Current Approaches in the Multimodal Management of Asthma in Adolescents—From Pharmacology to Personalized Therapy
Abstract
:1. Introduction
2. Epidemiology
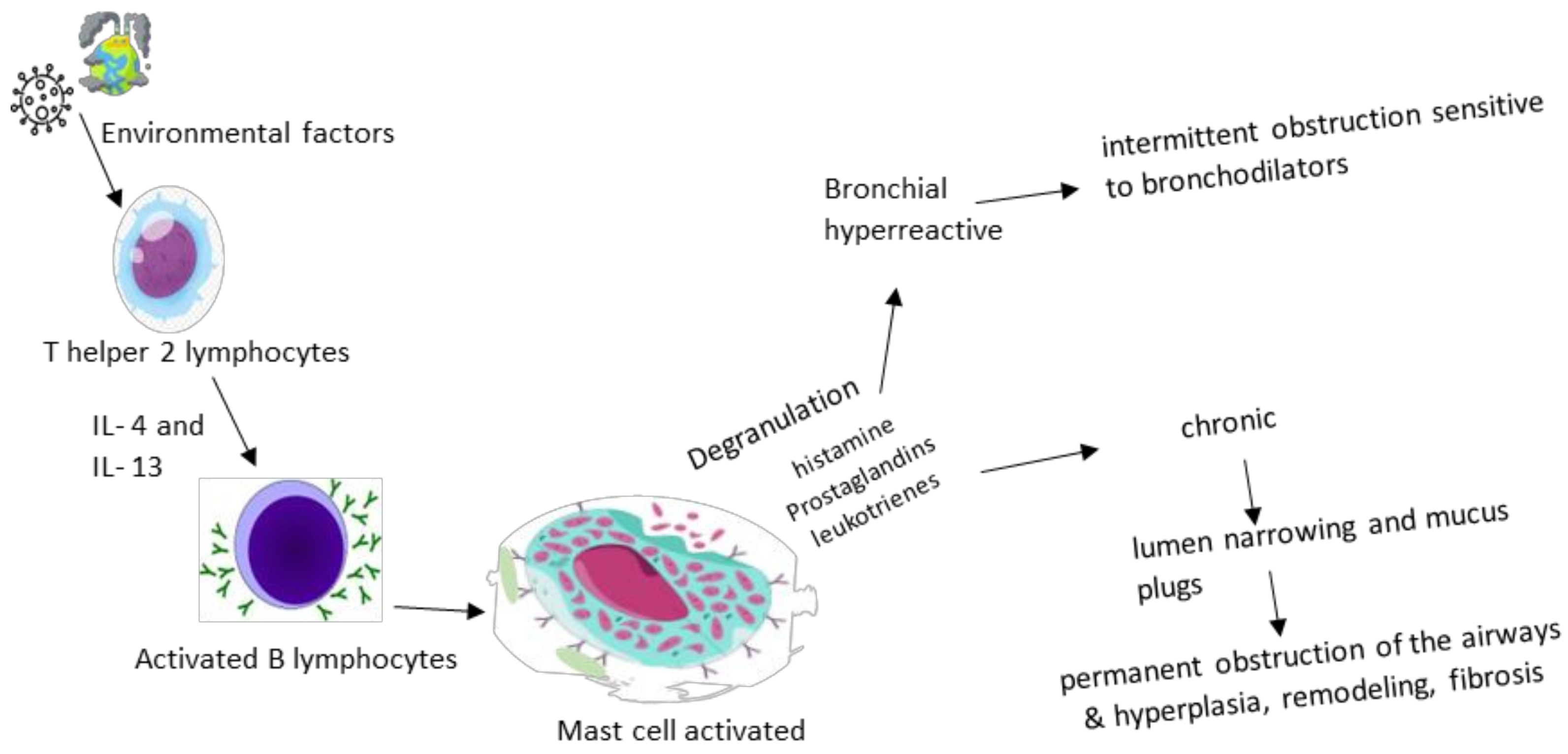
3. Pathogenesis
4. Diagnosis
5. Treatment
6. Challenges in Management
6.1. Pulmonary Function
6.2. Gender
6.3. Asthma Phenotype
6.4. Compliance with Therapy
6.5. Associated Comorbidities
6.6. The Psychological and Social Component
7. Before Closing
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Serebrisky, D.; Wiznia, A. Pediatric Asthma: A Global Epidemic. Ann. Glob. Health 2019, 85, 6. [Google Scholar]
- National Center for Environmental Health. Most Recent National Asthma Data. 2022. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 30 June 2023).
- Alruwaili, M.F.; Elwan, A. Prevalence of asthma among male 16 to 18-year-old adolescents in the Northern Borders Region of Saudi Arabia. Electron. Physician 2018, 10, 6920–6926. [Google Scholar] [CrossRef]
- Vilar de Assis, E.; Santana, M.D.R.; Feitosa, A.N.A.; Alves de Sousa, M.N.; Isidório, U.A.; Valenti, V.E.; Fonseca, F.L.A. Prevalence of Asthma symptoms and risk factors in adolescents. Journal of Human Growth and Development. J. Hum. Growth Dev. 2019, 29, 110–116. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef]
- Azmeh, R.; Greydanus, D.E.; Agana, M.G.; Dickson, C.A.; Patel, D.R.; Ischander, M.M.; Lloyd, R.D., Jr. Update in Pediatric Asthma: Selected Issues. Dis. Mon. 2020, 66, 100886. [Google Scholar] [CrossRef]
- Ullmann, N.; Mirra, V.; Di Marco, A.; Pavone, M.; Porcaro, F.; Negro, V.; Onofri, A.; Cutrera, R. Asthma: Differential Diagnosis and Comorbidities. Front. Pediatr. 2018, 6, 276. [Google Scholar] [CrossRef]
- Busse, W.W.; Kraft, M. Current unmet needs and potential solutions to uncontrolled asthma. Eur. Respir. Rev. 2022, 31, 210176. [Google Scholar]
- Breiteneder, H.; Peng, Y.Q.; Agache, I.; Diamant, Z.; Eiwegger, T.; Fokkens, W.J.; Traidl-Hoffmann, C.; Nadeau, K.; O’Hehir, R.E.; O’Mahony, L.; et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy 2020, 75, 3039–3068. [Google Scholar] [CrossRef]
- Zhang, Y.; McConnell, R.; Gilliland, F.; Berhane, K. Ethnic differences in the effect of asthma on pulmonary function in children. Am. J. Respir. Crit. Care Med. 2011, 183, 596–603. [Google Scholar] [CrossRef]
- Aaron, S.D.; Boulet, L.P.; Reddel, H.K.; Gershon, A.S. Underdiagnosis and Overdiagnosis of Asthma. Am. J. Respir. Crit. Care Med. 2018, 198, 1012–1020. [Google Scholar]
- Hallit, S.; Raherison, C.; Malaeb, D.; Hallit, R.; Waked, M.; Kheir, N.; Salameh, P. Development of an asthma risk factors scale (ARFS) for risk assessment asthma screening in children. Pediatr. Neonatol. 2019, 60, 156–165. [Google Scholar] [CrossRef]
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-summary report. J. Allergy Clin. Immunol. 2007, 120 (Suppl. S5), S94–S138. [Google Scholar] [CrossRef]
- Mims, J.W. Asthma: Definitions and pathophysiology. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. S1), S2–S6. [Google Scholar] [CrossRef]
- Landeo-Gutierrez, J.; Celedón, J.C. Chronic stress and asthma in adolescents. Ann. Allergy Asthma Immunol. 2020, 125, 393–398. [Google Scholar] [CrossRef]
- Tarrant, I.; Finlay, B.B. Like mother, like child: The maternal microbiome impacts offspring asthma. Cell Rep. Med. 2022, 3, 100722. [Google Scholar] [CrossRef]
- Sinyor, B.; Concepcion Perez, L. Pathophysiology of Asthma; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Malmström, K.; Lohi, J.; Sajantila, A.; Jahnsen, F.L.; Kajosaari, M.; Sarna, S.; Mäkelä, M.J. Immunohistology and remodeling in fatal pediatric and adolescent asthma. Respir. Res. 2017, 18, 94. [Google Scholar] [CrossRef]
- Barrios, R.J.; Kheradmand, F.; Batts, L.; Corry, D.B. Asthma: Pathology and pathophysiology. Arch. Pathol. Lab. Med. 2006, 130, 447–451. [Google Scholar] [CrossRef]
- Lemanske, R.F., Jr.; Busse, W.W. Asthma: Clinical expression and molecular mechanisms. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S95–S102. [Google Scholar] [CrossRef]
- Fireman, P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003, 24, 79–83. [Google Scholar]
- Adami, A.J.; Bracken, S.J. Breathing Better Through Bugs: Asthma and the Microbiome. Yale J. Biol. Med. 2016, 89, 309–324. [Google Scholar]
- Sullivan, A.; Hunt, E.; MacSharry, J.; Murphy, D.M. The Microbiome and the Pathophysiology of Asthma. Respir. Res. 2016, 17, 163. [Google Scholar] [CrossRef]
- Singanayagam, A.; Ritchie, A.I.; Johnston, S.L. Role of microbiome in the pathophysiology and disease course of asthma. Curr. Opin. Pulm. Med. 2017, 23, 41–47. [Google Scholar] [CrossRef]
- Lupu, A.; Jechel, E.; Mihai, C.M.; Mitrofan, E.C.; Fotea, S.; Starcea, I.M.; Ioniuc, I.; Mocanu, A.; Ghica, D.C.; Popp, A.; et al. The Footprint of Microbiome in Pediatric Asthma—A Complex Puzzle for a Balanced Development. Nutrients 2023, 15, 3278. [Google Scholar] [CrossRef]
- Gaillard, E.A.; Kuehni, C.E.; Turner, S.; Goutaki, M.; Holden, K.A.; de Jong, C.C.M.; Lex, C.; Lo, D.K.H.; Lucas, J.S.; Midulla, F.; et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur. Respir. J. 2021, 58, 2004173. [Google Scholar] [CrossRef]
- Fainardi, V.; Caffarelli, C.; Bergamini, B.M.; Biserna, L.; Bottau, P.; Corinaldesi, E.; Dondi, A.; Fornaro, M.; Guidi, B.; Lombardi, F.; et al. Management of Children with Acute Asthma Attack: A RAND/UCLA Appropriateness Approach. Int. J. Env. Res. Public. Health 2021, 18, 12775. [Google Scholar] [CrossRef]
- Kaminsky, D.A.; Chapman, D.G. Asthma and Lung Mechanics. Compr. Physiol. 2020, 10, 975–1007. [Google Scholar]
- Moral, L.; Monzo, M.A.; Benito, J.C.J.; Casanueva, C.O.; Calzon, N.M.P.; Garcia, M.I.P.; Fernandez-Oliva, C.R.R.; Ortega, J.S.; Navarrete, L.V.; Valverde-Molina, J. Pediatric Asthma: The REGAP consensus. An. Pediatr. (Engl. Ed.) 2021, 95, 125.e1–125.e11. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; de Mir, I.; Jain, N.; Sher, L.D.; Mao, X.; Liu, D.; et al. Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 2230–2240. [Google Scholar] [CrossRef]
- Haktanir Abul, M.; Phipatanakul, W. Severe asthma in children: Evaluation and management. Allergol. Int. 2019, 68, 150–157. [Google Scholar] [CrossRef]
- Just, J.; Deschildre, A.; Lejeune, S.; Amat, F. New perspectives of childhood asthma treatment with biologics. Pediatr. Allergy Immunol. 2019, 30, 159–171. [Google Scholar] [CrossRef]
- Ioniuc, I.; Miron, I.; Lupu, V.V.; Starcea, I.M.; Azoicai, A.; Alexoae, M.; Adam Raileanu, A.; Dragan, F.; Lupu, A. Challenges in the Pharmacotherapeutic Management of Pediatric Asthma. Pharmaceuticals 2022, 15, 1581. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am. J. Respir. Crit. Care Med. 2022, 205, 17–35. [Google Scholar] [CrossRef]
- Pearce, C.J.; Fleming, L. Adherence to medication in children and adolescents with asthma: Methods for monitoring and intervention. Expert. Rev. Clin. Immunol. 2018, 14, 1055–1063. [Google Scholar] [CrossRef]
- Engelkes, M.; Janssens, H.M.; de Jongste, J.C.; Sturkenboom, M.C.; Verhamme, K.M. Medication adherence and the risk of severe asthma exacerbations: A systematic review. Eur. Respir. J. 2015, 45, 396–407. [Google Scholar] [CrossRef]
- Habib, N.; Pasha, M.A.; Tang, D.D. Current Understanding of Asthma Pathogenesis and Biomarkers. Cells 2022, 11, 2764. [Google Scholar] [CrossRef]
- Guida, G.; Bagnasco, D.; Carriero, V.; Bertolini, F.; Ricciardolo, F.L.M.; Nicola, S.; Brussino, L.; Nappi, E.; Paoletti, G.; Canonica, G.W.; et al. Critical evaluation of asthma biomarkers in clinical practice. Front. Med. 2022, 9, 969243. [Google Scholar] [CrossRef]
- Price, D.; Castro, M.; Bourdin, A.; Fucile, S.; Altman, P. Short-course systemic corticosteroids in asthma: Striking the balance between efficacy and safety. Eur. Respir. Rev. 2020, 29, 190151. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hong, J.G.; Wandalsen, G.; Larenas-Linnemann, D.; El Beleidy, A.; Zaytseva, O.V.; Pedersen, S.E. Nebulized Inhaled Corticosteroids in Asthma Treatment in Children 5 Years or Younger: A Systematic Review and Global Expert Analysis. J. Allergy Clin. Immunol. Pract. 2020, 8, 1815–1827. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Gaffin, J.M.; Israel, E.; Phipatanakul, W. Asthma and Corticosteroid Responses in Childhood and Adult Asthma. Clin. Chest Med. 2019, 40, 163–177. [Google Scholar] [CrossRef]
- Daley-Yates, P.; Brealey, N.; Thomas, S.; Austin, D.; Shabbir, S.; Harrison, T.; Singh, D.; Barnes, N. Therapeutic index of inhaled corticosteroids in asthma: A dose-response comparison on airway hyperresponsiveness and adrenal axis suppression. Br. J. Clin. Pharmacol. 2021, 87, 483–493. [Google Scholar] [CrossRef]
- Schuh, S.; Sweeney, J.; Rumantir, M.; Coates, A.L.; Willan, A.R.; Stephens, D.; Atenafu, E.G.; Finkelstein, Y.; Thompson, G.; Zemek, R.; et al. Effect of Nebulized Magnesium vs. Placebo Added to Albuterol on Hospitalization Among Children With Refractory Acute Asthma Treated in the Emergency Department: A Randomized Clinical Trial. JAMA 2020, 324, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, R.; Khera, D.; Singh, S.; Toteja, N.; Choudhary, B.; Singh, K. Nebulized Magnesium Sulphate in Bronchiolitis: A Randomized Controlled Trial. Indian. J. Pediatr. 2021, 88, 1080–1085. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Vale, N. Salbutamol in the Management of Asthma: A Review. Int. J. Mol. Sci. 2022, 23, 14207. [Google Scholar] [CrossRef]
- Billington, C.K.; Penn, R.B.; Hall, I.P. β2 Agonists. Handb. Exp. Pharmacol. 2017, 237, 23–40. [Google Scholar]
- Quint, J.K.; Arnetorp, S.; Kocks, J.W.H.; Kupczyk, M.; Nuevo, J.; Plaza, V.; Cabrera, C.; Raherison-Semjen, C.; Walker, B.; Penz, E.; et al. Short-Acting Beta-2-Agonist Exposure and Severe Asthma Exacerbations: SABINA Findings from Europe and North America. J. Allergy Clin. Immunol. Pract. 2022, 10, 2297–2309.e10. [Google Scholar] [CrossRef]
- Crossingham, I.; Turner, S.; Ramakrishnan, S.; Fries, A.; Gowell, M.; Yasmin, F.; Richardson, R.; Webb, P.; O’Boyle, E.; Hinks, T.S. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst. Rev. 2021, 5, CD013518. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; FitzGerald, J.M.; Bateman, E.D.; Barnes, P.J.; Zhong, N.; Keen, C.; Jorup, C.; Lamarca, R.; Ivanov, S.; Reddel, H.K. Inhaled Combined Budesonide-Formoterol as Needed in Mild Asthma. N. Engl. J. Med. 2018, 378, 1865–1876. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Zhang, H.P.; Wang, L.; Kang, Y.; Barnes, P.J.; Wang, G. Corticosteroid plus β2-agonist in a single inhaler as reliever therapy in intermittent and mild asthma: A proof-of-concept systematic review and meta-analysis. Respir. Res. 2017, 18, 203. [Google Scholar] [CrossRef]
- Virchow, J.C.; Kuna, P.; Paggiaro, P.; Papi, A.; Singh, D.; Corre, S.; Zuccaro, F.; Vele, A.; Kots, M.; Georges, G.; et al. Single inhaler extrafine triple therapy in uncontrolled asthma (TRIMARAN and TRIGGER): Two double-blind, parallel-group, randomised, controlled phase 3 trials. Lancet 2019, 394, 1737–1749. [Google Scholar] [CrossRef]
- Soler, X.; Ramsdell, J. Anticholinergics/antimuscarinic drugs in asthma. Curr. Allergy Asthma Rep. 2014, 14, 484. [Google Scholar] [CrossRef]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Teoh, L.; Cates, C.J.; Hurwitz, M.; Acworth, J.P.; van Asperen, P.; Chang, A.B. Anticholinergic therapy for acute asthma in children. Cochrane Database Syst. Rev. 2012, 4, CD003797. [Google Scholar] [CrossRef]
- D’Amato, M.; Vitale, C.; Molino, A.; Lanza, M.; D’Amato, G. Anticholinergic drugs in asthma therapy. Curr. Opin. Pulm. Med. 2017, 23, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Dusser, D.; Ducharme, F.M. Safety of tiotropium in patients with asthma. Ther. Adv. Respir. Dis. 2019, 13, 1753466618824010. [Google Scholar] [CrossRef]
- Raissy, H.H.; Kelly, H.W. Tiotropium Bromide in Children and Adolescents with Asthma. Paediatr. Drugs 2017, 19, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Faulds, D. Montelukast. Drugs 1998, 56, 251–256; Discussion 257. [Google Scholar] [CrossRef] [PubMed]
- Özata, E.; Akelma, Z.; Günbey, S. Relationship between montelukast and behavioral problems in preschool children with asthma. Allergol. Immunopathol. 2022, 50, 85–91. [Google Scholar] [CrossRef]
- Del Giudice, M.M.; Pezzulo, A.; Capristo, C.; Alterio, E.; Caggiano, S.; de Benedictis, D.; Capristo, A.F. Leukotriene modifiers in the treatment of asthma in children. Ther. Adv. Respir. Dis. 2009, 3, 245–251. [Google Scholar] [CrossRef]
- Price, D. Tolerability of montelukast. Drugs 2000, 59 (Suppl. S1), 35–42; Discussion 43–45. [Google Scholar] [CrossRef]
- Muijsers, R.B.; Noble, S. Montelukast: A review of its therapeutic potential in asthma in children 2 to 14 years of age. Paediatr. Drugs 2002, 4, 123–139. [Google Scholar] [CrossRef]
- Pearlman, D.S.; Lampl, K.L.; Dowling, P.J., Jr.; Miller, C.J.; Bonuccelli, C.M. Effectiveness and tolerability of zafirlukast for the treatment of asthma in children. Clin. Ther. 2000, 22, 732–747. [Google Scholar] [CrossRef] [PubMed]
- Selvadurai, H.; Mellis, C. Antileukotriene drugs in childhood asthma: What is their place in therapy? Paediatr. Drugs 2000, 2, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Poeta, M.; Santamaria, F. New Drugs for Pediatric Asthma. Front. Pediatr. 2019, 6, 432. [Google Scholar] [CrossRef] [PubMed]
- Dorzin, S.E.; Halaby, C.; Quintos, M.L.; Noor, A.; El-Chaar, G. Antimicrobial Stewardship Program Using Plan-Do-Study-Act Cycles to Reduce Unjustified Antibiotic Prescribing in Children Admitted with an Asthma Exacerbation. J. Pediatr. Pharmacol. Ther. 2017, 22, 436–443. [Google Scholar] [CrossRef]
- Kesavelu, D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 20499361231154443. [Google Scholar] [CrossRef]
- Jewell, M.J.; Leyenaar, J.; Shieh, M.S.; Pekow, P.S.; Stefan, M.; Lindenauer, P.K. Unnecessary antibiotic prescribing in children hospitalised for asthma exacerbation: A retrospective national cohort study. BMJ Qual. Saf. 2021, 30, 292–299. [Google Scholar] [CrossRef]
- Baan, E.J.; Janssens, H.M.; Kerckaert, T.; Bindels, P.J.E.; de Jongste, J.C.; Sturkenboom, M.C.J.M.; Verhamme, K.M.C. Antibiotic use in children with asthma: Cohort study in UK and Dutch primary care databases. BMJ Open 2018, 8, e022979. [Google Scholar] [CrossRef]
- Williams, D.; Portnoy, J.M.; Meyerson, K. Strategies for improving asthma outcomes: A case-based review of successes and pitfalls. J. Manag. Care Pharm. 2010, 16 (Suppl. 1C), S3–S14; Quiz S16–S17. [Google Scholar] [CrossRef]
- Koefoed, H.J.L.; Vonk, J.M.; Koppelman, G.H. Predicting the course of asthma from childhood until early adulthood. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 115–122. [Google Scholar] [CrossRef]
- Koefoed, H.J.L.; Gehring, U.; Vonk, J.M.; Koppelman, G.H. Blood eosinophils associate with reduced lung function growth in adolescent asthmatics. Clin. Exp. Allergy 2021, 51, 556–563. [Google Scholar] [CrossRef]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The Gut⁻Lung Axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Mangova, M.; Lipek, T.; Vom Hove, M.; Körner, A.; Kiess, W.; Treudler, R.; Prenzel, F. Obesity-associated asthma in childhood. Allergol. Sel. 2020, 4, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Pijnenburg, M.W.; Roberts, G.; Pike, K.C.; Petsky, H.; Chang, A.B.; Szefler, S.J.; Gergen, P.; Vermeulen, F.; Vael, R.; et al. Does lung function change in the months after an asthma exacerbation in children? Pediatr. Allergy Immunol. 2021, 32, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Luisi, F.; Pinto, L.A.; Marostica, L.; Jones, M.H.; Stein, R.T.; Pitrez, P.M. Persistent pulmonary function impairment in children and adolescents with asthma. J. Bras. Pneumol. 2012, 38, 158–166, (In English and Portuguese). [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E. The impact of exercise on asthma. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 118–125. [Google Scholar] [CrossRef]
- Lochte, L.; Nielsen, K.G.; Petersen, P.E.; Platts-Mills, T.A. Childhood asthma and physical activity: A systematic review with meta-analysis and Graphic Appraisal Tool for Epidemiology assessment. BMC Pediatr. 2016, 16, 50. [Google Scholar] [CrossRef]
- Weiler, J.M.; Hallstrand, T.S.; Parsons, J.P.; Randolph, C.; Silvers, W.S.; Storms, W.W.; Bronstone, A. Improving screening and diagnosis of exercise-induced bronchoconstriction: A call to action. J. Allergy Clin. Immunol. Pract. 2014, 2, 275–280.e7. [Google Scholar] [CrossRef]
- Koper, I.; Hufnagl, K.; Ehmann, R. Gender aspects and influence of hormones on bronchial asthma—Secondary publication and update. World Allergy Organ. J. 2017, 10, 46. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Phillips, B.R.; Mauger, D.T.; Zein, J.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.M.; Myers, R.; Ross, K.R.; Chmiel, J.; et al. Effects of endogenous sex hormones on lung function and symptom control in adolescents with asthma. BMC Pulm. Med. 2018, 18, 58. [Google Scholar] [CrossRef]
- Miyasaka, T.; Dobashi-Okuyama, K.; Kawakami, K.; Masuda-Suzuki, C.; Takayanagi, M.; Ohno, I. Sex Plays a Multifaceted Role in Asthma Pathogenesis. Biomolecules 2022, 12, 650. [Google Scholar] [CrossRef]
- Chiarella, S.E.; Cardet, J.C.; Prakash, Y.S. Sex, Cells, and Asthma. Mayo Clin. Proc. 2021, 96, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Nappi, R.E.; Farolfi, A.; Tiranini, L.; Rossi, V.; Regalbuto, C.; Zuccotti, G. Perimenstrual Asthma in Adolescents: A Shared Condition in Pediatric and Gynecological Endocrinology. Children 2022, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; Lavoie, K.L.; Raherison-Semjen, C.; Kaplan, A.; Singh, D.; Jenkins, C.R. Addressing sex and gender to improve asthma management. npj Prim. Care Respir. Med. 2022, 32, 56. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Licari, A.; Castagnoli, R.; Ciprandi, R.; Marseglia, G.L. Asthma control in adolescents: The importance of assessing adherence. Acta Biomed. 2022, 93, e2022264. [Google Scholar]
- Levy, M.L.; Carroll, W.; Izquierdo Alonso, J.L.; Keller, C.; Lavorini, F.; Lehtimäki, L. Understanding Dry Powder Inhalers: Key Technical and Patient Preference Attributes. Adv. Ther. 2019, 36, 2547–2557. [Google Scholar] [CrossRef]
- Gillette, C.; Rockich-Winston, N.; Kuhn, J.A.; Flesher, S.; Shepherd, M. Inhaler Technique in Children with Asthma: A Systematic Review. Acad. Pediatr. 2016, 16, 605–615. [Google Scholar] [CrossRef]
- Payares-Salamanca, L.; Contreras-Arrieta, S.; Florez-García, V.; Barrios-Sanjuanelo, A.; Stand-Niño, I.; Rodriguez-Martinez, C.E. Metered-dose inhalers versus nebulization for the delivery of albuterol for acute exacerbations of wheezing or asthma in children: A systematic review with meta-analysis. Pediatr. Pulmonol. 2020, 55, 3268–3278. [Google Scholar] [CrossRef]
- Normansell, R.; Kew, K.M.; Mathioudakis, A.G. Interventions to improve inhaler technique for people with asthma. Cochrane Database Syst. Rev. 2017, 3, CD012286. [Google Scholar]
- Margolis, R.H.F.; Bellin, M.H.; Morphew, T.; Tsoukleris, M.; Bollinger, M.E.; Butz, A. Caregiver Depressive Symptoms and Primary Medication Nonadherence in Children with Asthma. J. Pediatr. Health Care 2022, 36, 136–143. [Google Scholar] [CrossRef]
- Fainardi, V.; Passadore, L.; Labate, M.; Pisi, G.; Esposito, S. An Overview of the Obese-Asthma Phenotype in Children. Int. J. Env. Res. Public. Health 2022, 19, 636. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Miron, I.; Tarca, E.; Trandafir, L.M.; Anton-Paduraru, D.T.; Moisa, S.M.; Starcea, M.; Cernomaz, A.; Miron, L.; Lupu, A. Gastroesophageal Reflux in Children with Asthma. Children 2022, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, K.; Boatright, R.O.; Gilger, M.A.; El-Serag, H.B. Gastroesophageal reflux and asthma in children: A systematic review. Pediatrics 2010, 125, e925–e930. [Google Scholar] [CrossRef] [PubMed]
- Khoshoo, V.; Haydel, R. Update on Gastroesophageal Reflux Disease and Asthma in Children. Curr. GERD Rep. 2007, 1, 65–72. [Google Scholar] [CrossRef]
- Wrigley, J.D.; Lemberg, D.A.; Jaffé, A.; Thomas, P.S. Asthma and gastroesophageal reflux in children: Cause or effect? Current and novel approaches. Pediatr. Health 2008, 2, 333–339. [Google Scholar] [CrossRef]
- Matucci, A.; Bormioli, S.; Nencini, F.; Chiccoli, F.; Vivarelli, E.; Maggi, E.; Vultaggio, A. Asthma and Chronic Rhinosinusitis: How Similar Are They in Pathogenesis and Treatment Responses? Int. J. Mol. Sci. 2021, 22, 3340. [Google Scholar] [CrossRef]
- Tsao, C.H.; Chen, L.C.; Yeh, K.W.; Huang, J.L. Concomitant chronic sinusitis treatment in children with mild asthma: The effect on bronchial hyperresponsiveness. Chest 2003, 123, 757–764. [Google Scholar] [CrossRef]
- Baran, H.; Ozcan, K.M.; Selcuk, A.; Cetin, M.A.; Cayir, S.; Ozcan, M.; Dere, H. Allergic Rhinitis and its Impact on Asthma classification correlations. J. Laryngol. Otol. 2014, 128, 431–437. [Google Scholar] [CrossRef]
- Prasad, B.; Nyenhuis, S.M.; Imayama, I.; Siddiqi, A.; Teodorescu, M. Asthma and Obstructive Sleep Apnea Overlap: What Has the Evidence Taught Us? Am. J. Respir. Crit. Care Med. 2020, 201, 1345–1357. [Google Scholar] [CrossRef]
- Porsbjerg, C.; Menzies-Gow, A. Co-morbidities in severe asthma: Clinical impact and management. Respirology 2017, 22, 651–661. [Google Scholar] [CrossRef]
- Madama, D.; Silva, A.; Matos, M.J. Overlap syndrome—Asthma and obstructive sleep apnea. Rev. Port. Pneumol. 2016, 22, 6–10. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, E.L.; Spieth, L.E.; Spirito, A. The pediatric psychologist’s role in differential diagnosis: Vocal-cord dysfunction presenting as asthma. J. Pediatr. Psychol. 1997, 22, 739–748. [Google Scholar] [CrossRef]
- Licari, A.; Castagnoli, R.; Ciprandi, R.; Brambilla, I.; Guasti, E.; Marseglia, G.L.; Ciprandi, G. Anxiety and depression in adolescents with asthma: A study in clinical practice. Acta Biomed. 2022, 93, e2022021. [Google Scholar] [PubMed]
- Özyurt, G.; Tuncel, T.; Eliaçık, K.; Şenol, H.D.; Öztürk, Y.; Özdoğru, E.E. Adolescents with asthma reported more peer victimization, more anger repression, and less anger expression. J. Asthma 2021, 58, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Maio, S.; Baldacci, S.; Simoni, M.; Angino, A.; Martini, F.; Cerrai, S.; Sarno, G.; Pala, A.; Bresciani, M.; Paggiaro, P.; et al. Impact of asthma and comorbid allergic rhinitis on quality of life and control in patients of Italian general practitioners. J. Asthma 2012, 49, 854–861. [Google Scholar] [CrossRef]
- Magnan, A.; Meunier, J.P.; Saugnac, C.; Gasteau, J.; Neukirch, F. Frequency and impact of allergic rhinitis in asthma patients in everyday general medical practice: A French observational cross-sectional study. Allergy 2008, 63, 292–298. [Google Scholar] [CrossRef]
- Bender, B.G. Depression symptoms and substance abuse in adolescents with asthma. Ann. Allergy Asthma Immunol. 2007, 99, 319–324. [Google Scholar] [CrossRef]
- Weekes, J.C.; Cotton, S.; McGrady, M.E. Predictors of substance use among black urban adolescents with asthma: A longitudinal assessment. J. Natl. Med. Assoc. 2011, 103, 392–398. [Google Scholar] [CrossRef]
- Gilliland, F.D.; Islam, T.; Berhane, K.; Gauderman, W.J.; McConnell, R.; Avol, E.; Peters, J.M. Regular smoking and asthma incidence in adolescents. Am. J. Respir. Crit. Care Med. 2006, 174, 1094–1100. [Google Scholar] [CrossRef]
- Di Cicco, M.; Sepich, M.; Beni, A.; Comberiati, P.; Peroni, D.G. How E-cigarettes and vaping can affect asthma in children and adolescents. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 86–94. [Google Scholar] [CrossRef]
- Alnajem, A.; Redha, A.; Alroumi, D.; Alshammasi, A.; Ali, M.; Alhussaini, M.; Almutairi, W.; Esmaeil, A.; Ziyab, A.H. Use of electronic cigarettes and secondhand exposure to their aerosols are associated with asthma symptoms among adolescents: A cross-sectional study. Respir. Res. 2020, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.; Sepich, M.; Ragazzo, V.; Peroni, D.G.; Comberiati, P. Potential effects of E-cigarettes and vaping on pediatric asthma. Minerva Pediatr. 2020, 72, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Chipps, B. Challenges in the treatment of asthma in children and adolescents. Ann. Allergy Asthma Immunol. 2018, 120, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Garcia, G.; de Larrard, A.; Cancalon, C.; Bénard, S.; Perez, V.; Mahieu, A.; Vieu, L.; Demoly, P. Real-life impact of uncontrolled severe asthma on mortality and healthcare use in adolescents and adults: Findings from the retrospective, observational RESONANCE study in France. BMJ Open 2022, 12, e060160. [Google Scholar] [CrossRef]
- Cekic, S.; Karali, Z.; Cicek, F.; Canitez, Y.; Sapan, N. The Impact of the COVID-19 Pandemic in Adolescents with Asthma. J. Korean Med. Sci. 2021, 36, e339. [Google Scholar] [CrossRef]
- Green, I.; Merzon, E.; Vinker, S.; Golan-Cohen, A.; Magen, E. COVID-19 Susceptibility in Bronchial Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 684–692.e1. [Google Scholar] [CrossRef]
- Boechat, J.L.; Wandalsen, G.F.; Kuschnir, F.C.; Delgado, L. COVID-19 and Pediatric Asthma: Clinical and Management Challenges. Int. J. Env. Res. Public. Health 2021, 18, 1093. [Google Scholar] [CrossRef]
- Olusanya, O.A.; Bednarczyk, R.A.; Davis, R.L.; Shaban-Nejad, A. Addressing Parental Vaccine Hesitancy and Other Barriers to Childhood/Adolescent Vaccination Uptake During the Coronavirus (COVID-19) Pandemic. Front. Immunol. 2021, 12, 663074. [Google Scholar] [CrossRef]
- Cupertino, V.; Bozzola, E.; De Luca, G.; Del Giudice, E.; De Martino, G.; Cannataro, P.; Tozzi, A.E.; Corsello, G. The awareness and acceptance of anti-COVID 19 vaccination in adolescence. Ital. J. Pediatr. 2022, 48, 194. [Google Scholar] [CrossRef]
- Basch, C.H.; Meleo-Erwin, Z.; Fera, J.; Jaime, C.; Basch, C.E. A global pandemic in the time of viral memes: COVID-19 vaccine misinformation and disinformation on TikTok. Hum. Vaccin. Immunother. 2021, 17, 2373–2377. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Wang, X.C.; Feng, L.Z.; Xie, Z.D.; Jiang, Y.; Lu, G.; Li, X.W.; Jiang, R.M.; Deng, J.K.; Liu, M.; et al. Expert consensus on COVID-19 vaccination in children. World J. Pediatr. 2021, 17, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Tano, E.; San Martin, S.; Girgis, S.; Martinez-Fernandez, Y.; Sanchez Vegas, C. Perimyocarditis in Adolescents After Pfizer-BioNTech COVID-19 Vaccine. J. Pediatr. Infect. Dis. Soc. 2021, 10, 962–966. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Remarks | Recommendation |
|---|---|---|
| Symptoms include wheeze, cough and breathing difficulty |
| Strong recommendation against the intervention. |
| Improvement in symptoms following preventer medication |
| Conditional recommendation against the intervention, based on clinical experience. |
| Spirometry testing | Diagnosis:
| Strong recommendation for the intervention. |
| BDR testing |
Consider BDR testing when baseline spirometry is normal if the clinical history is strongly suggestive of asthma. | Strong recommendation for the intervention, based on clinical experience, in all children with FEV1 < LLN or <80% pred and/or FEV1/FVC < LLN or <80%. |
| FeNO testing |
| Strong recommendation for the intervention. |
| PEFR variability |
| Conditional recommendation against the intervention. |
| Allergy testing (skin-prick tests to aeroallergens and serum total and specific IgE tests) | Overdiagnosis of asthma, particularly in children with other atopic diseases, and underdiagnosis if physicians rely on allergy tests for asthma diagnosis. | Strong recommendation against the intervention. |
| Direct bronchial challenge testing (methacholine and histamine) |
| Conditional recommendation for the intervention. |
| Indirect bronchial challenge testing (exercise and mannitol) |
| Conditional recommendation for the intervention. |
| SpO2 % | Lung Score | Respiratory Rate | Wheezing | Use of Accessory Muscles | Score | ||
|---|---|---|---|---|---|---|---|
| <6 Years | ≥6 Years | ||||||
| <30 | <20 | Not | Not | 0 | |||
| Easy | >94 | 0–3 | 31–45 | 21–35 | End of exhalation (with stethoscope) | Low | 1 |
| Moderate | 91–94 | 4–6 | 46–60 | 36–50 | Full exhalation (with stethoscope) | Moderate | 2 |
| Severe | <91 | 7–9 | >60 | >50 | Both inspiratory and expiratory (no stethoscope) | Excessive | 3 |
| Intermittent Asthma | It Does Not Require Treatment | |
|---|---|---|
| Persistent asthma | Step 1. | |
Preferred reliever:
| Alternative reliever:
| |
| Step 2. | ||
Preferred reliever:
| Alternative reliever:
| |
| Step 3. | ||
Preferred reliever:
| Alternative reliever:
| |
| Step 4. | ||
Preferred reliever:
| Alternative reliever:
| |
| Steps 5. | ||
Preferred reliever:
| Alternative reliever:
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupu, V.V.; Jechel, E.; Fotea, S.; Morariu, I.D.; Starcea, I.M.; Azoicai, A.; Mocanu, A.; Mitrofan, E.C.; Lupu, A.; Munteanu, D.; et al. Current Approaches in the Multimodal Management of Asthma in Adolescents—From Pharmacology to Personalized Therapy. Biomedicines 2023, 11, 2429. https://doi.org/10.3390/biomedicines11092429
Lupu VV, Jechel E, Fotea S, Morariu ID, Starcea IM, Azoicai A, Mocanu A, Mitrofan EC, Lupu A, Munteanu D, et al. Current Approaches in the Multimodal Management of Asthma in Adolescents—From Pharmacology to Personalized Therapy. Biomedicines. 2023; 11(9):2429. https://doi.org/10.3390/biomedicines11092429
Chicago/Turabian StyleLupu, Vasile Valeriu, Elena Jechel, Silvia Fotea, Ionela Daniela Morariu, Iuliana Magdalena Starcea, Alice Azoicai, Adriana Mocanu, Elena Cristina Mitrofan, Ancuta Lupu, Dragos Munteanu, and et al. 2023. "Current Approaches in the Multimodal Management of Asthma in Adolescents—From Pharmacology to Personalized Therapy" Biomedicines 11, no. 9: 2429. https://doi.org/10.3390/biomedicines11092429
APA StyleLupu, V. V., Jechel, E., Fotea, S., Morariu, I. D., Starcea, I. M., Azoicai, A., Mocanu, A., Mitrofan, E. C., Lupu, A., Munteanu, D., Badescu, M. C., Cuciureanu, M., & Ioniuc, I. (2023). Current Approaches in the Multimodal Management of Asthma in Adolescents—From Pharmacology to Personalized Therapy. Biomedicines, 11(9), 2429. https://doi.org/10.3390/biomedicines11092429








