A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis
Abstract
:1. Introduction
1.1. A New Epidemiological Threat?
1.2. Prognosis
1.3. Molecular Pathogenesis
1.4. Therapeutic Approaches in BM RCC
1.4.1. Hereditary versus Sporadic, and the Primary Management of RCC
1.4.2. Multidisciplinary Opportunities in BM RCC
1.4.3. A Focus on Neurosurgery
2. Materials and Methods
3. Results
3.1. Statistics and Replicability
3.2. Demographic Profile, Clinicopathological Characteristics, and Correlation Analysis
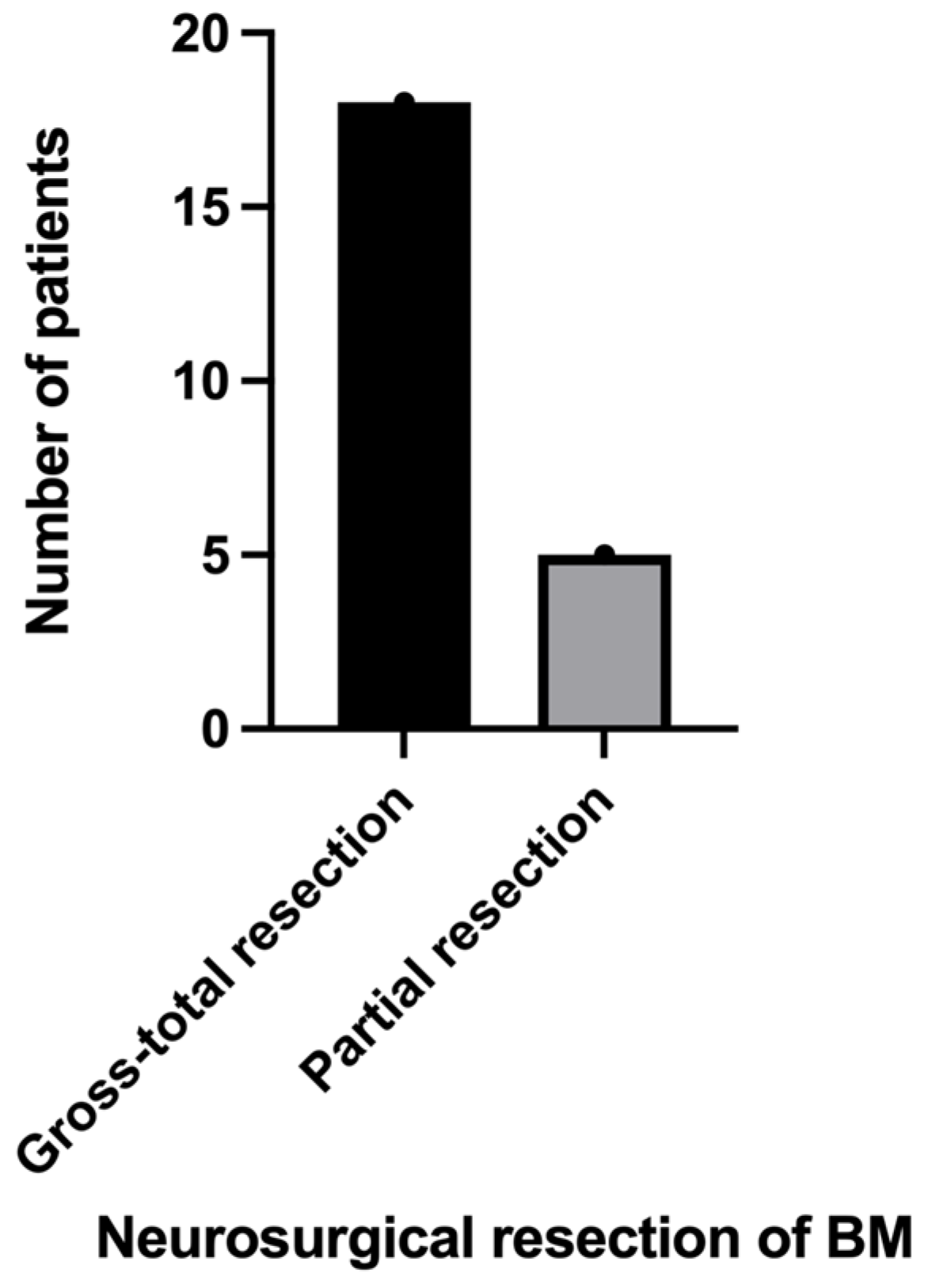
3.3. Neurosurgical Results
3.4. Histopathological Features
3.5. Survival Analysis
3.6. The Metabolism of Ferrous Iron: A Soft Spot in the Modulation of Cancer and Metastasis?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clemmensen, T.; Matoso, A.; Graham, T.; Lai, W.S.; Rais-Bahrami, S.; Gordetsky, J. Pathologic and clinical characteristics of early onset renal cell carcinoma. Hum. Pathol. 2018, 74, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- D’Avella, C.; Abbosh, P.; Pal, S.K.; Geynisman, D.M. Mutations in renal cell carcinoma. Urol. Oncol. 2020, 38, 763–773. [Google Scholar] [CrossRef]
- Liviu Preda, A.; Galieta Mincă, D. Cost-Effectiveness Analysis of Treatment for Metastatic Renal Carcinoma in Romania. J. Med. Life 2018, 11, 306–311. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Today. 2023. Available online: https://gco.iarc.fr/today/data/factsheets/populations/642-romania-fact-sheets.pdf (accessed on 25 July 2023).
- Kattan, J.; Rassy, E.E.; Assi, T.; Bakouny, Z.; Pavlidis, N. A comprehensive review of the role of immune checkpoint inhibitors in brain metastasis of renal cell carcinoma origin. Crit. Rev. Oncol. Hematol. 2018, 130, 60–69. [Google Scholar] [CrossRef]
- Incorvaia, L.; Madonia, G.; Corsini, L.R.; Cucinella, A.; Brando, C.; Gagliardo, C.; Santoni, M.; Fanale, D.; Inno, A.; Fazio, I.; et al. Challenges and advances for the treatment of renal cancer patients with brain metastases: From immunological background to upcoming clinical evidence on immune-checkpoint inhibitors. Crit. Rev. Oncol. Hematol. 2021, 163, 103390. [Google Scholar] [CrossRef]
- Dridi, M.; Bouleftour, W.; Rivoirard, R.; Dal Col, P.; Langrand-Escure, J.; Vassal, C.; Guillot, A. Leptomeningeal Metastases in Renal Cell Carcinoma at Initial Diagnosis: 2 Case Reports and Literature Review. Cancer Investig. 2019, 37, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Hasanov, E.; Yeboa, D.N.; Tucker, M.D.; Swanson, T.A.; Beckham, T.H.; Rini, B.; Ene, C.I.; Hasanov, M.; Derks, S.; Smits, M.; et al. An interdisciplinary consensus on the management of brain metastases in patients with renal cell carcinoma. CA Cancer J. Clin. 2022, 72, 454–489. [Google Scholar] [CrossRef]
- Roshkovan, L. Chapter 11—Radiologic assessment of tumor response to immunotherapy and its complications. In NK Cells in Cancer Immunotherapy: Successes and Challenges; Academic Press: Cambridge, MA, USA, 2023; pp. 239–261. [Google Scholar]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging-”how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef]
- Ferro, M.; Musi, G.; Marchioni, M.; Maggi, M.; Veccia, A.; Del Giudice, F.; Barone, B.; Crocetto, F.; Lasorsa, F.; Antonelli, A.; et al. Radiogenomics in Renal Cancer Management-Current Evidence and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4615. [Google Scholar] [CrossRef]
- Bharwani, N.; Miquel, M.E.; Powles, T.; Dilks, P.; Shawyer, A.; Sahdev, A.; Wilson, P.D.; Chowdhury, S.; Berney, D.M.; Rockall, A.G. Diffusion-weighted and multiphase contrast-enhanced MRI as surrogate markers of response to neoadjuvant sunitinib in metastatic renal cell carcinoma. Br. J. Cancer 2014, 110, 616–624. [Google Scholar] [CrossRef]
- Antunes, J.; Viswanath, S.; Rusu, M.; Valls, L.; Hoimes, C.; Avril, N.; Madabhushi, A. Radiomics Analysis on FLT-PET/MRI for Characterization of Early Treatment Response in Renal Cell Carcinoma: A Proof-of-Concept Study. Transl. Oncol. 2016, 9, 155–162. [Google Scholar] [CrossRef]
- Duclos, V.; Iep, A.; Gomez, L.; Goldfarb, L.; Besson, F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for Diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021, 22, 4159. [Google Scholar] [CrossRef]
- Lo Gullo, R.; Daimiel, I.; Morris, E.A.; Pinker, K. Combining molecular and imaging metrics in cancer: Radiogenomics. Insights Imaging 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.; Yazdian Anari, P.; Turkbey, E.; Jones, E.C.; Malayeri, A.A. The Next Paradigm Shift in the Management of Clear Cell Renal Cancer: Radiogenomics-Definition, Current Advances, and Future Directions. Cancers 2022, 14, 793. [Google Scholar] [CrossRef]
- Alessandrino, F.; Shinagare, A.B.; Bossé, D.; Choueiri, T.K.; Krajewski, K.M. Radiogenomics in renal cell carcinoma. Abdom. Radiol. 2019, 44, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Li, Y.; Yuan, Y.; Zhong, H.; Huang, C.; Huang, J.; Lin, Y.; Huang, J. Characterization of Molecular Heterogeneity Associated With Tumor Microenvironment in Clear Cell Renal Cell Carcinoma to Aid Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 736540. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, P.; Fu, X.; Lin, W. Circular RNAs in renal cell carcinoma: Implications for tumorigenesis, diagnosis, and therapy. Mol. Cancer 2020, 19, 149. [Google Scholar] [CrossRef]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of renal cell carcinoma: Findings and clinical implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef]
- Gui, C.P.; Wei, J.H.; Chen, Y.H.; Fu, L.M.; Tang, Y.M.; Cao, J.Z.; Chen, W.; Luo, J.H. A new thinking: Extended application of genomic selection to screen multiomics data for development of novel hypoxia-immune biomarkers and target therapy of clear cell renal cell carcinoma. Brief Bioinform. 2021, 22, bbab173. [Google Scholar] [CrossRef] [PubMed]
- Deleuze, A.; Saout, J.; Dugay, F.; Peyronnet, B.; Mathieu, R.; Verhoest, G.; Bensalah, K.; Crouzet, L.; Laguerre, B.; Belaud-Rotureau, M.A.; et al. Immunotherapy in Renal Cell Carcinoma: The Future Is Now. Int. J. Mol. Sci. 2020, 21, 2532. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Yim, S.; Park, H. The cancer driver genes IDH1/2, JARID1C/ KDM5C, and UTX/ KDM6A: Crosstalk between histone demethylation and hypoxic reprogramming in cancer metabolism. Exp. Mol. Med. 2019, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Montero, C.M.; Rini, B.I.; Finke, J.H. The immunology of renal cell carcinoma. Nat. Rev. Nephrol. 2020, 16, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Kaelin, W.G., Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1519–1530. [Google Scholar] [CrossRef]
- Bradley, A.J.; MacDonald, L.; Whiteside, S.; Johnson, R.J.; Ramani, V.A. Accuracy of preoperative CT T staging of renal cell carcinoma: Which features predict advanced stage? Clin. Radiol. 2015, 70, 822–829. [Google Scholar] [CrossRef]
- Perazella, M.A.; Dreicer, R.; Rosner, M.H. Renal cell carcinoma for the nephrologist. Kidney Int. 2018, 94, 471–483. [Google Scholar] [CrossRef]
- Petejova, N.; Martinek, A. Renal cell carcinoma: Review of etiology, pathophysiology and risk factors. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2016, 160, 183–194. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; Oudard, S.; Else, T.; Maranchie, J.K.; Welsh, S.J.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Leveridge, M.J.; Bostrom, P.J.; Koulouris, G.; Finelli, A.; Lawrentschuk, N. Imaging renal cell carcinoma with ultrasonography, CT and MRI. Nat. Rev. Urol. 2010, 7, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.E.; Harris, G.T. Renal Cell Carcinoma: Diagnosis and Management. Am. Fam. Physician 2019, 99, 179–184. [Google Scholar] [PubMed]
- Vander Velde, R.; Yoon, N.; Marusyk, V.; Durmaz, A.; Dhawan, A.; Miroshnychenko, D.; Lozano-Peral, D.; Desai, B.; Balynska, O.; Poleszhuk, J.; et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat. Commun. 2020, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.; Daugherty, E.; Jacob, J.; Shapiro, O.; Mollapour, M.; Bratslavsky, G. Renal cell carcinoma and brain metastasis: Questioning the dogma of role for cytoreductive nephrectomy. Urol. Oncol. 2019, 37, 182.e9–182.e15. [Google Scholar] [CrossRef]
- Vuong, L.; Kotecha, R.R.; Voss, M.H.; Hakimi, A.A. Tumor Microenvironment Dynamics in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2019, 9, 1349–1357. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Suarez-Sarmiento, A., Jr.; Nguyen, K.A.; Syed, J.S.; Nolte, A.; Ghabili, K.; Cheng, M.; Liu, S.; Chiang, V.; Kluger, H.; Hurwitz, M.; et al. Brain Metastasis From Renal-Cell Carcinoma: An Institutional Study. Clin. Genitourin. Cancer 2019, 17, e1163–e1170. [Google Scholar] [CrossRef]
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.M. The Management of Brain Metastases-Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef]
- Znaor, A.; Lortet-Tieulent, J.; Laversanne, M.; Jemal, A.; Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur. Urol. 2015, 67, 519–530. [Google Scholar] [CrossRef]
- Sankey, E.W.; Tsvankin, V.; Grabowski, M.M.; Nayar, G.; Batich, K.A.; Risman, A.; Champion, C.D.; Salama, A.K.S.; Goodwin, C.R.; Fecci, P.E. Operative and peri-operative considerations in the management of brain metastasis. Cancer Med. 2019, 8, 6809–6831. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Chen, C.H. Potential New Therapeutic Approaches for Renal Cell Carcinoma. Semin. Nephrol. 2020, 40, 86–97. [Google Scholar] [CrossRef] [PubMed]
- De Meerleer, G.; Khoo, V.; Escudier, B.; Joniau, S.; Bossi, A.; Ost, P.; Briganti, A.; Fonteyne, V.; Van Vulpen, M.; Lumen, N.; et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014, 15, e170–e177. [Google Scholar] [CrossRef] [PubMed]
- Dengina, N.; Tsimafeyeu, I.; Mitin, T. Current Role of Radiotherapy for Renal-Cell Carcinoma: Review. Clin. Genitourin. Cancer 2017, 15, 183–187. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Patel, B.; Kim, A.H. Laser Interstitial Thermal Therapy. Mo Med. 2020, 117, 50–55. [Google Scholar]
- Chitti, B.; Goyal, S.; Sherman, J.H.; Caputy, A.; Sarfaraz, M.; Cifter, G.; Aghdam, H.; Rao, Y.J. The role of brachytherapy in the management of brain metastases: A systematic review. J. Contemp. Brachytherapy 2020, 12, 67–83. [Google Scholar]
- Petrelli, F.; Coinu, A.; Vavassori, I.; Cabiddu, M.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma Treated With Targeted Therapies: A Systematic Review With a Meta-Analysis. Clin. Genitourin. Cancer 2016, 14, 465–472. [Google Scholar] [CrossRef]
- Napolitano, L.; Manfredi, C.; Cirillo, L.; Fusco, G.M.; Passaro, F.; Abate, M.; La Rocca, R.; Mastrangelo, F.; Spirito, L.; Pandolfo, S.D.; et al. Cytoreductive Nephrectomy and Metastatic Renal Cell Carcinoma: State of the Art and Future Perspectives. Medicina 2023, 59, 767. [Google Scholar] [CrossRef]
- Barbastefano, J.; Garcia, J.A.; Elson, P.; Wood, L.S.; Lane, B.R.; Dreicer, R.; Campbell, S.C.; Rini, B.I. Association of percentage of tumour burden removed with debulking nephrectomy and progression-free survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. BJU Int. 2010, 106, 1266–1269. [Google Scholar] [CrossRef]
- Pindoria, N.; Raison, N.; Blecher, G.; Catterwell, R.; Dasgupta, P. Cytoreductive nephrectomy in the era of targeted therapies: A review. BJU Int. 2017, 120, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O’Brien, T.; Larkin, J.; Horswell, S.; et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell 2018, 173, 581–594.e12. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; et al. Sunitinib Alone or After Nephrectomy for Patients with Metastatic Renal Cell Carcinoma: Is There Still a Role for Cytoreductive Nephrectomy? Eur. Urol. 2021, 80, 417–424. [Google Scholar] [CrossRef]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; van Thienen, J.V.; Blank, C.U.; van Velthoven, R.; Del Pilar Laguna, M.; Wood, L.; van Melick, H.H.E.; et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef]
- Kokorovic, A.; Rendon, R.A. Cytoreductive nephrectomy in metastatic kidney cancer: What do we do now? Curr. Opin. Support Palliat. Care 2019, 13, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Quaini, F.; Bersanelli, M.; Buti, S. Cytoreductive nephrectomy in the era of targeted- and immuno- therapy for metastatic renal cell carcinoma: An elusive issue? A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2021, 160, 103293. [Google Scholar] [CrossRef]
- Stellato, M.; Santini, D.; Verzoni, E.; De Giorgi, U.; Pantano, F.; Casadei, C.; Fornarini, G.; Maruzzo, M.; Sbrana, A.; Di Lorenzo, G.; et al. Impact of Previous Nephrectomy on Clinical Outcome of Metastatic Renal Carcinoma Treated With Immune-Oncology: A Real-World Study on Behalf of Meet-URO Group (MeetUro-7b). Front. Oncol. 2021, 11, 682449. [Google Scholar] [CrossRef]
- Van Praet, C.; Slots, C.; Vasdev, N.; Rottey, S.; Fonteyne, V.; Andras, I.; Albersen, M.; De Meerleer, G.; Bex, A.; Decaestecker, K. Current role of cytoreductive nephrectomy in metastatic renal cell carcinoma. Turk. J. Urol. 2021, 47 (Suppl. 1), S79–S84. [Google Scholar] [CrossRef]
- Singla, N.; Ghandour, R.A.; Margulis, V. Is cytoreductive nephrectomy relevant in the immunotherapy era? Curr. Opin. Urol. 2019, 29, 526–530. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef]
- Kotecha, R.R.; Flippot, R.; Nortman, T.; Guida, A.; Patil, S.; Escudier, B.; Motzer, R.J.; Albiges, L.; Voss, M.H. Prognosis of Incidental Brain Metastases in Patients With Advanced Renal Cell Carcinoma. J. Natl. Compr. Canc. Netw. 2021, 19, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Sirbu, C.; Lucas, B.D., Jr.; Jubelirer, S.J.; Khalid, A.; Mei, L. A Retrospective Study of Brain Metastases From Solid Malignancies: The Effect of Immune Checkpoint Inhibitors. Front. Oncol. 2021, 11, 667847. [Google Scholar] [CrossRef]
- Lin, N.U.; Lee, E.Q.; Aoyama, H.; Barani, I.J.; Barboriak, D.P.; Baumert, B.G.; Bendszus, M.; Brown, P.D.; Camidge, D.R.; Chang, S.M.; et al. Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol. 2015, 16, e270–e278. [Google Scholar] [CrossRef]
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2957–2995. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y. Current Multimodality Treatments Against Brain Metastases from Renal Cell Carcinoma. Cancers 2020, 12, 2875. [Google Scholar] [CrossRef] [PubMed]
- Hasanov, E.; Jonasch, E. Management of Brain Metastases in Metastatic Renal Cell Carcinoma. Hematol. Oncol. Clin. N. Am. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Wildrick, D.M.; Sawaya, R. Management of Cerebral Metastases: The Role of Surgery. Cancer Control 1998, 5, 124–129. [Google Scholar] [CrossRef]
- Hassaneen, W.; Suki, D.; Salaskar, A.L.; Wildrick, D.M.; Lang, F.F.; Fuller, G.N.; Sawaya, R. Surgical management of lateral-ventricle metastases: Report of 29 cases in a single-institution experience. J. Neurosurg. 2010, 112, 1046–1055. [Google Scholar] [CrossRef]
- Suki, D.; Hatiboglu, M.A.; Patel, A.J.; Weinberg, J.S.; Groves, M.D.; Mahajan, A.; Sawaya, R. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery 2009, 64, 664–674; discussion 674–676. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Andrews, D.W.; Scott, C.B.; Sperduto, P.W.; Flanders, A.E.; Gaspar, L.E.; Schell, M.C.; Werner-Wasik, M.; Demas, W.; Ryu, J.; Bahary, J.P.; et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet 2004, 363, 1665–1672. [Google Scholar] [CrossRef]
- Minniti, G.; Scaringi, C.; Paolini, S.; Lanzetta, G.; Romano, A.; Cicone, F.; Osti, M.; Enrici, R.M.; Esposito, V. Single-Fraction Versus Multifraction (3 × 9 Gy) Stereotactic Radiosurgery for Large (>2 cm) Brain Metastases: A Comparative Analysis of Local Control and Risk of Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Wardak, Z.; Christie, A.; Bowman, A.; Stojadinovic, S.; Nedzi, L.; Barnett, S.; Patel, T.; Mickey, B.; Whitworth, T.; Hannan, R.; et al. Stereotactic Radiosurgery for Multiple Brain Metastases From Renal-Cell Carcinoma. Clin. Genitourin. Cancer 2019, 17, e273–e280. [Google Scholar] [CrossRef] [PubMed]
- Bastos, D.C.A.; Fuentes, D.T.; Traylor, J.; Weinberg, J.; Kumar, V.A.; Stafford, J.; Li, J.; Rao, G.; Prabhu, S.S. The use of laser interstitial thermal therapy in the treatment of brain metastases: A literature review. Int. J. Hyperth. 2020, 37, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.S.; Deng, D.; Vera, A.; Chiang, V.L. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J. Neurooncol. 2019, 142, 309–317. [Google Scholar] [CrossRef]
- Pollock, B.E.; Brown, P.D.; Foote, R.L.; Stafford, S.L.; Schomberg, P.J. Properly selected patients with multiple brain metastases may benefit from aggressive treatment of their intracranial disease. J. Neurooncol. 2003, 61, 73–80. [Google Scholar] [CrossRef]
- Kayama, T.; Sato, S.; Sakurada, K.; Mizusawa, J.; Nishikawa, R.; Narita, Y.; Sumi, M.; Miyakita, Y.; Kumabe, T.; Sonoda, Y.; et al. Effects of Surgery With Salvage Stereotactic Radiosurgery Versus Surgery With Whole-Brain Radiation Therapy in Patients with One to Four Brain Metastases (JCOG0504): A Phase III, Noninferiority, Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, Jco2018786186. [Google Scholar] [CrossRef]
- Cohen, H.T.; McGovern, F.J. Renal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2477–2490. [Google Scholar] [CrossRef]
- Jiang, H.; Muir, R.K.; Gonciarz, R.L.; Olshen, A.B.; Yeh, I.; Hann, B.C.; Zhao, N.; Wang, Y.H.; Behr, S.C.; Korkola, J.E.; et al. Ferrous iron-activatable drug conjugate achieves potent MAPK blockade in KRAS-driven tumors. J. Exp. Med. 2022, 219, e20210739. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Iron: Key player in cancer and cell cycle? J. Trace Elem. Med. Biol. 2020, 62, 126582. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and Cancer: 2020 Vision. Cancer Res. 2020, 80, 5435–5448. [Google Scholar] [CrossRef] [PubMed]
- Crisman, C.M.; Patel, A.R.; Winston, G.; Brennan, C.W.; Tabar, V.; Moss, N.S. Clinical Outcomes in Patients with Renal Cell Carcinoma Metastases to the Choroid Plexus. World Neurosurg. 2020, 140, e7–e13. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mooi, J.; Lawrentschuk, N.; McKay, R.R.; Hannan, R.; Lo, S.S.; Hall, W.A.; Siva, S. The Role of Stereotactic Ablative Body Radiotherapy in Renal Cell Carcinoma. Eur. Urol. 2022, 82, 613–622. [Google Scholar] [CrossRef]
- All, S.; Garant, A.; Hannan, R. Stereotactic Ablative Radiation (SAbR) for Oligometastatic RCC. Semin. Radiat. Oncol. 2021, 31, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kothari, G.; Foroudi, F.; Gill, S.; Corcoran, N.M.; Siva, S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol. 2015, 54, 148–157. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: Results from a large, multicenter study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Hoff, C.M. Importance of hemoglobin concentration and its modification for the outcome of head and neck cancer patients treated with radiotherapy. Acta Oncol. 2012, 51, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Littlewood, T.J. The impact of hemoglobin levels on treatment outcomes in patients with cancer. Semin. Oncol. 2001, 28 (Suppl. 8), 49–53. [Google Scholar] [CrossRef]
- Edgren, G.; Bagnardi, V.; Bellocco, R.; Hjalgrim, H.; Rostgaard, K.; Melbye, M.; Reilly, M.; Adami, H.O.; Hall, P.; Nyrén, O. Pattern of declining hemoglobin concentration before cancer diagnosis. Int. J. Cancer 2010, 127, 1429–1436. [Google Scholar] [CrossRef]
- Clarke, H.; Pallister, C.J. The impact of anaemia on outcome in cancer. Clin. Lab. Haematol. 2005, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.R.; Gopal, N.; Ball, M.W. Tumorigenesis Mechanisms Found in Hereditary Renal Cell Carcinoma: A Review. Genes 2022, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Yap, N.Y.; Rajandram, R.; Ng, K.L.; Pailoor, J.; Fadzli, A.; Gobe, G.C. Genetic and Chromosomal Aberrations and Their Clinical Significance in Renal Neoplasms. Biomed. Res. Int. 2015, 2015, 476508. [Google Scholar] [CrossRef] [PubMed]












| Characteristic | Variable | Value (total n = 24) |
|---|---|---|
| Male | 19 (79.1%) | |
| Sex | Female | 5 (20.8%) |
| Distribution area | Rural/Urban | 14 (58.3%)/10 (41.6%) |
| Age at RCC diagnosis | Median age | 62 years |
| Age at BM RCC diagnosis | Median age | 62.5 years |
| BM localization | Frontal lobe | 8 (33.3%) |
| Temporal lobe | 6 (25%) | |
| Parietal lobe | 1 (4.1%) | |
| Occipital lobe | 1 (4.1%) | |
| Cerebellum | 7 (29.1%) | |
| Sellar region | 1 (4.1%) | |
| Single or multiple BM RCC | Single BM RCC 2 or more BM RCC | 20 (83.3%) 4 (16.6%) |
| Number of BM RCC | Mean/Median (min-max) | 1.29/1 (1–4) |
| Size of BM RCC (mm) | Mean/Median (min-max) | 32.54 mm/31 mm (13–63) |
| Symptoms at presentation | Yes No | 22 (91.6%) 2 (8.3%) |
| Clinical symptoms/manifestations | None | 2 (8.3%) |
| Raised intracranial Pressure syndrome | 7 (29.1%) | |
| Headache | 11 (45.8%) | |
| Cranial nerve palsies | 5 (20.8%) | |
| Pituitary dysfunction | 1 (4.1%) | |
| Limb paralysis | 10 (41.6%) | |
| Aphasia | 3 (12.5%) | |
| Seizures | 2 (8.3%) | |
| Karnofsky Performance Status Scale at admission | ≥80 <80 | 13 (54.1%) 11 (45.8%) |
| Admission to the neurosurgical department | First time Recurrence | 20 (83.3%) 4 (16.6%) |
| Extracranial metastases 1 | Yes No | 7 (29.1%) 17 (70.8%) |
| Yes | 7 (29.1%) | |
| Systemic therapy | No | 17 (70.8%) |
| Yes | 9 (37.5%) | |
| Prior nephrectomy | No | 15 (62.5%) |
| Yes | 3 (12.5%) | |
|
Stereotactic Radiosurgery (SRS) | No | 21 (87.5%) |
| IMDC Risk | Patients N (%) |
|---|---|
| Favorable-risk group | 5 (20.8%) |
| Intermediate-risk group | 16 (66.6%) |
| Poor-risk group | 3 (12.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenescu, L.E.; Tataranu, L.G.; Dricu, A.; Ciubotaru, G.V.; Radoi, M.P.; Rodriguez, S.M.B.; Kamel, A. A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis. Biomedicines 2023, 11, 2485. https://doi.org/10.3390/biomedicines11092485
Semenescu LE, Tataranu LG, Dricu A, Ciubotaru GV, Radoi MP, Rodriguez SMB, Kamel A. A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis. Biomedicines. 2023; 11(9):2485. https://doi.org/10.3390/biomedicines11092485
Chicago/Turabian StyleSemenescu, Liliana Eleonora, Ligia Gabriela Tataranu, Anica Dricu, Gheorghe Vasile Ciubotaru, Mugurel Petrinel Radoi, Silvia Mara Baez Rodriguez, and Amira Kamel. 2023. "A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis" Biomedicines 11, no. 9: 2485. https://doi.org/10.3390/biomedicines11092485
APA StyleSemenescu, L. E., Tataranu, L. G., Dricu, A., Ciubotaru, G. V., Radoi, M. P., Rodriguez, S. M. B., & Kamel, A. (2023). A Neurosurgical Perspective on Brain Metastases from Renal Cell Carcinoma: Multi-Institutional, Retrospective Analysis. Biomedicines, 11(9), 2485. https://doi.org/10.3390/biomedicines11092485





