Optimizing TMS Coil Placement Approaches for Targeting the Dorsolateral Prefrontal Cortex in Depressed Adolescents: An Electric Field Modeling Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Motor Hotspot and Resting Motor Threshold Determination
2.3. Imaging
2.4. Treatment Intervention
2.5. E-Field Modeling
2.5.1. E-Field Computation and Head Model Generation
2.5.2. Structural Mask Definition
2.5.3. Coil Placement and Optimization
2.5.4. Estimation of for the Standard Motor Threshold
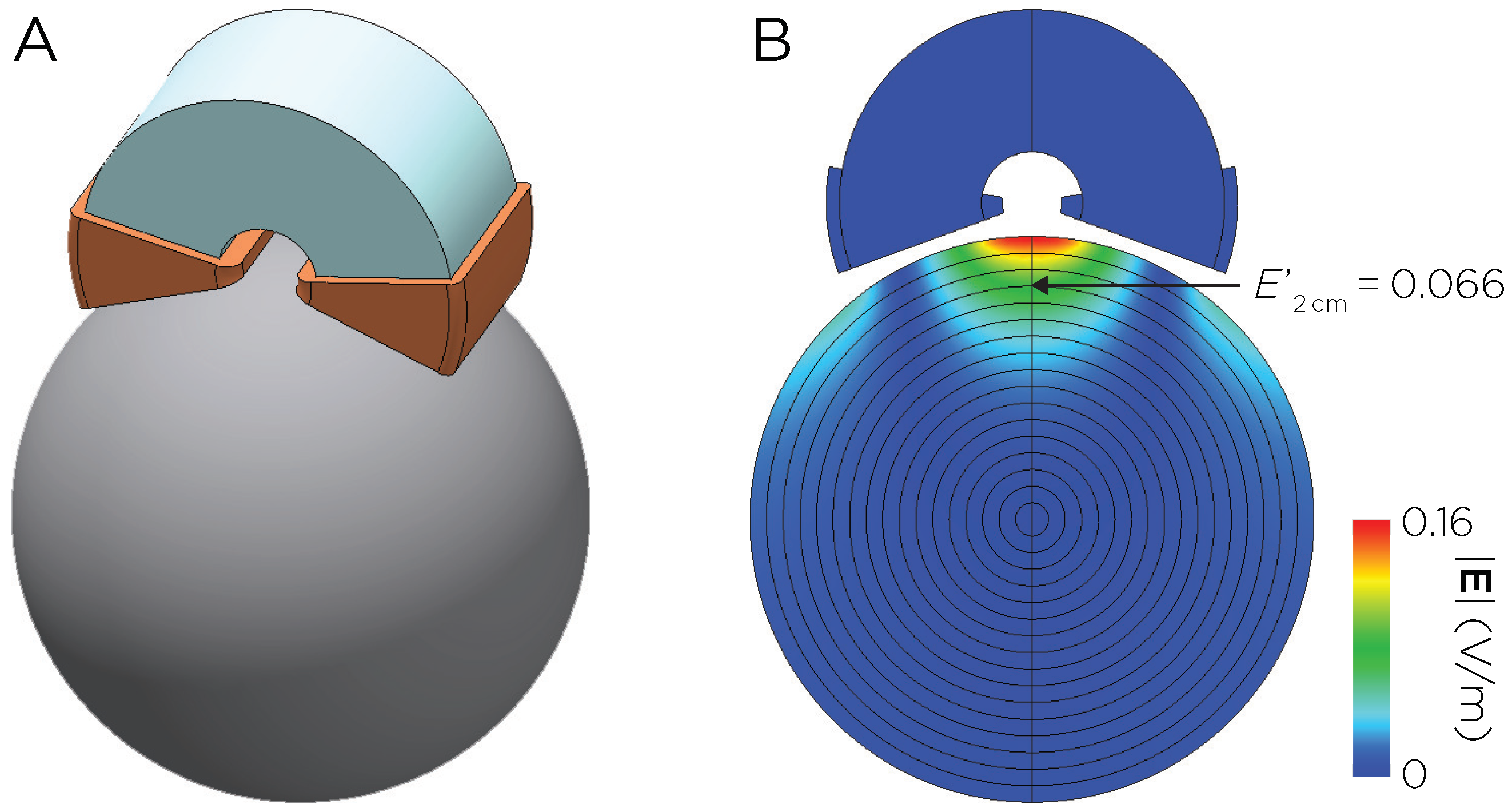
2.5.5. E-Field Postprocessing
3. Results
3.1. Dose–Response Relationship between E-Field and Clinical Outcome
3.2. Comparison between Alternative Coil Placements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health; HHS Publication No. PEP21-07-01-003; HHS: Washington, DC, USA, 2020.
- Donaldson, A.E.; Gordon, M.S.; Melvin, G.A.; Barton, D.A.; Fitzgerald, P.B. Addressing the needs of adolescents with treatment resistant depressive disorders: A systematic review of rTMS. Brain Stimul. 2014, 7, 7–12. [Google Scholar] [CrossRef]
- Krishnan, C.; Santos, L.; Peterson, M.D.; Ehinger, M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015, 8, 76–87. [Google Scholar] [CrossRef]
- Magavi, L.R.; Reti, I.M.; Vasa, R.A. A review of repetitive transcranial magnetic stimulation for adolescents with treatment-resistant depression. Int. Rev. Psychiatry 2017, 29, 79–88. [Google Scholar] [CrossRef]
- Croarkin, P.E.; Nakonezny, P.A.; Deng, Z.D.; Romanowicz, M.; Vande Voort, J.L.; Doruk Camsari, D.; Schak, K.M.; Port, J.D.; Lewis, C.P. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J. Affect. Disord. 2018, 239, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Hett, D.; Rogers, J.; Humpston, C.; Marwaha, S. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in adolescence: A systematic review. J. Affect. Disord. 2021, 278, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, R.; Luo, X.; Zhang, S.; Zhong, X.; Ning, Y.; Zhang, B. Repetitive transcranial magnetic stimulation target location methods for depression. Front. Neurosci. 2021, 15, 695423. [Google Scholar] [CrossRef]
- Croarkin, P.E.; Nakonezny, P.A.; Wall, C.A.; Murphy, L.L.; Sampson, S.M.; Frye, M.A.; Port, J.D. Transcranial magnetic stimulation potentiates glutamatergic neurotransmission in depressed adolescents. Psychiatry Res. Neuroimaging 2016, 247, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.A.; Croarkin, P.E.; Maroney-Smith, M.J.; Haugen, L.M.; Baruth, J.M.; Frye, M.A.; Sampson, S.M.; Port, J.D. Magnetic resonance imaging-guided, open-label, high-frequency repetitive transcranial magnetic stimulation for adolescents with major depressive disorder. J. Child Adolesc. Psychopharmacol. 2016, 26, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.A.; Croarkin, P.E.; Sim, L.A.; Husain, M.M.; Janicak, P.G.; Kozel, F.A.; Emslie, G.J.; Dowd, S.M.; Sampson, S.M. Adjunctive use of repetitive transcranial magnetic stimulation in depressed adolescents: A prospective, open pilot study. J. Clin. Psychiatry 2011, 72, 1263–1269. [Google Scholar] [CrossRef]
- Deng, Z.D.; Liston, C.; Gunning, F.M.; Dubin, M.J.; Fridgeirsson, E.A.; Lilien, J.; van Wingen, G.; van Waarde, J.A. Chapter Electric Field Modeling for Transcranial Magnetic Stimulation and Electroconvulsive therapy. In Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018; Springer Nature: Cham, Switzerland, 2019; pp. 75–84. [Google Scholar]
- Herwig, U.; Padberg, F.; Unger, J.; Spitzer, M.; Schönfeldt-Lecuona, C. Transcranial magnetic stimulation in therapy studies: Examination of the reliability of “standard” coil position by neuronavigation. Biol. Psychiatry 2001, 50, 58–61. [Google Scholar] [CrossRef]
- Roche, A.F.; Mukherjee, D.; Guo, S.M.; Moore, W.M. Head circumference reference data: Birth to 18 years. Pediatrics 1987, 79, 706–712. [Google Scholar] [CrossRef]
- Croarkin, P.E.; Elmaadawi, A.; Aaronson, S.T.; Schrodt, R.R., Jr.; Holbert, R.C.; Verdoliva, S.; Heart, K.L.; Demitrack, M.A.; Strawn, J.R. Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: A double-blind, randomized, sham-controlled trial. Neuropsychopharmacology 2021, 46, 462–469. [Google Scholar] [CrossRef]
- Homan, R.W.; Herman, J.; Purdy, P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Beam, W.; Borchardt, J.J.; Reeves, S.T.; George, M.S. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Maller, J.J.; Hoy, K.E.; Thomson, R.; Daskalakis, Z.J. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009, 2, 234–237. [Google Scholar] [CrossRef]
- Mir-Moghtadaei, A.; Caballero, R.; Fried, P.; Fox, M.D.; Lee, K.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul. 2015, 8, 965–973. [Google Scholar] [CrossRef]
- Gomez, L.J.; Dannhauer, M.; Peterchev, A.V. Fast computational optimization of TMS coil placement for individualized electric field targeting. Neuroimage 2021, 228, 117696. [Google Scholar] [CrossRef] [PubMed]
- Dannhauer, M.; Huang, Z.; Beynel, L.; Wood, E.; Bukhari-Parlakturk, N.; Peterchev, A.V. TAP: Targeting and analysis pipeline for optimization and verification of coil placement in transcranial magnetic stimulation. J. Neural Eng. 2022, 19, 026050. [Google Scholar] [CrossRef]
- Kaufman, J.; Birmaher, B.; Brent, D.; Rao, U.; Flynn, C.; Moreci, P.; Williamson, D.; Ryan, N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 980–988. [Google Scholar] [CrossRef]
- Poznanski, E.O.; Grossman, J.A.; Buchsbaum, Y.; Banegas, M.; Freeman, L.; Gibbons, R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J. Am. Acad. Child Psychiatry 1984, 23, 191–197. [Google Scholar] [CrossRef]
- Sackeim, H.A. The definition and meaning of treatment-resistant depression. J. Clin. Psychiatry 2001, 62, 10–17. [Google Scholar] [PubMed]
- Plonsey, R.; Heppner, D.B. Considerations of quasi-stationarity in electrophysiological systems. Bull. Math. Biophys. 1967, 29, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 222–225. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gomez, L.J.; Dannhauer, M.; Koponen, L.M.; Peterchev, A.V. Conditions for numerically accurate TMS electric field simulation. Brain Stimul. 2020, 13, 157–166. [Google Scholar] [CrossRef]
- Opitz, A.; Legon, W.; Rowlands, A.; Bickel, W.K.; Paulus, W.; Tyler, W.J. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 2013, 81, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakim, R.; Fallon, J.; Nain, D.; Melonakos, J.; Tannenbaum, A. A dorsolateral prefrontal cortex semi-automatic segmenter. In Proceedings of the SPIE, San Diego, CA, USA, 11–16 February 2006; Volume 6144, pp. 170–177. [Google Scholar] [CrossRef]
- Seibt, O.; Brunoni, A.R.; Huang, Y.; Bikson, M. The pursuit of DLPFC: Non-neuronavigated methods to target the left dorsolateral pre-frontal cortex with symmetric bicephalic transcranial direct current stimulation (tDCS). Brain Stimul. 2015, 8, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Neuronetics, Inc. NeuroStar® System Instructions for Use; 52-4US1E-030 IFU Revision H; Neuronetics, Inc.: Malvern, PA, USA, 2020; Available online: https://neurostar.com/wp-content/uploads/2021/07/52-4US1E-030-IFU-NS-3.5.pdf (accessed on 1 November 2020).
- Davey, K.R.; Riehl, M.E. Designing transcranial magnetic stimulation systems. IEEE Trans. Magn. 2005, 41, 1142–1148. [Google Scholar] [CrossRef]
- Deng, Z.D.; Lisanby, S.H.; Peterchev, A.V. Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimul. 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Zhang, B.B.B.; Stöhrmann, P.; Godbersen, G.M.; Unterholzner, J.; Kasper, S.; Kranz, G.S.; Lanzenberger, R. Normal component of TMS-induced electric field is correlated with depressive symptom relief in treatment-resistant depression. Brain Stimul. 2022, 15, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Saturnino, G.B.; Puonti, O.; Nielsen, J.D.; Antonenko, D.; Madsen, K.H.; Thielscher, A. SimNIBS 2.1: A Comprehensive Pipeline for Individualized Electric Field Modelling for Transcranial Brain Stimulation; Springer: Cham, Switzerland, 2019; Chapter 1; pp. 3–25. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Pascual-Leone, A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br. J. Psychiatry 2000, 177, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Beck, J.; Schuepbach, D.; Hell, D.; Boesiger, P.; Bermpohl, F.; Niehaus, L.; Boeker, H.; Northoff, G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol. Psychiatry 2008, 63, 369–376. [Google Scholar] [CrossRef]
- Kimbrell, T.A.; Little, J.T.; Dunn, R.T.; Frye, M.A.; Greenberg, B.D.; Wassermann, E.M.; Repella, J.D.; Danielson, A.L.; Willis, M.W.; Benson, B.E.; et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. Biol. Psychiatry 1999, 46, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Liu, H.; Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 2013, 66, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Wu, Y.; Fitzgerald, P.B.; Zalesky, A. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Hum. Brain Mapp. 2021, 42, 4155–4172. [Google Scholar] [CrossRef]
- Neacsiu, A.D.; Luber, B.M.; Davis, S.W.; Bernhardt, E.; Strauman, T.J.; Lisanby, S.H. On the concurrent use of self-system therapy and functional magnetic resonance imaging-guided transcranial magnetic stimulation as treatment for depression. J. ECT 2018, 34, 266–273. [Google Scholar] [CrossRef]
- Iseger, T.A.; van Bueren, N.E.R.; Kenemans, J.L.; Gevirtz, R.; Arns, M. A frontal-vagal network theory for major depressive disorder: Implications for optimizing neuromodulation techniques. Brain Stimul. 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Iseger, T.A.; Padberg, F.; Kenemans, J.L.; van Dijk, H.; Arns, M. Neuro-Cardiac-Guided TMS (NCG TMS): A replication and extension study. Biol. Psychol. 2021, 162, 108097. [Google Scholar] [CrossRef]
- Downar, J.; Daskalakis, Z.J. New targets for rTMS in depression: A review of convergent evidence. Brain Stimul. 2013, 6, 231–240. [Google Scholar] [CrossRef]
- Downar, J.; Geraci, J.; Salomons, T.V.; Dunlop, K.; Wheeler, S.; McAndrews, M.P.; Bakker, N.; Blumberger, D.M.; Dasklakis, Z.J.; Kennedy, S.H.; et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol. Psychiatry 2014, 76, 176–185. [Google Scholar] [CrossRef]
- Bakker, N.; Shahab, S.; Giacobbe, P.; Blumberger, D.M.; Daskalakis, Z.J.; Kennedy, S.H.; Downar, J. rTMS of the dorsomedial prefrontal cortex for major depression: Safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul. 2015, 8, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, K.; Sheen, J.; Schulze, L.; Fettes, P.; Mansouri, F.; Feffer, K.; Blumberger, D.M.; Daskalakis, Z.J.; Kennedy, S.H.; Giacobbe, P.; et al. Dorsomedial prefrontal cortex repetitive transcranial magnetic stimulation for treatment-refractory major depressive disorder: A three-arm, blinded, randomized controlled trial. Brain Stimul. 2020, 13, 337–340. [Google Scholar] [CrossRef]
- Feffer, K.; Fette, P.; Giacobbe, P.; Daskalakis, Z.J.; Blumberger, D.M.; Downar, J. 1Hz rTMS of the right orbitofrontal cortex for major depression: Safety, tolerability and clinical outcomes. Eur. Neuropsychopharmacol. 2018, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Trapp, N.T.; Bruss, J.; Johnson, M.K.; Uitermarkt, B.D.; Garrett, L.; Heinzerling, A.; Wu, C.; Koscik, T.R.; Ten Eyck, P.; Boes, A.D. Reliability of targeting methods in TMS for depression: Beam F3 vs. 5.5 cm. Brain Stimul. 2020, 13, 578–581. [Google Scholar] [CrossRef]
- Johnson, K.A.; Baig, M.; Ramsey, D.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Li, X.; Bernhardt, E.R.; Haynor, D.R.; Holtzheimer, P.E., 3rd; et al. Prefrontal rTMS for treating depression: Location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013, 6, 108–117. [Google Scholar] [CrossRef]
- Westin, G.G.; Bassi, B.D.; Lisanby, S.H.; Luber, B. Determination of motor threshold using visual observation overestimates transcranial magnetic stimulation dosage: Safety implications. Clin. Neurophysiol. 2014, 125, 142–147. [Google Scholar] [CrossRef]
- Young, I.M.; Osipowicz, K.; Mackenzie, A.; Clarke, O.; Taylor, H.; Nicholas, P.; Ryan, M.; Holle, J.; Tanglay, O.; Doyen, S.; et al. Comparison of consistency between image guided and craniometric trancranial magnetic stimulation coil placement. Brain Stimul. 2022, 15, 1465–1466. [Google Scholar] [CrossRef]
- Mir-Moghtadaei, A.; Siddiqi, S.H.; Mir-Moghtadaei, K.; Blumberger, D.M.; Vila-Rodriguez, F.; Daskalakis, Z.J.; Fox, M.D.; Downar, J. Updated scalp heuristics for localizing the dorsolateral prefrontal cortex based on convergent evidence of lesion and brain stimulation studies in depression. Brain Stimul. 2022, 15, 291–295. [Google Scholar] [CrossRef]
- Trojak, B.; Meille, V.; Jonval, L.; Schuffenecker, N.; Haffen, E.; Schwan, R.; Bonin, B.; Chauvet-Gelinier, J.C. Interest of targeting either cortical area Brodmann 9 or 46 in rTMS treatment for depression: A preliminary randomized study. Clin. Neurophysiol. 2014, 125, 2384–2389. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Hoy, K.; McQueen, S.; Maller, J.J.; Herring, S.; Segrave, R.; Bailey, M.; Been, G.; Kulkarni, J.; Daskalakis, Z.J. A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 2009, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.; Horn, A.; Caballero, R.; Cooke, D.; Stern, A.P.; Taylor, S.F.; Press, D.; Pascual-Leone, A.; Fox, M.D. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 2018, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Shen, Z.; Jiao, J.; Chen, J.; Li, S.; Lu, J.; Duan, J.; Wei, N.; Shang, D.; Hu, S.; et al. Neuronavigation-guided rTMS for the treatment of depressive patients with suicidal ideation: A double-blind, randomized, sham-controlled trial. Clin. Pharmacol. Ther. 2020, 108, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Z.; Zhao, Y.; Xiao, X.; Zhang, W.; Sun, P.; Yang, Y.; Zhu, C. Targeting brain functions from the scalp: Transcranial brain atlas based on large-scale fMRI data synthesis. Neuroimage 2020, 210, 116550. [Google Scholar] [CrossRef] [PubMed]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Fitzgerald, P.B.; Zalesky, A. Functional magnetic resonance imaging-guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry 2021, 78, 337–399. [Google Scholar] [CrossRef]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S.; Stimpson, K.H.; Nejad, R.; Barmak, F.; Veerapal, C.; Khan, N.; Cherian, K.; Felber, E.; et al. Stanford Neuromodulation Therapy (SNT): A double-blind randomized controlled tria. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef]
- Alawi, M.; Lee, P.F.; Deng, Z.D.; Goh, Y.K.; Croarkin, P.E. Modelling the differential effects of age on transcranial magnetic stimulation induced electric fields. J. Neural Eng. 2023, 20, 026016. [Google Scholar] [CrossRef]
- Balderston, N.L.; Roberts, C.; Beydler, E.M.; Deng, Z.D.; Radman, T.; Luber, B.; Lisanby, S.H.; Ernst, M.; Grillon, C. A generalized workflow for conducting electric field-optimized, fMRI-guided, transcranial magnetic stimulation. Nat. Protoc. 2020, 15, 3595–3614. [Google Scholar] [CrossRef]
- Lynch, C.J.; Elbau, I.G.; Ng, T.H.; Wolk, D.; Zhu, S.; Ayaz, A.; Power, P.D.; Zebley, B.; Gunning, F.M.; Liston, C. Automated optimization of TMS coil placement for personalized functional network engagement. Neuron 2022, 110, 3263–3277. [Google Scholar] [CrossRef]
- Harita, S.; Momi, D.; Mazza, F.; Griffiths, J.D. Mapping inter-individual functional connectivity variability in TMS targets in major depressive disorder. Front. Psychiatry 2022, 13, 902089. [Google Scholar] [CrossRef]
- Cao, Z.; Xiao, X.; Zhao, Y.; Jiang, Y.; Xie, C.; Paillère-Martinot, M.L.; Artiges, E.; Li, Z.; Daskalakis, Z.J.; Yang, Y.; et al. Targeting the pathological network: Feasibility of network-based optimization of transcranial magnetic stimulation coil placement for treatment of psychiatric disorders. Front. Neurosci. 2023, 16, 1079078. [Google Scholar] [CrossRef] [PubMed]
- Arian, M.; Haque, M.; Johal, L.; Mathur, P.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461. [Google Scholar] [CrossRef]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial magnetic and direct current stimulation in children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.-D.; Robins, P.L.; Dannhauer, M.; Haugen, L.M.; Port, J.D.; Croarkin, P.E. Optimizing TMS Coil Placement Approaches for Targeting the Dorsolateral Prefrontal Cortex in Depressed Adolescents: An Electric Field Modeling Study. Biomedicines 2023, 11, 2320. https://doi.org/10.3390/biomedicines11082320
Deng Z-D, Robins PL, Dannhauer M, Haugen LM, Port JD, Croarkin PE. Optimizing TMS Coil Placement Approaches for Targeting the Dorsolateral Prefrontal Cortex in Depressed Adolescents: An Electric Field Modeling Study. Biomedicines. 2023; 11(8):2320. https://doi.org/10.3390/biomedicines11082320
Chicago/Turabian StyleDeng, Zhi-De, Pei L. Robins, Moritz Dannhauer, Laura M. Haugen, John D. Port, and Paul E. Croarkin. 2023. "Optimizing TMS Coil Placement Approaches for Targeting the Dorsolateral Prefrontal Cortex in Depressed Adolescents: An Electric Field Modeling Study" Biomedicines 11, no. 8: 2320. https://doi.org/10.3390/biomedicines11082320
APA StyleDeng, Z. -D., Robins, P. L., Dannhauer, M., Haugen, L. M., Port, J. D., & Croarkin, P. E. (2023). Optimizing TMS Coil Placement Approaches for Targeting the Dorsolateral Prefrontal Cortex in Depressed Adolescents: An Electric Field Modeling Study. Biomedicines, 11(8), 2320. https://doi.org/10.3390/biomedicines11082320





