The Association between Vitamin D, Interleukin-4, and Interleukin-10 Levels and CD23+ Expression with Bronchial Asthma in Stunted Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Sample Study
2.2. Anthropometric Measurement
2.3. Vitamin D Level Measurement
2.4. Measurement of IL-4 and IL-10 Levels
2.5. CD23+ Expression Measurement
2.6. Data Analysis
3. Results
3.1. Participant Characteristics
3.2. Vitamin D, IL-4, and IL-10 Levels and CD23+ Expression in Participants
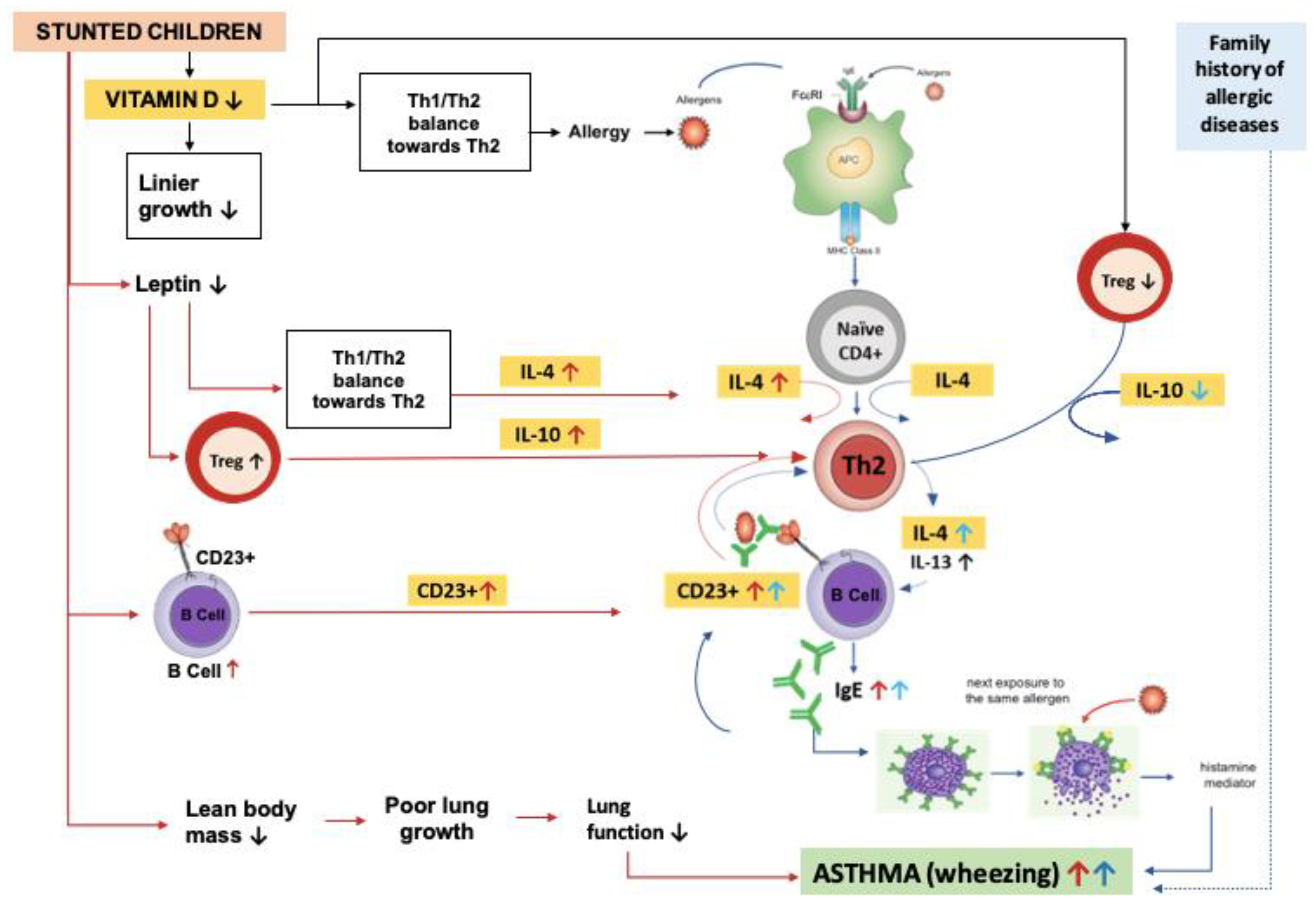
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNICEF; WHO; World Bank Group. Levels and Trends in Child Malnutrition; UNICEF: New York, NY, USA; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Kemenkes, R.I. Hasil Utama Riset Kesehatan Dasar (RISKESDAS) Tahun 2018; Kemenkes RI: Jakarta, Indonesia, 2018. [Google Scholar]
- WHO. Reducing Stunting in Children: Equity Considerations for Achieving the Global Nutrition Targets 2025; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Hawlader, M.; Noguchi, E.; Arifeen, S.; Persson, L.; Moore, S.; Raqib, R.; Wagatsuma, Y. Nutritional status and childhood wheezing in rural Bangladesh. Public Health Nutr. 2013, 17, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, R.; Holick, M.; Sempertegui, F.; Griffiths, J.; Estrella, B.; Moore, L.; Fox, M.; Hamer, H.D. Vitamin D status is associated with underweight and stunting in children aged 6–36 months residing in the Ecuadorian Andes. Public Health Nutr. 2017, 21, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Braman, S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef] [PubMed]
- Nurmatov, U.; Devereux, G.; Sheikh, A. Nutrients and foods for the primary prevention of asthma and allergy: Systematic review and meta-analysis. J. Allergy Clin. Immunol. 2011, 127, 724–733. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Weiss, S.T. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007, 120, 1031–1035. [Google Scholar] [CrossRef]
- Esfandiar, N.; Alaei, F.; Fallah, S.; Babaie, D.; Sedghi, N. Vitamin D deficiency and its impact on asthma severity in asthmatic children. Ital. J. Pediatr. 2016, 42, 108. [Google Scholar] [CrossRef]
- Adrian, S.; Chad, W.; Chandrika, C.; Mario, C. Mechanisms of remodeling in asthmatic airways. J. Allergy 2012, 2012, 316049. [Google Scholar] [CrossRef]
- Vasiliou, J.E.; Lui, S.; Walker, S.A.; Chohan, V.; Xystrakis, E.; Bush, A.; Hawrylowicz, C.M.; Saglani, S.; Lloyd, C.M. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy 2014, 69, 1380–1389. [Google Scholar] [CrossRef]
- Gorman, S.; Judge, M.; Hart, P. Topical 1,25-dihydroxyvitamin D3 subverts the priming ability of draining lymph node dendritic cells. Immunology 2010, 131, 415–425. [Google Scholar] [CrossRef]
- Taher, Y.; Esch, B.v.; Hofman, G.; Henricks, P.; Oosterhout, A.v. 1alpha,25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: Role for IL-10 and TGF-beta. J. Immunol. 2008, 180, 5211–5221. [Google Scholar] [CrossRef]
- Lama, M.; Chatterjee, M.; Nayak, C.R.; Chaudhuri, T.K. Increased interleukin-4 and decreased interferon-γ levels in serum of children with asthma. Cytokine 2011, 55, 335–338. [Google Scholar] [CrossRef]
- Maes, T.; Joos, G.F.; Brusselle, G.G. Targeting Interleukin-4 in Asthma: Lost in Translation? Am. J. Respir. Cell Mol. Biol. 2012, 47, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Abd-Alrahman, S.; Draz, H.; Alkharfy, K.; Mohammed, A.K.; Clerici, M.S.; Alokail, M.S. Increased IL-4 mRNA expression and poly-aromatic hydrocarbon concentrations from children with asthma. BMC Pediatr. 2014, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Daher, S.; Santos, L.; Solé, D.; Lima, M.D.; Naspitz, C.; Musatti, C.C. Interleukin-4 and soluble CD23 serum levels in asthmatic atopic children. J. Investig. Allergol. Clin. Immunol. 1995, 5, 251–254. [Google Scholar] [PubMed]
- van der Heijden, F.L.; Joost van Neerven, R.J.; van Katwijk, M.; Bos, J.D.; Kapsenberg, M.L. Serum-IgE-facilitated allergen presentation in atopic disease. J. Immunol. 1993, 150, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Gagroa, A.; Rabatic, S.; Tresdeca, A.; Dekarisa, D.; Medar-Lasicb, M. Expression of lymphocytes FcsRII/CD23 in allergic children undergoing hyposensitization. Int. Arch. Allergy Immunol. 1993, 101, 203–208. [Google Scholar] [CrossRef]
- Sánchez-Ortega, H.; Jiménez-Cortegana, C.; Novalbos-Ruiz, J.P.; Gómez-Bastero, A.; Soto-Campos, J.G.; Sánchez-Margalet, V. Role of Leptin as a Link between Asthma and Obesity: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 24, 546. [Google Scholar] [CrossRef]
- Gerriets, V.A.; MacIver, N.J. Role of T cells in malnutrition and obesity. Front. Immunol. 2014, 5, 379. [Google Scholar] [CrossRef]
- Rodriguez, L.; González, C.; Flores, L.; Jimenez-Zamudio, L.; Graniel, J.; Ortiz, R.o. Assessment by flow cytometry of cytokine production in malnourished children. Clin. Diagn. Lab. Immunol. 2005, 12, 502–507. [Google Scholar] [CrossRef]
- Palacio, A.; Lopez, M.; Perez-Bravo, F.; Monkeberg, F.; Schlesinger, L. Leptin Levels Are Associated with Immune Response in Malnourished Infants. J. Clin. Endocrinol. Metab. 2002, 87, 3040–3046. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Nahar, B.; Haque, A.; Mondal, D.; Mahfuz, M.; Naila, N.N.; Gazi, A.; Hasan, M. Serum adipokines, growth factors, and cytokines Are independently associated with stunting in bangladeshi children. Nutrients 2019, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Hagel, I.; Lynch, N.; Puccio, F.; Rodriguez, O.; Luzondo, R.; Rodríguez, P.; Sánchez, P.; Cabrera, M.; Prisco, M. Defective regulation of the protective IgE response against intestinal helminth Ascaris lumbricoides in malnourished children. J. Trop. Pediatr. 2003, 49, 136–142. [Google Scholar] [CrossRef]
- Sapartini, G.; Wong, G.W.K.; Indrati, A.R.; Kartasasmita, C.B.; Setiabudiawan, B. Stunting as a Risk Factor for Asthma: The Role of Vitamin D, Leptin, IL-4, and CD23. Medicina 2022, 58, 1236. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Borland, G.; Edkins, A.L.; Maclellan, L.M.; Matheson, J.; Ozanne, B.W.; Cushley, W. CD23/FcεRII: Molecular multi-tasking. Clin. Exp. Immunol. 2010, 162, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Duffy, D. Genetics and gene-environment interactions in childhood and adult onset asthma. Front. Pediatr. 2019, 7, 499. [Google Scholar] [CrossRef]
- Nikhita, B.; Padmalatha, P. Study on risk factors for asthma In children admitted In Ggh,Guntur. GJRA 2022, 11, 18. [Google Scholar] [CrossRef]
- Di Cicco, M.; D’Elios, S.; Peroni, D.G.; Comberiati, P. The role of atopy in asthma development and persistence. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 131–137. [Google Scholar] [CrossRef]
- Ali, Z.; Jemec, G.B.E.; Ulrik, C.S. Associations between maternal and environmental exposures on atopic disease in the offspring of mothers with asthma. Immun. Inflamm. Dis. 2021, 9, 862–870. [Google Scholar] [CrossRef]
- Lebold, K.M.; Jacoby, D.B.; Drake, M.G. Inflammatory mechanisms linking maternal and childhood asthma. J. Leukoc. Biol. 2020, 108, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Abarca, N.E.; Garro, A.C.; Pearlman, D.N. Relationship between breastfeeding and asthma prevalence in young children exposed to adverse childhood experiences. J. Asthma 2019, 56, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Saif, N.T.; Kleiner, G.I.; Forster, L.Q.; Hershorin, E.R.; Colin, A.A.; Mirsaeidi, M.; Kumar, N. Allergies, allergic comorbidities and the home environment in pediatric asthma in southern florida. Int. J. Environ. Res. Public. Health 2021, 18, 4142. [Google Scholar] [CrossRef]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of air pollution on asthma outcomes. Int. J. Environ. Res. 2020, 17, 6212. [Google Scholar] [CrossRef]
- Kim, K.-y.; Ko, H.J.; Choi, K.Y.; Seo, S. Effect of Environmental Relationship between Fungal Exposure and Asthma in Children; ISEE: Ottawa, ON, Canada, 2018. [Google Scholar]
- Taniguchi, Y.; Kobayashi, M. Exposure to dogs and cats and risk of asthma: A retrospective study. PLoS ONE 2023, 18, e0282184. [Google Scholar] [CrossRef]
- Budha, M.; Naning, R.; Wati, D. The relationship between contact to cat and the development of asthma in children. Paediatr. Indones. 2009, 49, 379-6. [Google Scholar] [CrossRef]
- Neophytou, A.M.; Oh, S.S.; White, M.J.; Mak, A.C.Y.; Hu, D.; Huntsman, S.; Eng, C.; Serebrisky, D.; Borrell, L.N.; Farber, H.J.; et al. Secondhand smoke exposure and asthma outcomes among African-American and Latino children with asthma. Thorax 2018, 73, 1041–1048. [Google Scholar] [CrossRef]
- Sturm, J.J.; Yeatts, K.; Loomis, D. Effects of tobacco smoke exposure on asthma prevalence and medical care use in North Carolina middle school children. Am. J. Public Health 2004, 94, 308–313. [Google Scholar] [CrossRef]
- Harju, M.; Keski-Nisula, L.; Georgiadis, L.; Heinonen, S. Parental smoking and cessation during pregnancy and the risk of childhood asthma. BMC Public Health 2016, 16, 428. [Google Scholar] [CrossRef]
- Cao, S.; Xie, M.; Jia, C.; Zhang, Y.; Gong, J.; Wang, B.; Qin, N.; Zhao, L.; Yu, D.; Duan, X. Household second-hand smoke exposure and stunted growth among Chinese school-age children. Environ. Technol. Innov. 2022, 17, 5524. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Chouchane, A.I. Role of vitamin D beyond the skeletal function: A review of the molecular and clinical studies. Int. J. Mol. Sci. 2018, 19, 1618. [Google Scholar] [CrossRef] [PubMed]
- Marasinghe, E.; Chackrewarthy, S.; Abeysena, C.; Rajindrajith, S. Micronutrient status and its relationship with nutritional status in preschool children in urban Sri Lanka. Asia Pac. J. Clin. Nutr. 2015, 24, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Walli, N.Z.; Munubhi, E.K.; Aboud, S.; Manji, K.P. Vitamin D Levels in Malnourished Children under 5 Years in a Tertiary Care Center at Muhimbili National Hospital, Dar es Salaam, Tanzania-A Cross-sectional Study. J. Trop. Pediatr. 2017, 63, 203–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Stuijvenberg, M.E.; Nel, J.; Schoeman, S.E.; Lombard, C.J.; du Plessis, L.M.; Dhansay, M.A. Low intake of calcium and vitamin D, but not zinc, iron or vitamin A, is associated with stunting in 2- to 5-year-old children. Nutrition 2015, 31, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Nurhayati, E.; Paramashanti, B.A.; Astiti, D.; Aji, A.S. Dietary diversity, vitamin D intake and childhood stunting: A case-control study in Bantul, Indonesia. Mal. J. Nutr. 2020, 26, 273–287. [Google Scholar] [CrossRef]
- Knuschke, P. Sun exposure and vitamin D. Curr. Probl. Dermatol. 2021, 55, 296–315. [Google Scholar] [CrossRef]
- Nurmatov, U.; Nwaru, B.; Devereux, G.; Sheikh, A. Confounding and effect modification in studies of diet and childhood asthma and allergies. Allergy 2012, 67, 1041–1056. [Google Scholar] [CrossRef]
- Malheiro, A.P.G.; Gianfrancesco, L.; Nogueira, R.J.N.; Grotta, M.B.; Morcillo, A.M.; Ribeiro, J.D.; Toro, A. Association between serum Vitamin D levels and asthma severity and control in children and adolescents. Lung 2023, 201, 181–187. [Google Scholar] [CrossRef]
- Jat, K.R.; Khairwa, A. Vitamin D and asthma in children: A systematic review and meta-analysis of observational studies. Lung India 2017, 34, 355–363. [Google Scholar] [CrossRef]
- Havan, M.; Razi, C.H.; Bulus, A.D.; Koksal, A.O.; Andiran, N. Effects of 25 hydroxy vitamin D levels on the severity and asthma control in school age asthma patients. Arch. Argent. Pediatr. 2017, 115, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Bendik, I.; Friedel, A.; Roos, F.F.; Weber, P.; Eggersdorfer, M. Vitamin D: A critical and essential micronutrient for human health. Front. Physiol. 2014, 5, 248. [Google Scholar] [CrossRef]
- Gorman, S.; Judge, M.; Burchell, J.; Turner, D.; Hart, P. 1,25-dihydroxyvitamin D3 enhances the ability of transferred cd4+ cd25+ cells to modulate t helper type 2-driven asthmatic responses. Immunology 2010, 130, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Lama, M.; Chatterjee, M.; Chaudhuri, T.K. Total serum immunoglobulin e in children with asthma. Indian. J. Clin. Biochem. 2013, 28, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Pelaia, C.; Heffler, E.; Crimi, C.; Maglio, A.; Vatrella, A.; Pelaia, G.; Canonica, G.W. Interleukins 4 and 13 in asthma: Key pathophysiologic cytokines and druggable molecular targets. Front. Pharmacol. 2022, 13, 851940. [Google Scholar] [CrossRef] [PubMed]
- Elsaid, A.; Shoaib, R.M.S.; Badr, S.S.; Wahba, Y.; Ayyad, S.-E.N. Polymorphisms of interleukin 4 and interleukin 4 receptor genes and bronchial asthma risk among Egyptian children. Clin. Biochem. 2021, 93, 66–72. [Google Scholar] [CrossRef]
- Al eterie, S.; Al khouatli, K. Serum Levels of IL-4 and IgE in bronchial asthma in syrian children. JCHPS 2017, 10, 880–883. [Google Scholar]
- Makieieva, N.; Malakhova, V.; Vasylchenko, Y.; Tsymbal, V. Are level of IL-13 and IL-4 predictive for formation of chronic inflammation in children with asthma? Adv. Respir. Med. 2020, 88, 320–326. [Google Scholar] [CrossRef]
- Rodríguez, L.; Graniel, J.; Ortiz, R. Effect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected children. Clin. Exp. Immunol. 2007, 148, 478–485. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Hawrylowicz, C.M.; O’Garra, A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005, 5, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Li, C.C.; Xu, D.P.; Lin, A.; Bao, W.G.; Yang, G.S.; Yan, W.H. Analysis of the plasma soluble human leukocyte antigen–G and interleukin-10 levels in childhood atopic asthma. Hum. Immunol. 2010, 71, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, B.; Aydemir, C.; Ustundag, G.; Eldes, N.; Kutsal, E.; Can, M. The effect of treatment with montelukast on levels of serum interleukin-10, eosinophil cationic protein, blood eosinophil counts, and clinical parameters in children with asthma. Turk. J. Pediat. 2009, 51, 460–465. [Google Scholar]
- Tsai, T.-C.; Lu, J.-H.; Chen, S.-J.; Tang, R.-B. Soluble interleukin-10 and transforming growth factor-β in children with acute exacerbation of allergic asthma. J. Asthma 2009, 46, 21–24. [Google Scholar] [CrossRef]
- Kehry, M.; Yamashita, L. Low-affinity IgE receptor (CD23) function on mouse B cells: Role in IgE-dependent antigen focusing. Proc. Natl. Acad. Sci. USA 1989, 86, 7556–7560. [Google Scholar] [CrossRef]
- van Neerven, R.; Knol, E.; Ejrnaes, A.; Wurtzen, P. IgE-mediated allergen presentation and blocking antibodies: Regulation of T-cell activation in allergy. Int. Arch. Allergy Immunol. 2006, 141, 119–129. [Google Scholar] [CrossRef]
- Chary, A.V.; Hemalatha, R.; Murali, M.V.; Jayaprakash, D.; Kumar, B.D. Association of T-regulatory cells and CD23/CD21 expression with vitamin D in children with asthma. Ann. Allergy Asthma Immunol. 2016, 116, 447–454.e2. [Google Scholar] [CrossRef]
- Aberlc, N.; Gagro, A.; Rabalic, S.; Rcinci-Banovac, Z.; Dckaris, D. Expression of CD23 antigen and its ligands in children with intrinsic and extrinsic asthma. Allergy 1997, 52, 1238–1242. [Google Scholar] [CrossRef]
- Oettgen, H.C.; Geha, R.S. IgE regulation and roles in asthma pathogenesis. J. Allergy Clin. Immunol. 2001, 107, 429–440. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Kabesch, M. Current concepts of IgE regulation and impact of genetic determinants. Clin. Exp. Allergy 2012, 42, 852–871. [Google Scholar] [CrossRef]

| Characteristic | Stunted Asthmatic (n = 37) | Stunted without Asthma (n = 38) | Non-Stunted Asthmatic (n = 24) | p-Value b |
|---|---|---|---|---|
| Sex | ||||
| Male | 22 (59.5%) | 17 (44.7%) | 15 (62.5%) | 0.294 |
| Female | 15 (40.5%) | 21 (55.3%) | 9 (37.5%) | |
| Age (months) Mean (SD) | 41.4 (9.9) | 39.7(9.6) | 45.5 (10.3) | 0.082 |
| Weight for height | ||||
| Normal | 36 (97.3%) | 36 (94.7%) | 24 (100%) | 0.364 |
| Wasted | 1 (2.7%) | 2 (5.3%) | 0 | |
| Asthma severity | ||||
| Intermittent | 26 (70.3%) | - | 16 (66.7%) | 0.767 |
| Mild Persistent | 11 (29.7%) | - | 8 (33.3%) | |
| Allergen exposure | ||||
| Cat ownership when 0–12 months old | 4 (10.8%) | 2 (5.3%) | 3 (12.5%) | 0.565 |
| Cat ownership in the past 12 months | 4 (10.8%) | 3 (7.9%) | 3 (12.5%) | 0.828 |
| Dog ownership when 0–12 months old | 0 | 0 | 1 | 0.206 |
| Dog ownership in the past 12 month | 0 | 0 | 0 | - |
| History of allergic diseases | ||||
| Father with atopy | 10 (27.0%) | 2 (5.3%) | 5 (20.8%) | 0.038 |
| Mother with atopy | 20 (54.1%) | 5 (13.2%) | 11 (45.8%) | 0.001 |
| Both father and mother with atopy | 5 (13.5%) | 1 (1.6%) | 2 (8.3%) | 0.224 |
| Siblings with atopy | 12 (32.4%) | 3 (7.9%) | 4 (16.7%) | 0.025 |
| Smoking exposure | ||||
| Yes | 33 (89.2%) | 32 (84.2%) | 18 (75.0%) | 0.338 |
| No | 4 (10.8%) | 6 (15.8%) | 6 (25.0%) | |
| Delivery | ||||
| Vaginal | 33 (89.2%) | 31 (81.6%) | 19 (79.2%) | 0.519 |
| Caesarean section | 4 (10.8%) | 7 (18.4%) | 2 (20.8%) | |
| Lactation | ||||
| Exclusive Breastfeeding | 5 (13.5%) | 8 (21.1%) | 17 (70.8%) | <0.001 |
| Breastfeeding and Formula milk | 32 (86.5%) | 30 (78.9%) | 7 (29.2%) | |
| Formula milk | - | - | - | |
| Birth weight | ||||
| <2500 g | 5 (13.5%) | 3 (7.9%) | 2 (8.3%) | 0.684 |
| ≥2500 g | 32 (86.5%) | 35 (92.1%) | 22 (91.7%) | |
| Gestational age | ||||
| Preterm | 2 (5.4%) | 3 (7.9%) | 1 (4.2%) | 0.807 |
| Term | 34 (91.9%) | 32 (84.2%) | 21 (87.5%) | |
| Post-term | 1 (2.7%) | 3 (7.9%) | 2 (8.3%) | |
| Leukocyte count (×103 cells/mm3) Mean (SD) | 10.50 (2.98) | 9.78 (2.26) | 10.97 (2.94) | 0.288 |
| Group | Stunted Asthmatic (n = 37) | Stunted without Asthma (n = 38) | Non-Stunted Asthmatic (n = 24) | p-Values | |
|---|---|---|---|---|---|
| Category a | |||||
| Deficiency ≤20 ng/mL | 18 (48.6%) | 17 (44.7%) | 5 (20.8%) | 0.171 b | |
| Insufficiency 21–29 ng/mL | 15 (40.5%) | 14 (36.8%) | 12 (50%) | ||
| Sufficient ≥30 ng/mL | 4 (10.8%) | 7 (18.4%) | 7 (29.2%) | ||
| Group | Stunted Asthmatic (n = 37) | Stunted without Asthma (n = 38) | Non-Stunted Asthmatic (n = 24) | p-Value b | |
|---|---|---|---|---|---|
| Variable | |||||
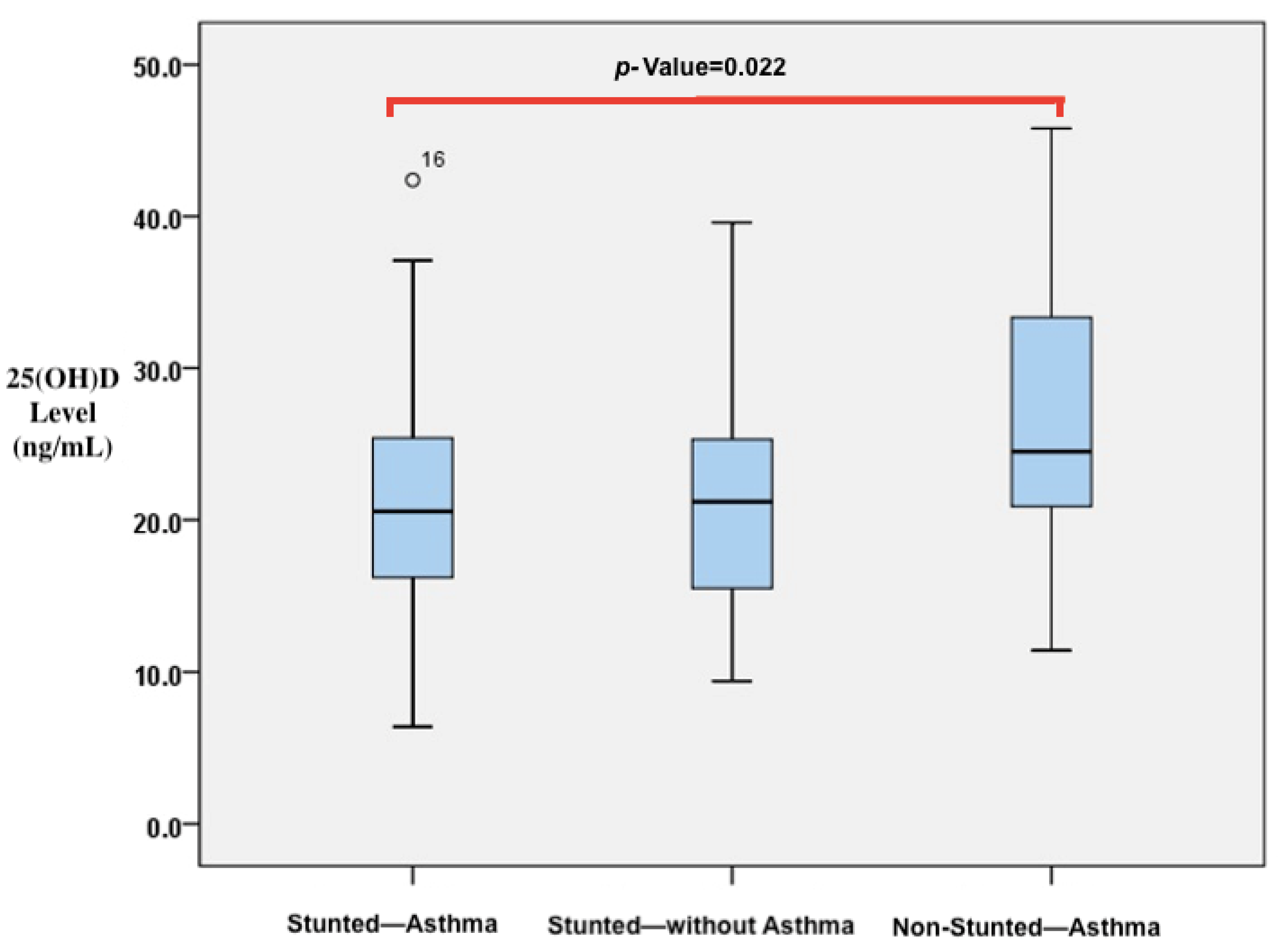
| 25(OH)D level (ng/mL) | 20.55 (16.18–25.55) c | 21.2 (15.45–25.4) c | 24.50 (20.90–34.02) d | 0.042 | |
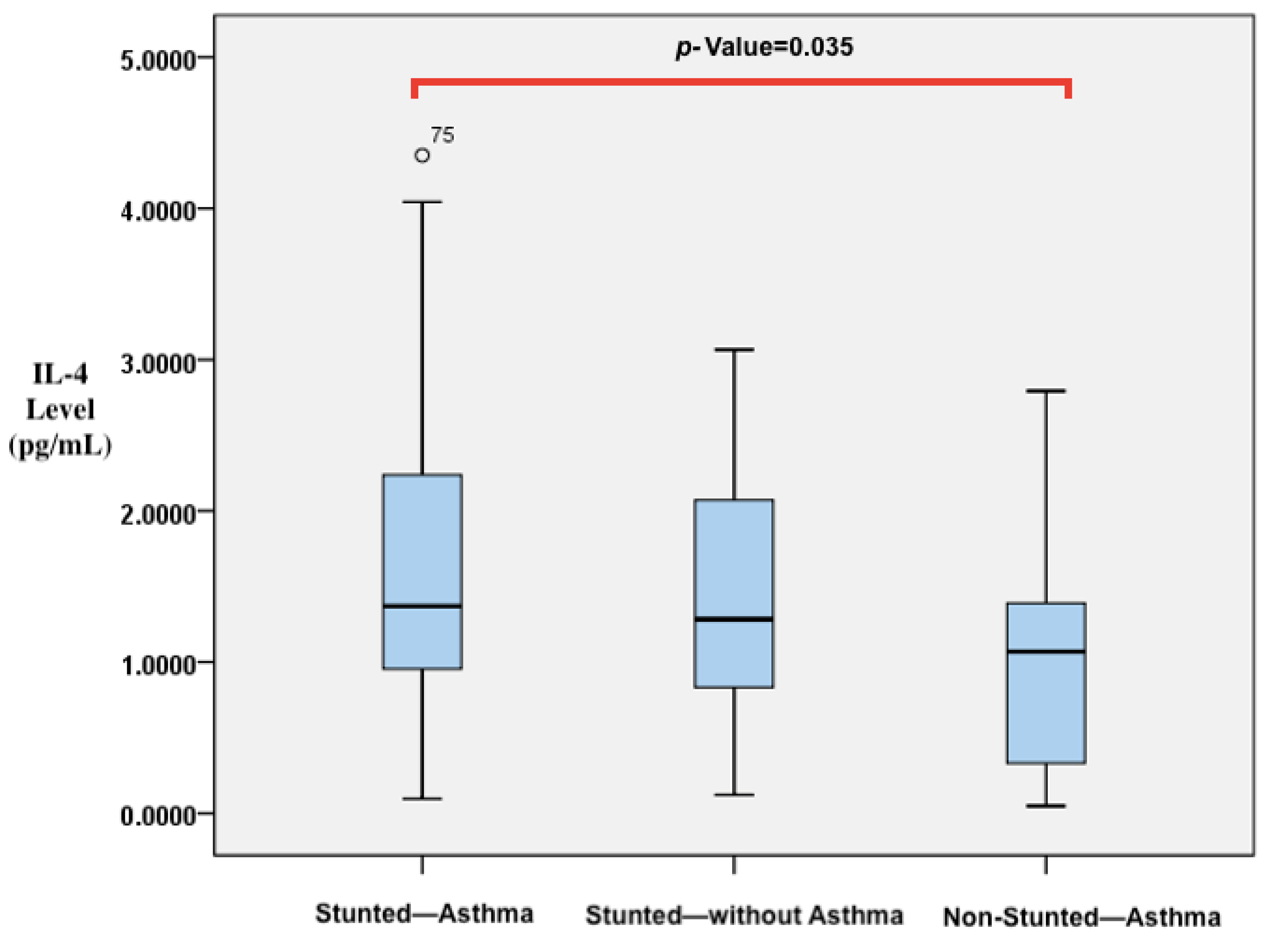
| IL-4 level (pg/mL) | 1.41 (0.95–2.40) c | 1.34 (0.83–2.17) c | 1.09 (0.30–1.58) d | 0.038 | |
| IL-10 level (pg/mL) | 0.76 (0.26–1.68) c | 0.87 (0.26–1.81) c | 1.07 (0.18–1.64) c | 0.956 | |
| Ratio IL-4/IL-10 | 1.963 (0.744–5.610) c | 1.716 (0.848–6.353) c | 1.221 (0.423–2.084) c | 0.099 | |
| Expression of CD23+/Percentage | 39.1 (30.65–50.55) c | 38.75 (32.02–50.45) c | 37.05 (12.02–48.78) c | 0.393 | |
| Expression of CD23+/MFI | 982 (747–1367) c | 939 (770–1272) c | 763 (611–1228) c | 0.091 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapartini, G.; Wong, G.W.K.; Indrati, A.R.; Kartasasmita, C.B.; Setiabudiawan, B. The Association between Vitamin D, Interleukin-4, and Interleukin-10 Levels and CD23+ Expression with Bronchial Asthma in Stunted Children. Biomedicines 2023, 11, 2542. https://doi.org/10.3390/biomedicines11092542
Sapartini G, Wong GWK, Indrati AR, Kartasasmita CB, Setiabudiawan B. The Association between Vitamin D, Interleukin-4, and Interleukin-10 Levels and CD23+ Expression with Bronchial Asthma in Stunted Children. Biomedicines. 2023; 11(9):2542. https://doi.org/10.3390/biomedicines11092542
Chicago/Turabian StyleSapartini, Gartika, Gary W. K. Wong, Agnes Rengga Indrati, Cissy B. Kartasasmita, and Budi Setiabudiawan. 2023. "The Association between Vitamin D, Interleukin-4, and Interleukin-10 Levels and CD23+ Expression with Bronchial Asthma in Stunted Children" Biomedicines 11, no. 9: 2542. https://doi.org/10.3390/biomedicines11092542
APA StyleSapartini, G., Wong, G. W. K., Indrati, A. R., Kartasasmita, C. B., & Setiabudiawan, B. (2023). The Association between Vitamin D, Interleukin-4, and Interleukin-10 Levels and CD23+ Expression with Bronchial Asthma in Stunted Children. Biomedicines, 11(9), 2542. https://doi.org/10.3390/biomedicines11092542







