Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue
Abstract
:1. Introduction
2. Case Presentation
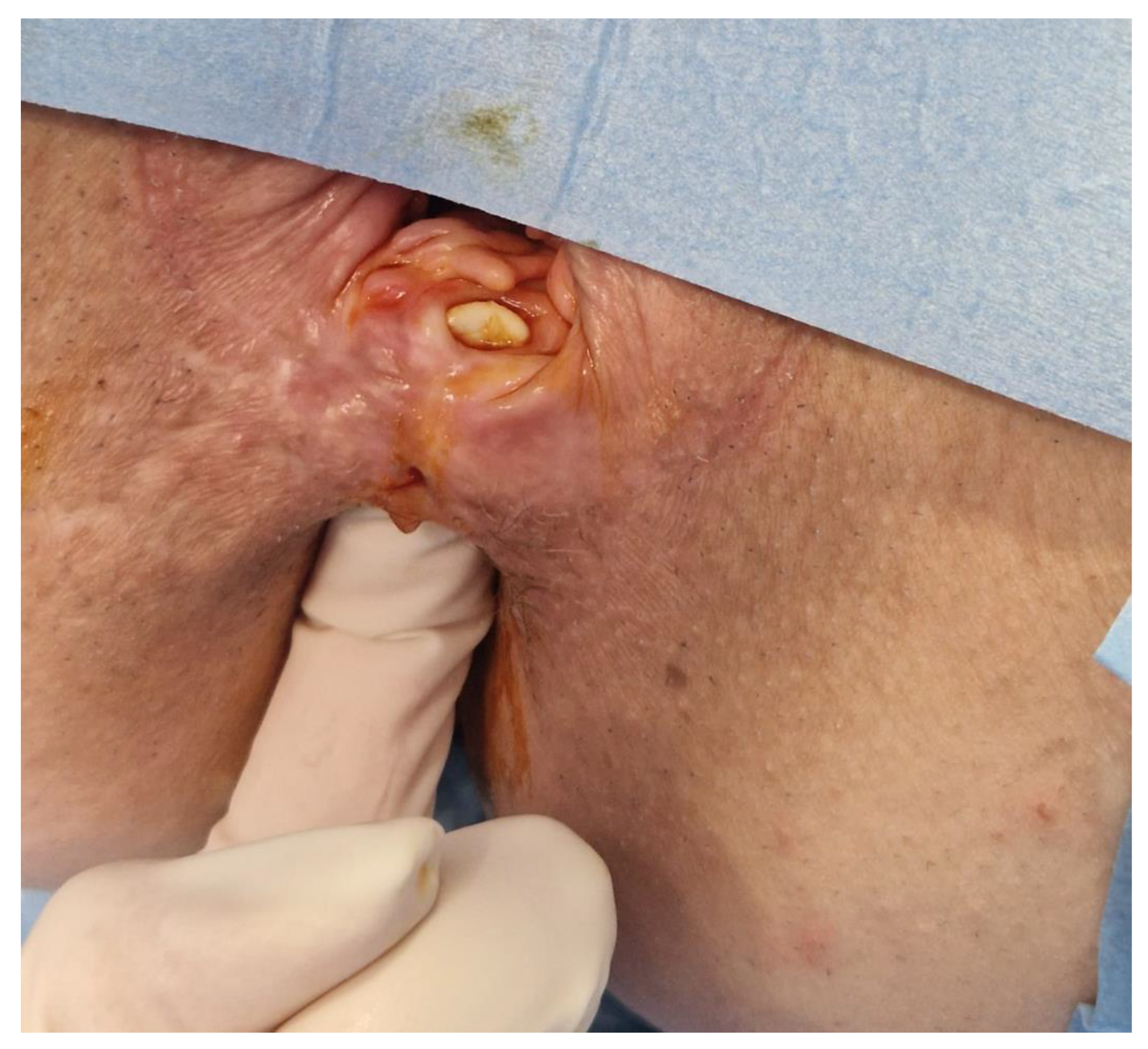
2.1. Clinical Findings
2.2. Diagnostic Assessment
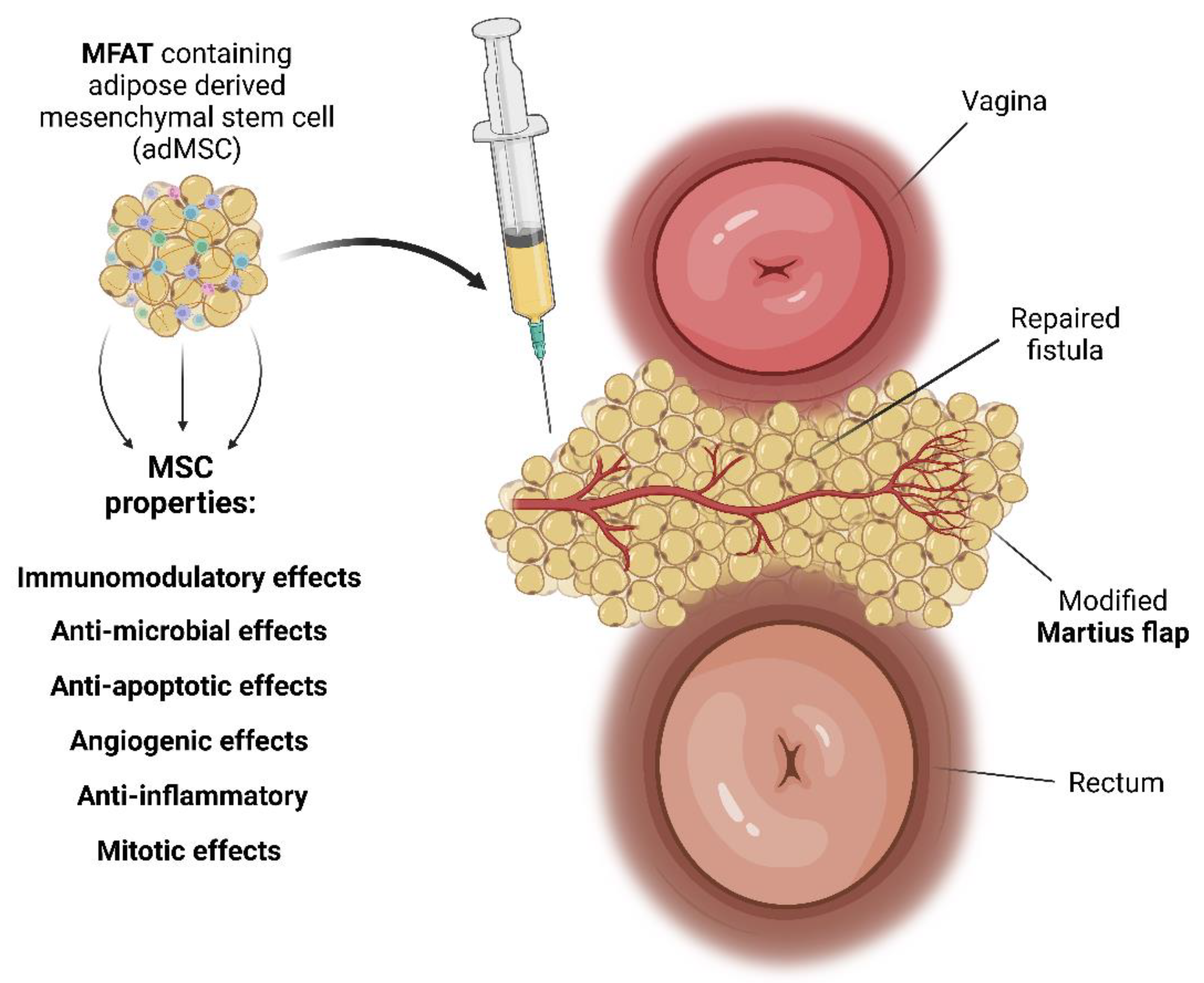
2.3. Preparation of MFAT Containing MSC
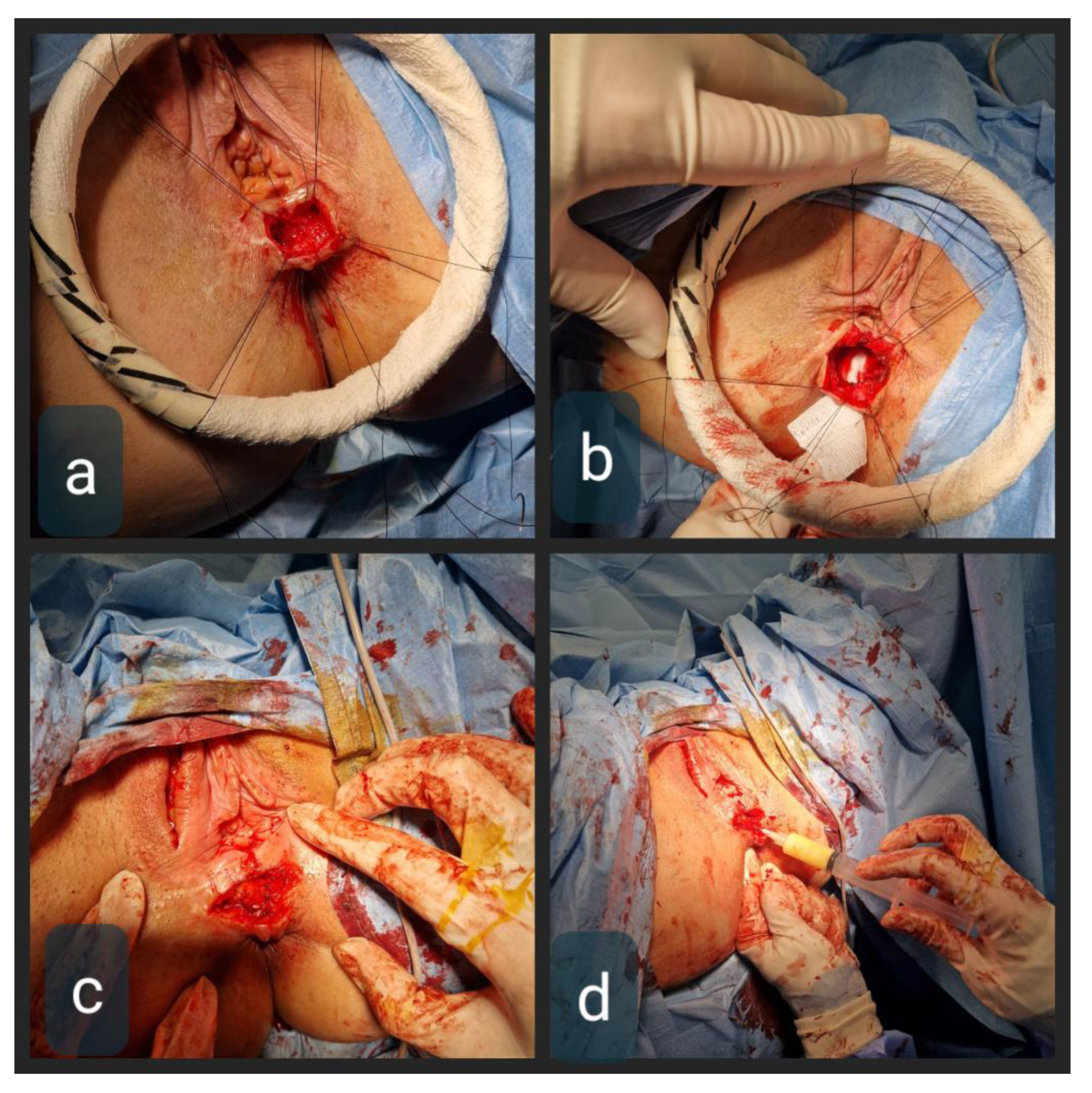
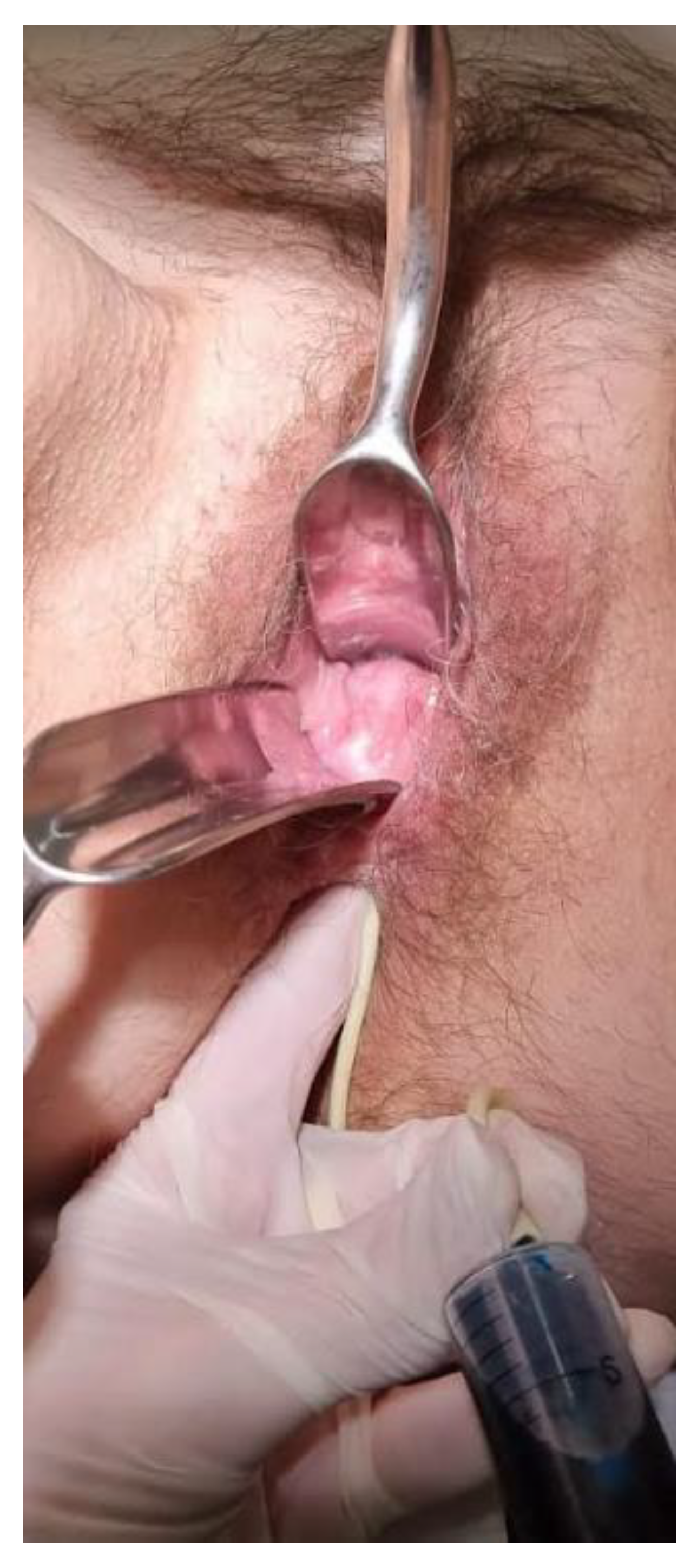
2.4. Therapeutic Intervention
2.5. Follow-Up and Outcome
3. Discussion
4. Patient Perspective in an Original Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsang, C.B.; Rothenberger, D.A. Rectovaginal fistulas. Therapeutic options. Surg. Clin. N. Am. 1997, 77, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A.; Nair, A.; Hughes, L.E. Anovaginal and rectovaginal fistula in patients with Crohn’s disease. Br. J. Surg. 1992, 79, 1379–1380. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, S.; Yazigi, R.; Köhler, A.; Helmes, C. Recovery rates and functional results after repair for rectovaginal fistula in Crohn’s disease: A comparison of different techniques. Int. J. Colorectal Dis. 2007, 22, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Löffler, T.; Welsch, T.; Mühl, S.; Hinz, U.; Schmidt, J.; Kienle, P. Long-term success rate after surgical treatment of anorectal and rectovaginal fistulas in Crohn’s disease. Int. J. Colorectal Dis. 2009, 24, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, L.H.; Frank, M.S.; Brandt, L.J.; Boley, S.J. Healing of perineal Crohn’s disease with metronidazole. Gastroenterology 1980, 79, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.J.; Bernstein, L.H.; Boley, S.J.; Frank, M.S. Metronidazole therapy for perineal Crohn’s disease: A follow-up study. Gastroenterology 1982, 83, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Ng, S.C.; Durdey, P.; Burt, C.; Torkington, J.; Rao, P.K.; Mayberry, J.; Moshkovska, T.; Stone, C.D.; Carapeti, E.; et al. Randomized clinical trial of metronidazole ointment versus placebo in perianal Crohn’s disease. Br. J. Surg. 2010, 97, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Ris, F.; Parkes, M.; Davies, J. Rectovaginal Fistula in Crohn’s Disease: When and How to Operate? Clin. Colon. Rectal Surg. 2022, 35, 10–20. [Google Scholar] [CrossRef]
- Dejaco, C.; Harrer, M.; Waldhoer, T.; Miehsler, W.; Vogelsang, H.; Reinisch, W. Antibiotics and azathioprine for the treatment of perianal fistulas in Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 18, 1113–1120. [Google Scholar] [CrossRef]
- Present, D.H.; Korelitz, B.I.; Wisch, N.; Glass, J.L.; Sachar, D.B.; Pasternack, B.S. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N. Engl. J. Med. 1980, 302, 981–987. [Google Scholar] [CrossRef]
- Korelitz, B.; Present, D. Favorable effect of 6-mercaptopurine on fistulae of Crohn’s Disease. Dig. Dis. Sci. 1985, 30, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Present, D.H.; Lichtiger, S. Efficacy of cyclosporine in treatment of fistula of Crohn’s disease. Dig. Dis. Sci. 1994, 39, 374–380. [Google Scholar] [CrossRef]
- Parsi, M.A.; Lashner, B.A.; Achkar, J.P.; Connor, J.T.; Brzezinski, A. Type of fistula determines response to infliximab in patients with fistulous Crohn’s disease. Am. J. Gastroenterol. 2004, 99, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegraven, A.A.; Sloots, C.E.; Felt-Bersma, R.J.; Meuwissen, S.G. Endosonographic evidence of persistence of Crohn’s disease associated fistulas after inflixmab treatment, irrespective of clinical response. Dis. Colon. Rectum 2002, 45, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ricart, E.; Panaccione, R.; Loftus, E.V.; Tremaine, W.J.; Sandborn, W.J. Infliximab for Crohn’s disease in clinical practice at the Mayo Clinic: The first 100 patients. Am. J. Gastroenterol. 2001, 96, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.J.; Shah, S.A.; Lodhavia, P.J.; Alsahli, M.; Falchuk, K.R.; Michetti, P.; Peppercorn, M.A. Clinical experience with infliximab therapy in 100 patients with Crohn’s Disease. Am. J. Gastroenterol. 2000, 95, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Hannaway, C.D.; Hull, T.L. Current considerations in the management of rectovaginal fistula from Crohn’s disease. Colorectal Dis. 2008, 10, 747–755; discussion 755–756. [Google Scholar] [CrossRef]
- Fry, R.D.; Shemesh, E.I.; Kodner, I.J.; Timmcke, A. Techniques and results in the management of anal and perianal Crohn’s disease. Surg. Gynecol. Obstet. 1989, 168, 42–48. [Google Scholar]
- Lichtenstein, G.R. Treatment of fistulizing Crohn’s disease. Gastroenterology 2000, 119, 1132–1147. [Google Scholar] [CrossRef]
- Farkas, A.M.; Gingold, B.S. Repair of rectovaginal fistula in Crohn’s disease by rectal mucosal advancement flap. Mt. Sinai J. Med. 1983, 50, 420–423. [Google Scholar]
- Berman, I.R. Sleeve advancement anorectoplasty for complicated anorectal/vaginal fistula. Dis. Colon. Rectum 1991, 34, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.H.; Geis, M.; Glatzle, J.; Kasparek, M.; Meile, T.; Jehle, E.C.; Kreis, M.E.; Zittel, T.T. Risk of fecal diversion in complicated perianal Crohn’s disease. J. Gastrointest. Surg. 2007, 11, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Heyen, F.; Winslet, M.C.; Andrews, H.; Alexander-Williams, J.; Keighley, M.R. Vaginal fistulas in Crohn’s disease. Dis. Colon. Rectum 1989, 32, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Aniwan, S.; Scott Harmsen, W.; Tremaine, W.J.; Lightner, A.L.; Faubion, W.A.; Loftus, E.V., Jr. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm. Bowel Dis. 2019, 25, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Sher, M.E.; Bauer, J.J.; Gelernt, I. Surgical repair of rectovaginal fistulas in patients with Crohn’s disease: Transvaginal approach. Dis. Colon. Rectum 1991, 34, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.J.; Sher, M.E.; Jaffin, H.; Present, D.; Gelerent, I. Transvaginal approach for repair of rectovaginal fistulae complicating Crohn’s disease. Ann. Surg. 1991, 213, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Al-Suleiman, S.A.; El-Yahia, A.R.; Rahman, J. Surgical treatment of rectovaginal fistula of obstetric origin: A review of 15 years’ experience in a teaching hospital. J. Obstet. Gynaecol. 2003, 23, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Shah, S.; Nar, A.S.; Bawa, A. The role of fibrin glue in the treatment of high and low fistulas in ano. J. Clin. Diagn. Res. 2013, 7, 876–879. [Google Scholar] [CrossRef]
- Grimaud, J.C.; Munoz-Bongrand, N.; Siproudhis, L.; Abramowitz, L.; Sénéjoux, A.; Vitton, V.; Gambiez, L.; Flourié, B.; Hébuterne, X.; Louis, E.; et al. Fibrin glue is effective healing perianal fistulas in patients with Crohn’s disease. Gastroenterology 2010, 138, 2275–2281.e1. [Google Scholar] [CrossRef]
- Loungnarath, R.; Dietz, D.W.; Mutch, M.G.; Birnbaum, E.H.; Kodner, I.J.; Fleshman, J.W. Fibrin glue treatment of complex anal fistulas has low success rate. Dis. Colon. Rectum. 2004, 47, 432–436. [Google Scholar] [CrossRef]
- Abel, M.E.; Chiu, Y.S.; Russell, T.R.; Volpe, P.A. Autologous fibrin glue in the treatment of rectovaginal and complex fistulas. Dis. Colon. Rectum. 1993, 36, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.N. Outcomes after repair of rectovaginal fistulas using bioprosthetics. Dis. Colon. Rectum. 2008, 51, 1084–1088. [Google Scholar] [CrossRef]
- Erceg Ivkošić, I.; Fureš, R.; Ćosić, V.; Mikelin, N.; Bulić, L.; Dobranić, D.; Brlek, P.; Primorac, D. Unlocking the Potential of Mesenchymal Stem Cells in Gynecology: Where Are We Now? J. Pers. Med. 2023, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal Stem Cell Mechanisms of Action and Clinical Effects in Osteoarthritis: A Narrative Review. Genes 2022, 13, 949. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Boric, I.; Rod, E.; Jeleč, Ž.; Radić, A.; Vrdoljak, T.; Skelin, A.; Lauc, G.; Trbojević Akmačić, I.; Plečko, M.; et al. The Effect of Intra-articular Administration of Autologous Microfragmented Fat Tissue with Adipose-derived Mesenchymal Stem Cells on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes. 2017, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Pitel, S.; Lefevre, J.H.; Parc, Y.; Chafai, N.; Shields, C.; Tiret, E. Martius advancement flap for low rectovaginal fistula: Short- and long-term results. Colorectal Dis. 2011, 13, e112–e115. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmo, D.; Gilaberte, I.; Binek, M.; Lindner, D.; Selvaggi, F.; Spinelli, A.; Panés, J. Follow-up Study to Evaluate the Long-term Safety and Efficacy of Darvadstrocel (Mesenchymal Stem Cell Treatment) in Patients With Perianal Fistulizing Crohn’s Disease: ADMIRE-CD Phase 3 Randomized Controlled Trial. Dis. Colon. Rectum 2022, 65, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Klotz, U.; Teml, A.; Schwab, M. Clinical pharmacokinetics and use of infliximab. Clin. Pharmacokinet. 2007, 46, 645–660. [Google Scholar] [CrossRef]
- Sakha, Y.A.; Ehsani, E.; Roshandel, E.; Jalili, A.; Vahdani, N.; Hajifathali, A. Assessment of the Effect of Infliximab on Immunomodulation Properties of Mesenchymal Stem Cells In Vitro. Adv. Pharm. Bull. 2021, 11, 739–745. [Google Scholar] [CrossRef]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: A prospective study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Jeleč, Ž.; Rod, E.; Borić, I.; Plečko, M.; Primorac, D. The Future of Cartilage Repair. In Personalized Medicine in Healthcare Systems: Legal, Medical and Economic Implications; Bodiroga-Vukobrat, N., Rukavina, D., Pavelić, K., Sander, G.G., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 375–411. [Google Scholar]
- Thornton, M.; Solomon, M.J. Long-term indwelling seton for complex anal fistulas in Crohn’s disease. Dis. Colon. Rectum 2005, 48, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Martius, H. Die operative Wiederhellstellung der volkommen fehlenden Harnhohare unde des Sclessmuskels derselben. Zentralbl. Gynakol. 1928, 52, 480–486. [Google Scholar]
- Elkins, T.E.; DeLancey, J.O.; McGuire, E.J. The use of modified Martius graft as an adjunctive technique in vesicovaginal and rectovaginal fistula repair. Obstet. Gynecol. 1990, 75, 727–733. [Google Scholar] [PubMed]
- Sajjadi, S.G.; Hortváth, Ö.P.; Kalmár, K. Martius flap: Historical and anatomical considerations. Eur. J. Plast. Surg. 2012, 35, 711–716. [Google Scholar] [CrossRef]
- Polancec, D.; Zenic, L.; Hudetz, D.; Boric, I.; Jelec, Z.; Rod, E.; Vrdoljak, T.; Skelin, A.; Plecko, M.; Turkalj, M.; et al. Immunophenotyping of a Stromal Vascular Fraction from Microfragmented Lipoaspirate Used in Osteoarthritis Cartilage Treatment and Its Lipoaspirate Counterpart. Genes 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Zenic, L.; Polancec, D.; Hudetz, D.; Jelec, Z.; Rod, E.; Vidovic, D.; Staresinic, M.; Sabalic, S.; Vrdoljak, T.; Petrovic, T.; et al. Polychromatic Flow Cytometric Analysis of Stromal Vascular Fraction from Lipoaspirate and Microfragmented Counterparts Reveals Sex-Related Immunophenotype Differences. Genes 2021, 12, 1999. [Google Scholar] [CrossRef] [PubMed]
- Zenić, L.; Polančec, D.; Hudetz, D.; Jeleč, Z.; Rod, E.; Vidović, D.; Starešinić, M.; Sabalić, S.; Vrdoljak, T.; Petrović, T.; et al. Medicinal signaling cells niche in stromal vascular fraction from lipoaspirate and microfragmented counterpart. Croat. Med. J. 2022, 63, 265–272. [Google Scholar] [CrossRef]
- Williams, A.R.; Hare, J.M. Mesenchymal stem cells: Biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011, 109, 923–940. [Google Scholar] [CrossRef]
- Le Blanc, K.; Mougiakakos, D.; Celius, E.G. The history of mesenchymal stromal cells (MSCs): From discovery to clinical trials. Bone Marrow Transpl. 2020, 55, 1–10. [Google Scholar]
- English, K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol. Cell Biol. 2013, 91, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2016, 2, 3–20. [Google Scholar] [CrossRef]
- Trallori, E.; Ghelardini, C.; Di Cesare Mannelli, L. Mesenchymal stem cells, implications for pain therapy. Neural Regen. Res. 2019, 14, 1915–1916. [Google Scholar] [CrossRef] [PubMed]
- Carvello, M.; Lightner, A.; Yamamoto, T.; Kotze, P.G.; Spinelli, A. Mesenchymal Stem Cells for Perianal Crohn’s Disease. Cells 2019, 8, 764. [Google Scholar] [CrossRef] [PubMed]
- Laureti, S.; Gionchetti, P.; Cappelli, A.; Vittori, L.; Contedini, F.; Rizzello, F.; Golfieri, R.; Campieri, M.; Poggioli, G. Refractory Complex Crohn’s Perianal Fistulas: A Role for Autologous Microfragmented Adipose Tissue Injection. Inflamm. Bowel Dis. 2020, 26, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yao, Q.; Chen, W.; Gao, F.; Li, P.; Wu, J.; Yu, J.; Cao, H. Stem cell therapy for Crohn’s disease: Systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res. Ther. 2021, 12, 463. [Google Scholar] [CrossRef]
- Hull, T. Rectovaginal Fistula. In Current Therapy in Colon and Rectal Surgery, 2nd ed.; Fazio, V.F., Church, J.M., Delaney, C.P., Eds.; Mosby, Inc.: Philadelphia, PA, USA, 2005; p. 39. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimova, A.; Erceg Ivkošić, I.; Brlek, P.; Dimov, S.; Pavlović, T.; Bokun, T.; Primorac, D. Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue. Biomedicines 2023, 11, 2509. https://doi.org/10.3390/biomedicines11092509
Dimova A, Erceg Ivkošić I, Brlek P, Dimov S, Pavlović T, Bokun T, Primorac D. Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue. Biomedicines. 2023; 11(9):2509. https://doi.org/10.3390/biomedicines11092509
Chicago/Turabian StyleDimova, Ana, Ivana Erceg Ivkošić, Petar Brlek, Stefan Dimov, Tomislav Pavlović, Tomislav Bokun, and Dragan Primorac. 2023. "Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue" Biomedicines 11, no. 9: 2509. https://doi.org/10.3390/biomedicines11092509
APA StyleDimova, A., Erceg Ivkošić, I., Brlek, P., Dimov, S., Pavlović, T., Bokun, T., & Primorac, D. (2023). Novel Approach in Rectovaginal Fistula Treatment: Combination of Modified Martius Flap and Autologous Micro-Fragmented Adipose Tissue. Biomedicines, 11(9), 2509. https://doi.org/10.3390/biomedicines11092509






