
- Hojbjerre, L.; Skov-Jensen, C.; Kaastrup, P.; Pedersen, P.E.; Stallknecht, B. Effect of steel and teflon infusion catheters on subcutaneous adipose tissue blood flow and infusion counter pressure in humans. Diabetes Technol. Ther. 2009, 11, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Benasi, K.; Ferrari, G.; Evans, M.G.; Shanmugham, S.; Wilson, D.M.; Buckingham, B.A. Randomized trial of infusion set function: Steel versus teflon. Diabetes Technol. Ther. 2014, 16, 15–19. [Google Scholar] [PubMed] [Green Version]
- Hauzenberger, J.R.; Munzker, J.; Kotzbeck, P.; Asslaber, M.; Bubalo, V.; Joseph, J.I.; Pieber, T.R. Systematic in vivo evaluation of the time-dependent inflammatory response to steel and Teflon insulin infusion catheters. Sci. Rep. 2018, 8, 1132. [Google Scholar]
- Teska, B.M.; Alarcon, J.; Pettis, R.J.; Randolph, T.W.; Carpenter, J.F. Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J. Pharm. Sci. 2014, 103, 2255–2267. [Google Scholar]
- Lewis, B.E.; Mulka, A.; Mao, L.; Sharafieh, R.; Qiao, Y.; Kesserwan, S.; Wu, R.; Kreutzer, D.; Klueh, U. Insulin Derived Fibrils Induce Cytotoxicity in vitro and Trigger Inflammation in Murine Models. J. Diabetes Sci. Technol. 2021, 17, 19322968211033868. [Google Scholar]
- Kesserwan, S.; Mulka, A.; Sharafieh, R.; Qiao, Y.; Wu, R.; Kreutzer, D.L.; Klueh, U. Advancing continuous subcutaneous insulin infusion in vivo: New insights into tissue challenges. J. Biomed. Mater. Res. A 2021, 109, 1065–1079. [Google Scholar] [PubMed]
- Mulka, A.; Lewis, B.E.; Mao, L.; Sharafieh, R.; Kesserwan, S.; Wu, R.; Kreutzer, D.L.; Klueh, U. Phenolic Preservative Removal from Commercial Insulin Formulations Reduces Tissue Inflammation while Maintaining Euglycemia. ACS Pharmacol. Transl. Sci. 2021, 4, 1161–1174. [Google Scholar]
- Weber, C.; Kammerer, D.; Streit, B.; Licht, A.H. Phenolic excipients of insulin formulations induce cell death, pro-inflammatory signaling and MCP-1 release. Toxicol. Rep. 2015, 2, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kesserwan, S.; Lewis, B.E.; Mao, L.; Sharafieh, R.; Atwood, T.; Kreutzer, D.L.; Klueh, U. Inflammation at Site of Insulin Infusion Diminishes Glycemic Control. J. Pharm. Sci. 2022, 111, 1952–1961. [Google Scholar] [CrossRef]
- Kesserwan, S.; Mao, L.; Sharafieh, R.; Kreutzer, D.L.; Klueh, U. A pharmacological approach assessing the role of mast cells in insulin infusion site inflammation. Drug Deliv. Transl. Res. 2022, 12, 1711–1718. [Google Scholar] [CrossRef]
- Arumugam, T.; Ramachandran, V.; Logsdon, C.D. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J. Natl. Cancer Inst. 2006, 98, 1806–1818. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Marichal, T.; Tchougounova, E.; Reber, L.L.; Pejler, G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv. Immunol. 2015, 126, 45–127. [Google Scholar]
- Wilgus, T.A.; Ud-Din, S.; Bayat, A. A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. Int. J. Mol. Sci. 2020, 21, 9673. [Google Scholar] [CrossRef]
- Otsuka, A.; Nonomura, Y.; Kabashima, K. Roles of basophils and mast cells in cutaneous inflammation. Semin. Immunopathol. 2016, 38, 563–570. [Google Scholar] [CrossRef]
- Benyon, R.C. The human skin mast cell. Clin. Exp. Allergy 1989, 19, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Chen, C.C.; Piliponsky, A.M.; Tsai, M.; Tam, S.Y.; Galli, S.J. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 2005, 167, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Reber, L.L.; Marichal, T.; Galli, S.J. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, D.L.; Kozak, C.A.; Mano, H.; Chapman, V.M.; Bucan, M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum. Mol. Genet. 1995, 4, 2073–2079. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The ingenious mast cell: Contemporary insights into mast cell behavior and function. Allergy 2022, 77, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Nigrovic, P.A.; Gray, D.H.; Jones, T.; Hallgren, J.; Kuo, F.C.; Chaletzky, B.; Gurish, M.; Mathis, D.; Benoist, C.; Lee, D.M. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am. J. Pathol. 2008, 173, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Piliponsky, A.M.; Chen, C.C.; Grimbaldeston, M.A.; Burns-Guydish, S.M.; Hardy, J.; Kalesnikoff, J.; Contag, C.H.; Tsai, M.; Galli, S.J. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 2010, 176, 926–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.K.; Huan, Y. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2008, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Lilla, J.N.; Chen, C.C.; Mukai, K.; BenBarak, M.J.; Franco, C.B.; Kalesnikoff, J.; Yu, M.; Tsai, M.; Piliponsky, A.M.; Galli, S.J. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 2011, 118, 6930–6938. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. The Staining of Mast Cells: A Historical Overview. Int. Arch. Allergy Immunol. 2018, 176, 55–60. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Tellechea, A.; Leal, E.C.; Kafanas, A.; Auster, M.E.; Kuchibhotla, S.; Ostrovsky, Y.; Tecilazich, F.; Baltzis, D.; Zheng, Y.; Carvalho, E.; et al. Mast Cells Regulate Wound Healing in Diabetes. Diabetes 2016, 65, 2006–2019. [Google Scholar] [CrossRef] [Green Version]
- Klueh, U.; Kaur, M.; Qiao, Y.; Kreutzer, D.L. Critical role of tissue mast cells in controlling long-term glucose sensor function in vivo. Biomaterials 2010, 31, 4540–4551. [Google Scholar] [CrossRef] [Green Version]
- Orenstein, S.B.; Saberski, E.R.; Klueh, U.; Kreutzer, D.L.; Novitsky, Y.W. Effects of mast cell modulation on early host response to implanted synthetic meshes. Hernia J. Hernias Abdom. Wall Surg. 2010, 14, 511–516. [Google Scholar] [CrossRef]
- Maurer, M.; Taube, C.; Schröder, N.W.J.; Ebmeyer, J.; Siebenhaar, F.; Geldmacher, A.; Schubert, N.; Roers, A. Mast cells drive IgE-mediated disease but might be bystanders in many other inflammatory and neoplastic conditions. J. Allergy Clin. Immunol. 2019, 144, S19–S30. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Meyer, N.; Jiao, Q.; Scheffel, J.; Zimmermann, C.; Metz, M.; Zenclussen, A.; Maurer, M.; Siebenhaar, F. Chymase-Cre; Mcl-1(fl/fl) Mice Exhibit Reduced Numbers of Mucosal Mast Cells. Front. Immunol. 2019, 10, 2399. [Google Scholar] [CrossRef] [Green Version]
- Peschke, K.; Dudeck, A.; Rabenhorst, A.; Hartmann, K.; Roers, A. Cre/loxP-based mouse models of mast cell deficiency and mast cell-specific gene inactivation. Methods Mol. Biol. 2015, 1220, 403–421. [Google Scholar] [PubMed]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, J.P.; Stylianou, M.; Backman, E.; Holmberg, S.; Ekoff, M.; Nilsson, G.; Urban, C.F. Cryptococcus neoformans Induces MCP-1 Release and Delays the Death of Human Mast Cells. Front. Cell Infect. Microbiol. 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.R.; Yu, J.-J.; Navara, C.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Contribution of FcɛRI-associated vesicles to mast cell–macrophage communication following Francisella tularensis infection. Innate Immun. 2016, 22, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.; Soleimani, H.; Rezaei, F.; Ghoreishi, S.K.; Chien, S.; Bayat, M. The Combined Effect of Photobiomodulation and Curcumin on Acute Skin Wound Healing in Rats. J. Lasers Med. Sci. 2021, 12, e9. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.C.; Allannic, H.; Genetet, B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Kumar, M.; Roe, K.; Nerurkar, P.V.; Orillo, B.; Thompson, K.S.; Verma, S.; Nerurkar, V.R. Reduced immune cell infiltration and increased pro-inflammatory mediators in the brain of Type 2 diabetic mouse model infected with West Nile virus. J. Neuroinflammat. 2014, 11, 80. [Google Scholar] [CrossRef] [Green Version]

| Tukey’s Multiple Comparisons Test | Total Cells | PMNs | MQ/Mo | LYMPH | |||||
|---|---|---|---|---|---|---|---|---|---|
| Summary | Summary | Summary | Summary | ||||||
| Group 1 | Group 2 | 3-Day | 7-Day | 3-Day | 7-Day | 3-Day | 7-Day | 3-Day | 7-Day |
| Saline C57BL/6J | Saline Kit W-Sh/W-Sh | **** | ns | * | ns | ** | ns | ns | ns |
| Saline C57BL6 | Diluent C57BL6 | **** | ** | **** | * | * | ns | *** | ns |
| Saline C57BL6 | Diluent Kit W-Sh/W-Sh | *** | ns | * | ns | * | ns | ns | ns |
| Saline Kit W-Sh/W-Sh | Diluent C57BL6 | **** | ** | **** | ns | **** | * | **** | ns |
| Saline Kit W-Sh/W-Sh | Diluent Kit W-Sh/W-Sh | ns | ns | ns | ns | ns | ns | ns | ns |
| Diluent C57BL6 | Diluent Kit W-Sh/W-Sh | **** | ** | **** | * | **** | * | **** | ns |
| Tukey’s Multiple Comparisons Test | Total Cells | PMNs | MQ/Mo | LYMPH | MC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Summary | Summary | Summary | Summary | Summary | |||||||
| Group 1 | Group 2 | 3-Day | 7-Day | 3-Day | 7-Day | 3-Day | 7-Day | 3-Day | 7-Day | 3-Day | 7-Day |
| Saline CPA3-Cre; Mcl-1+/+ | Saline CPA3-Cre; Mcl-1fl/fl | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Saline CPA3-Cre; Mcl-1+/+ | Diluent CPA3-Cre; Mcl-1+/+ | *** | * | ** | * | *** | * | ns | ns | * | ns |
| Saline CPA3-Cre; Mcl-1+/+ | Diluent Cpa3-Cre; Mcl-1fl/fl | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| SalineCpa3-Cre; Mcl-1fl/fl | Diluent CPA3-Cre; Mcl-1+/+ | *** | * | ** | * | *** | ns | ns | ns | * | ns |
| Saline Cpa3-Cre; Mcl-1fl/fl | Diluent Cpa3-Cre; Mcl-1fl/fl | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Diluent CPA3-Cre; Mcl-1+/+ | Diluent Cpa3-Cre; Mcl-1fl/fl | *** | ** | ** | ** | *** | * | ns | ns | * | * |
| Tukey’s Multiple Comparisons Test | Total Cells | PMNs | MQ/Mo | LYMPH | |
|---|---|---|---|---|---|
| Group 1 | Group 2 | Summary | Summary | Summary | Summary |
| Saline C57BL/6J | Saline Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline C57BL/6J | Diluent C57BL/6J | * | * | ns | ns |
| Saline C57BL/6J | Diluent Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline C57BL/6J | Insulin C57BL/6J | *** | ns | ** | ns |
| Saline C57BL/6J | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline Kit W-Sh/W-Sh | Diluent C57BL/6J | * | ** | ns | ns |
| Saline Kit W-Sh/W-Sh | Diluent Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline Kit W-Sh/W-Sh | Insulin C57BL/6J | *** | ns | *** | ns |
| Saline Kit W-Sh/W-Sh | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Diluent C57BL/6J | Diluent Kit W-Sh/W-Sh | ** | ** | ns | ns |
| Diluent C57BL/6J | Insulin C57BL/6J | ns | ns | ns | ns |
| Diluent C57BL/6J | Insulin Kit W-Sh/W-Sh | ** | ** | * | ns |
| Diluent Kit W-Sh/W-Sh | Insulin C57BL/6J | **** | ns | *** | * |
| Diluent Kit W-Sh/W-Sh | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Insulin C57BL/6J | Insulin Kit W-Sh/W-Sh | **** | ns | **** | * |
| Tukey’s Multiple Comparisons Test | Total Cells | PMNs | MQ/Mo | LYMPH | |
|---|---|---|---|---|---|
| Group 1 | Group 2 | Summary | Summary | Summary | Summary |
| Saline C57BL/6J | Saline Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline C57BL/6J | Diluent C57BL/6J | ** | * | ** | ns |
| Saline C57BL/6J | Diluent Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline C57BL/6J | Insulin C57BL/6J | * | ns | * | ns |
| Saline C57BL/6J | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline Kit W-Sh/W-Sh | Diluent C57BL/6J | ** | ns | ** | ns |
| Saline Kit W-Sh/W-Sh | Diluent Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Saline Kit W-Sh/W-Sh | Insulin C57BL/6J | * | ns | * | ns |
| Saline Kit W-Sh/W-Sh | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Diluent C57BL/6J | Diluent Kit W-Sh/W-Sh | * | ns | ** | ns |
| Diluent C57BL/6J | Insulin C57BL/6J | ns | ns | ns | ns |
| Diluent C57BL/6J | Insulin Kit W-Sh/W-Sh | * | * | ** | ns |
| Diluent Kit W-Sh/W-Sh | Insulin C57BL/6J | ns | ns | ns | ns |
| Diluent Kit W-Sh/W-Sh | Insulin Kit W-Sh/W-Sh | ns | ns | ns | ns |
| Insulin C57BL/6J | Insulin Kit W-Sh/W-Sh | * | ns | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kesserwan, S.; Sadagurski, M.; Mao, L.; Klueh, U. Mast Cell Deficiency in Mice Attenuates Insulin Phenolic Preservative-Induced Inflammation. Biomedicines 2023, 11, 2258. https://doi.org/10.3390/biomedicines11082258
Kesserwan S, Sadagurski M, Mao L, Klueh U. Mast Cell Deficiency in Mice Attenuates Insulin Phenolic Preservative-Induced Inflammation. Biomedicines. 2023; 11(8):2258. https://doi.org/10.3390/biomedicines11082258
Chicago/Turabian StyleKesserwan, Shereen, Marianna Sadagurski, Li Mao, and Ulrike Klueh. 2023. "Mast Cell Deficiency in Mice Attenuates Insulin Phenolic Preservative-Induced Inflammation" Biomedicines 11, no. 8: 2258. https://doi.org/10.3390/biomedicines11082258
APA StyleKesserwan, S., Sadagurski, M., Mao, L., & Klueh, U. (2023). Mast Cell Deficiency in Mice Attenuates Insulin Phenolic Preservative-Induced Inflammation. Biomedicines, 11(8), 2258. https://doi.org/10.3390/biomedicines11082258







