Vasodilatory Peripheral Response and Pain Levels following Radiofrequency Stressor Application in Women with Fibromyalgia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Definition of Variables
2.2.1. Peripheral Vascular Assessment and Central Thermoregulation
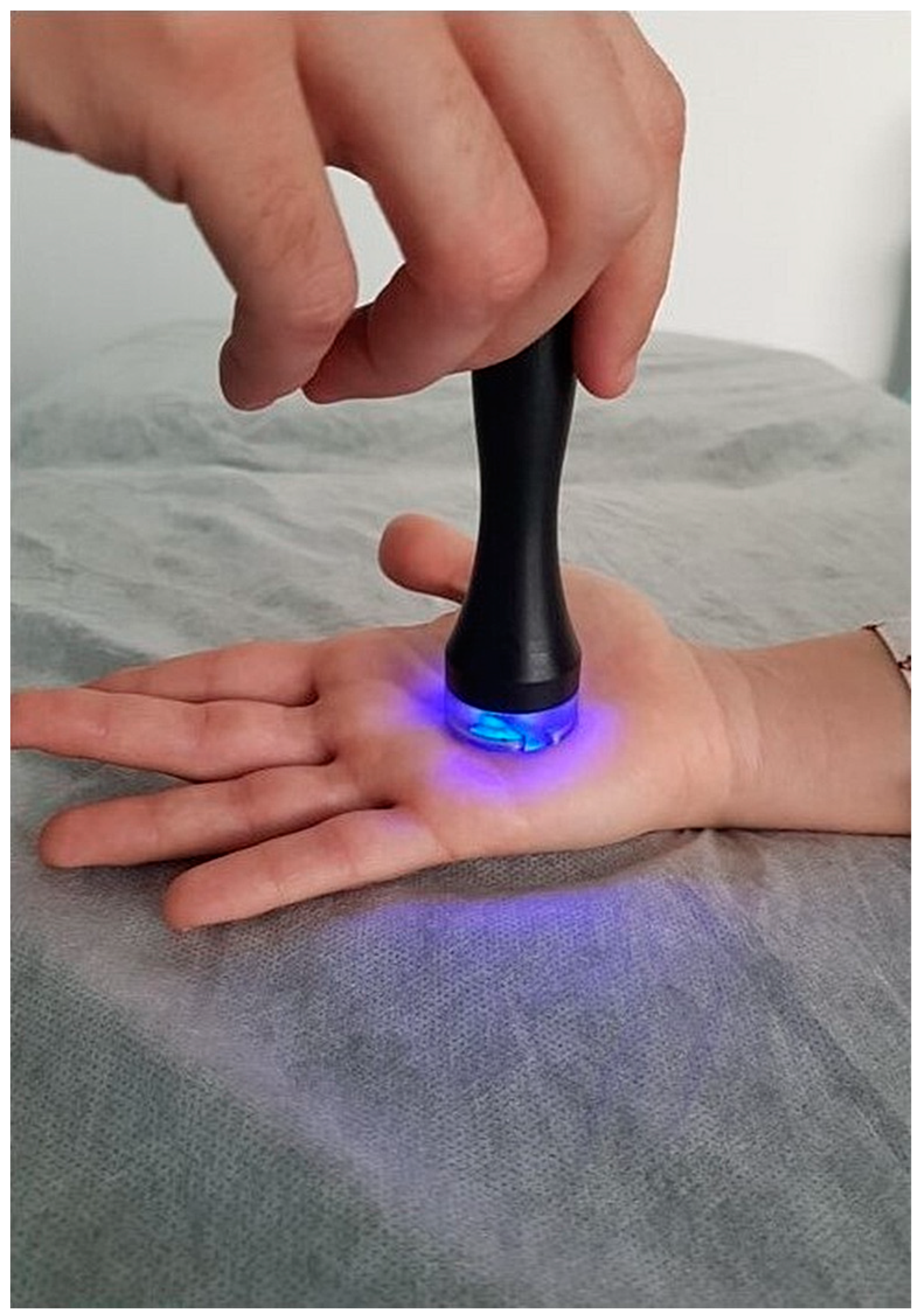
2.2.2. Radiofrequency Vasodilation
2.2.3. Subjective Perception of Pain and Determination of Pressure Pain Thresholds
2.2.4. Assessment of Pain in Response to Electrical Stimuli
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Differences in Pain Levels following Thermal Heat Stimulus
3.3. Differences in Vascular Response following Thermal Heat Stimulus
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Gayà, T.F.; Ferrer, C.B.; Mas, A.J.; Seoane-Mato, D.; Reyes, F.Á.; Sánchez, M.D.; Dubois, C.M.; Sánchez-Fernández, S.A.; Vargas, L.M.R.; Morales, P.G.; et al. Prevalence of fibromyalgia and associated factors in Spain. Clin. Exp. Rheumatol. 2020, 38, 47–52. [Google Scholar]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Ablin, J.; Fitzcharles, M.-A.; Littlejohn, G.; Luciano, J.V.; Usui, C. Fibromyalgia. Nat. Rev. Dis. Primers 2020, 1, 15022. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, P.J.; Hou, Q.; Argoff, C.E.; Storey, J.R.; Wymer, J.P.; Rice, F.L. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: Implications for widespread deep tissue pain and fatigue. Pain Med. 2013, 14, 895–915. [Google Scholar] [CrossRef]
- Choi, D.H.; Kim, H.S. Quantitative analysis of nailfold capillary morphology in patients with fibromyalgia. Korean J. Intern. Med. 2015, 30, 531–537. [Google Scholar] [CrossRef]
- Walløe, L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature 2016, 3, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Schlereth, T.; Schukraft, J.; Krämer-Best, H.H.; Geber, C.; Ackermann, T.; Birklein, F. Interaction of calcitonin gene related peptide (CGRP) and substance P (SP) in human skin. Neuropeptides 2016, 59, 5762. [Google Scholar] [CrossRef]
- Grayston, R.; Czanner, G.; Elhadd, K.; Goebel, A.; Frank, B.; Üçeyler, N.; Malik, R.A.; Alam, U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Semin. Arthritis Rheum. 2019, 48, 933–940. [Google Scholar] [CrossRef]
- Evdokimov, D.; Dinkel, P.; Frank, J.; Sommer, C.; Üçeyler, N. Characterization of dermal skin innervation in fibromyalgia syndrome. PLoS ONE 2020, 15, e0227674. [Google Scholar] [CrossRef]
- Kasikcioglu, E.; Dinler, M.; Berker, E. Reduced tolerance of exercise in fibromyalgia may be a consequence of impaired microcirculation initiated by deficient action of nitric oxide. Med. Hypotheses 2006, 66, 950–952. [Google Scholar] [CrossRef]
- Katz, D.L.; Greene, L.; Ali, A.; Faridi, Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med. Hypotheses 2007, 69, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, T.; Van Lantschoot, A.; Van Boxem, K.; Van Zundert, J. Pulsed radiofrequency in chronic pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.; Catapano, M.; Sahni, S.; Ma, F.; Abd-Elsayed, A.; Visnjevac, O. Pulsed Radiofrequency in Interventional Pain Management: Cellular and Molecular Mechanisms of Action—An Update and Review. Pain Physician 2021, 24, 525–532. [Google Scholar]
- Brusselmans, G.; Nogueira, H.; De Schamphelaere, E.; Devulder, J.; Crombez, G. Skin Temperature during Cold Pressor Test in Fibromyalgia: An Evaluation of the Autonomic Nervous System? Acta Anaesthesiol. Belg. 2015, 66, 19–27. [Google Scholar]
- Smith, B.W.; Tooley, E.M.; Montague, E.Q.; Robinson, A.E.; Cosper, C.J.; Mullins, P.G. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain 2008, 140, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J. Fibromyalgia. JAMA 2014, 311, 1547. [Google Scholar] [CrossRef]
- Casas-Barragán, A.; García-Ríos, M.C.; Rus, A.; Tapia-Haro, R.M.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Associations among serum VEGF and CGRP levels with the peripheral vascular blood flow of the skin of the hands in women with Fibromyalgia. J. Therm. Biol. 2023, 112, 103469. [Google Scholar] [CrossRef]
- Gasim, G.I.; Musa, I.R.; Abdien, M.T.; Adam, I. Accuracy of tympanic temperature measurement using an infrared tympanic membrane thermometer. BMC Res. Notes 2013, 6, 194. [Google Scholar] [CrossRef]
- Ibáñez-Vera, A.J.; García-Romero, J.C.; Alvero-Cruz, J.R.; Lomas-Vega, R. Effects of monopolar dielectric radiofrequency signals on the symptoms of fibromyalgia: A single-blind randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 2465. [Google Scholar] [CrossRef]
- Jensen, M.P.; Castarlenas, E.; Roy, R.; Tomé Pires, C.; Racine, M.; Pathak, A.; Miró, J. The Utility and Construct Validity of Four Measures of Pain Intensity: Results from a University-Based Study in Spain. Pain Med. 2019, 20, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Assumpção, A.; Matsutani, L.A.; Pereira, C.A.B.; Lage, L. Pain in fibromyalgia and discrimination power of the instruments: Visual Analog Scale, Dolorimetry and the McGill Pain Questionnaire. Acta Reumatol. Port. 2008, 33, 345–351. [Google Scholar] [PubMed]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Alstergren, P.; Förström, J. Acute oral pain intensity and pain threshold assessed by intensity matching to pain induced by electrical stimuli. J. Orofac. Pain 2003, 17, 151–159. [Google Scholar]
- Käll, K.B.; Kowalski, J.; Stener-Victorin, E. Assessing pain perception using the Painmatcher in patients with whiplash-associated disorders. J. Rehabil. Med. 2008, 40, 171–177. [Google Scholar] [CrossRef]
- Lund, I.; Lundeberg, T.; Kowalski, J.; Sandberg, L.; Norrbrink-Budh, C.; Svensson, E. Evaluation of variations in sensory and pain threshold assessments by electrocutaneous stimulation. Physiother. Theory Pract. 2005, 21, 81–92. [Google Scholar] [CrossRef]
- Tsilioni, I.; Russell, I.J.; Stewart, J.M.; Gleason, R.M.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 3, 664–672. [Google Scholar] [CrossRef]
- Hagiwara, S.; Iwasaka, H.; Takeshima, N.; Noguchi, T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: Roles of descending adrenergic and serotonergic systems. Eur. J. Pain 2009, 13, 249–252. [Google Scholar] [CrossRef]
- Nagasaka, T.; Hirata, K.; Nunomura, T. Contribution of arteriovenous anastomoses to vasoconstriction induced by local heating of the human finger. Jpn. J. Physiol. 1987, 37, 425–433. [Google Scholar] [CrossRef]
- Bergersen, T.K.; Eriksen, M.; Walløe, L. Effect of local warming on hand and finger artery blood velocities. Am. J. Physiol. 1995, 269, R325–R330. [Google Scholar] [CrossRef]
- Rein, E.B.; Walløe, L. No Evidence of Heat-Induced Vasoconstriction in Human Skin Using Ultrasound Dop-pler after Passive Whole-Body Heat Stress. J. Cardiovasc. Med. 2020, 105, 1–7. [Google Scholar] [CrossRef]
- Hales, J.R.S.; Jessen, C.; Fawcett, A.A.; King, R.B. Skin AVA and capillary dilatation and constriction induced by local skin heating. Arch. Eur. J. Physiol. 1985, 404, 203–207. [Google Scholar] [CrossRef]
- De Tommaso, M.; Vecchio, E.; Nolano, M. The puzzle of fibromyalgia between central sensitization syndrome and small fiber neuropathy: A narrative review on neurophysiological and morphological evidence. Neurol. Sci. 2022, 3, 1667–1684. [Google Scholar] [CrossRef]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C nociceptors in fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lavín, M. Fibromyalgia and small fiber neuropathy: The plot thickens! Clin. Rheumatol. 2018, 37, 3167–3171. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Hollis, S.; Campbell, F.; Moore, T.; Jayson, M.; Herrick, A. The “distal-dorsal difference” as a possible predictor of secondary Raynaud’s phenomenon. J. Rheumatol. 1999, 26, 1125–1128. [Google Scholar]
- Mariotti, A.; Grossi, G.; Amerio, P.; Orlando, G.; Mattei, P.A.; Tulli, A.; Romani, G.L.; Merla, A. Finger thermoregulatory model assessing functional impairment in Raynaud’s phenomenon. Ann. Biomed. Eng. 2009, 37, 2631–2639. [Google Scholar] [CrossRef]




| Women with FM (n = 29) | Healthy Women (n = 17) | ||
|---|---|---|---|
| Variable | Mean ± SD/Frequency (%) | Mean ± SD/Frequency (%) | p-Value |
| Age (years) | 54.90 ± 7.52 | 58.12 ± 4.83 | 0.121 |
| Height (cm) | 159.24 ± 5.47 | 159.24 ± 7.28 | 0.997 |
| Weight (kg) | 68.66 ± 13.20 | 66.20 ± 11.80 | 0.530 |
| BMI (kg/cm2) | 28.29 ± 7.48 | 26.17 ± 4.69 | 0.298 |
| Duration of FM (years) | 15.44 ± 9.17 | - | |
| Menopause status | |||
| Pre-menopausal | 21 (72.41) | 14 (82.35) | 0.446 |
| Post-menopausal | 8 (27.59) | 3 (17.65) | |
| Smoking history | |||
| Active smoker | 14 (48.28) | 10 (58.82) | |
| Non-smoker | 15 (51.72) | 7 (41.18) | |
| Comorbidity | |||
| Chronic fatigue | 10 (34.48) | 0 | 0.001 * |
| Chemical hypersensitivity | 3 (10.35) | 0 | |
| Painful bladder syndrome | 7 (24.13) | 2 (11.76) | |
| Non-comorbidity | 9 (31.04) | 15 (88.24) | |
| Pharmacologic history | |||
| Corticosteroids | 2 (6.90) | 0 | 0.268 |
| Analgesics | 8 (27.59) | 1 (5.88) | |
| Antidepressants | 4 (13.80) | 1 (5.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Barragán, A.; Muñoz-Revilla, A.; Tapia-Haro, R.M.; Molina, F.; Correa-Rodríguez, M.; Aguilar-Ferrándiz, M.E. Vasodilatory Peripheral Response and Pain Levels following Radiofrequency Stressor Application in Women with Fibromyalgia. Biomedicines 2024, 12, 142. https://doi.org/10.3390/biomedicines12010142
Casas-Barragán A, Muñoz-Revilla A, Tapia-Haro RM, Molina F, Correa-Rodríguez M, Aguilar-Ferrándiz ME. Vasodilatory Peripheral Response and Pain Levels following Radiofrequency Stressor Application in Women with Fibromyalgia. Biomedicines. 2024; 12(1):142. https://doi.org/10.3390/biomedicines12010142
Chicago/Turabian StyleCasas-Barragán, Antonio, Alba Muñoz-Revilla, Rosa María Tapia-Haro, Francisco Molina, María Correa-Rodríguez, and María Encarnación Aguilar-Ferrándiz. 2024. "Vasodilatory Peripheral Response and Pain Levels following Radiofrequency Stressor Application in Women with Fibromyalgia" Biomedicines 12, no. 1: 142. https://doi.org/10.3390/biomedicines12010142
APA StyleCasas-Barragán, A., Muñoz-Revilla, A., Tapia-Haro, R. M., Molina, F., Correa-Rodríguez, M., & Aguilar-Ferrándiz, M. E. (2024). Vasodilatory Peripheral Response and Pain Levels following Radiofrequency Stressor Application in Women with Fibromyalgia. Biomedicines, 12(1), 142. https://doi.org/10.3390/biomedicines12010142





