Abstract
Fibromyalgia (FM) is a chronic pain syndrome hypothesized to arise from a state of neurogenic inflammation. Mechanisms responsible for pain, as well as psychological variables, are typically altered in this condition. The main objective of this research was to explore somatosensory and psychological alterations in women with FM. The secondary objective was to carry out a secondary analysis to correlate the different variables studied and delve into the influences between them. The relationship between different psychological variables in fibromyalgia is not clear in the previous scientific literature. Forty-four individuals participated, of which twenty-two were controls and twenty-two were women with fibromyalgia. The main outcome measures were the Numeric Pain Rating Scale, Fibromyalgia Impact Questionnaire, pressure pain threshold, conditioned pain modulation, anxiety and depression symptoms, catastrophizing and kinesiophobia cognitions. The main analysis showed that there is a moderate correlation between the psychological variables of depression and fear of movement and the ability to modulate pain. There is also a moderately inverse correlation between pain catastrophizing cognitions and pain intensity/disability. Multiple moderate and strong correlations were found among the various psychological variables studied. FM patients exhibit somatosensory alterations alongside negative psychological symptoms that influence the experience of pain, and they may perpetuate the state of neurogenic inflammation.
1. Introduction
Fibromyalgia syndrome (FM) is a neurogenic inflammation condition characterized by chronic pain as the main symptom, associated with the presence of other symptoms of similar relevance, such as cognitive disorders, fatigue, restless sleep or the presence of psychological symptoms [1,2]. The diagnosis of FM is exclusively clinical since its etiology remains unknown. The clinical status of FM is heterogeneous in the population; even in the same person, the symptoms can fluctuate daily [3]. In Madrid, Spain, 5% of women aged 46 to 60 are affected, according to epidemiological research [4]. The presence of psychological symptoms can aggravate the painful experience suffered by FM patients. Both negative emotional states such as depression and the presence of cognitive distortions such as pain catastrophizing and fear-related movement may be risk factors for suffering from chronic pain for a longer period, increasing the intensity and the impact on functionality [5,6,7,8,9]. Unfortunately, as shown in recent longitudinal epidemiological studies [10,11], psychological symptoms have worsened owing to the health crisis caused by the COVID-19 pandemic, which may have influenced the clinical condition of FM patients [10,11].
Research into the pathophysiological mechanisms responsible for the pain suffered by patients with FM is of great interest in the scientific community [12]. Several alterations in the nociceptive system have been identified, including the transmission, processing and modulation of painful stimuli [13,14,15,16,17,18,19]. One of the most relevant clinical characteristics in patients with FM is the dysfunction in the pain inhibitory systems. It is suggested that the endogenous capacity to modulate pain is reduced, which translates into greater central pain processing [19].
Conditioned pain modulation (CPM) is a dynamic psychophysical test that reflects the capacity of the descending pain modulatory systems to decrease pain [20]. The CPM effect can be quantified by comparing the pain response to a noxious test stimulus applied before and during (or immediately after) a noxious conditioning stimulus in another body region. In healthy individuals, the painful conditioning stimulus should increase the pain threshold by triggering an efficient response from the descending inhibitory system. Nevertheless, results obtained in the CPM show great inter-individual variability, possibly due to differences establishing CPM protocols [21,22]. The CPM test may be reliable to demonstrate a state of neurogenic inflammation present in patients with FM. Thereby, the dysfunction of pain inhibitory mechanisms is evident, which is characteristic of neurogenic inflammation conditions.
On the other hand, emotional psychological symptoms such as anxiety and depression and cognitive psychological symptoms including catastrophism or fear-related movement are shown in neurogenic inflammation conditions [23]. Even though all these are existing variables in patients with FM, their influence on each other remains unclear (e.g., whether or not suffering from anxiety can trigger cognitive alterations such as fear of movement). Furthermore, especially in patients with FM, it is unknown whether these alterations are secondary to a state of chronic pain or whether these alterations might instead cause a condition of neurogenic inflammation and central sensitization. The main objective of this research was to explore somatosensory and psychological alterations in women with FM. The secondary objective was to carry out a secondary analysis to correlate the different variables studied and delve into the influences between them. The role of psychological factors in FM is often studied, although the direction of the relationship remains unclear. Given that the evidence remains weak, increasing knowledge of the relationship between psychological aspects and somatosensory variables of pain can help improve clinical decision-making in patients with FM and help us to find the most appropriate treatment for each case.
2. Materials and Methods
2.1. Participants
The study involved twenty-two female FM patients (FM) and twenty-two healthy female control subjects recruited through local support group advertisements and presentations. Data collection occurred from February to December 2023 and received approval from the Rey Juan Carlos University Ethical Review Board (2605202012920) following the Declaration of Helsinki. Convenience sampling method was used. All participants provided written informed consent. Inclusion criteria for FM patients included: (1) medical diagnosis of fibromyalgia by a rheumatologist specialist, (2) experiencing pain for over 3 months and (3) fluency in spoken and written Spanish. The criteria of speaking and understanding Spanish correctly did not exclude any participant for any ethnic or social reason. A researcher was present to ensure that participants understood the asked tasks and to answer any questions related to the self-administered questionnaires. Exclusion criteria for FM patients comprised cognitive inability to understand or complete measurement variables. Control participants met criteria for having no pain (NPRS = 0) and fluency in spoken and written Spanish. Exclusions for control subjects included recent musculoskeletal pain episodes within the last 12 weeks and any rheumatologic diseases. A flowchart is shown in Figure 1.
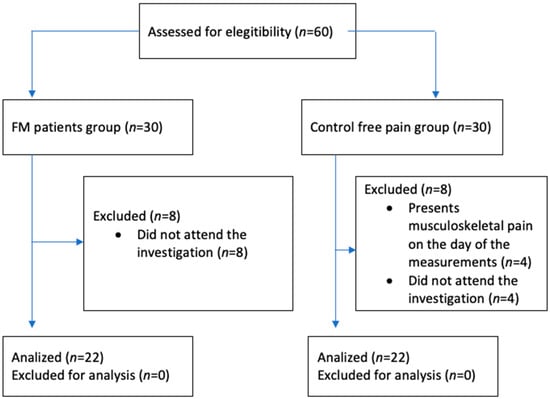
Figure 1.
Study design. The subsequent flowchart illustrates the allocation of participants in both the FM study and control groups. Instances that were excluded due to failure to meet selection criteria or withdrawal from the investigation are depicted.
2.2. Clinical Status
Pain intensity was assessed using the Numeric Pain Rating Scale (NPRS), a validated tool extensively employed for self-reported evaluation of perceived pain intensity among individuals coping with chronic pain [24]. It consists of an 11-point scale where 0 indicates no pain and 10 indicates the worst pain imaginable. NPRS scores are interpreted as follows: 0 = no pain; 1–3 = mild pain; 4–6 = moderate pain; 7–10 = severe pain. The recent scientific literature strongly recommends the NPRS as the foremost choice, attributing to its heightened sensitivity and consistent measurement of pain intensity [25].
The impact of FM on patients’ daily functioning and the resulting disability was appraised using the Spanish iteration of the Fibromyalgia Impact Questionnaire (FIQ). It is composed of 10 items that allow us to assess the degree of interference from FM symptoms in the person’s daily functioning during the last week. The first four items assess physical function, well-being and work performance using Likert-type scales. The rest of the items are answered using a 10 cm visual analogue scale (VAS) and collect information on pain, fatigue, rest quality, stiffness, anxiety and depression. The score of the Likert scale is transformed to be expressed in a range from 0 to 10. In VAS, the given value is the score for each scale. The global impact index is obtained by adding the transformed scores on the ten scales described, ranging from 0 to 100, 100 being the highest impairment caused by the syndrome. This adapted questionnaire has undergone validation for the Spanish population, demonstrating commendable sensitivity, specificity and internal consistency [26,27].
2.3. Pressure Pain Threshold (PPT)
Pressure pain threshold (PPT) is defined as the minimum pressure required, under standardized test conditions, to elicit the slightest sensation of pain. It stands as a reliable gauge for evaluating pain sensitivity. A digital algometer (Model FXD 10, Wagner Instruments, Greenwich, CT, USA) measuring pressure in kg/cm2 was employed for this purpose. Pressure was incrementally increased on the right upper trapezius muscle at a rate of one kilogram per second until the subject reported the sensation becoming painful. Each measurement was conducted thrice, and the resulting mean value was used as the final recorded measurement. This methodology has demonstrated notably high intra-examiner reliability (ICC values = 0.97) and substantial inter-examiner reliability (ICC values = 0.79) in the upper trapezius muscle region among healthy individuals. In individuals with FM, this method has been validated to assess mechanical hyperalgesia, yielding an ICC value of 0.88 [28,29,30].
2.4. Conditioned Pain Modulation
The cold pressor test paradigm was conducted following these steps: initially, a PPT measurement was taken on the upper area of the right trapezius muscle, as previously described. Subsequently, the left arm was immersed in a constant 12 °C water bath for 60 s. Finally, another PPT measurement was obtained on the upper part of the right trapezius muscle. The Conditioned Pain Modulation (CPM) value is calculated by subtracting the PPT value during the conditioning stimulus from the PPT value without the conditioning stimulus. In healthy individuals whose descending inhibitory pain system works correctly, it is expected that the second algometry measurement shows increased pressure tolerance with respect to the initial measurement before reaching the painful threshold. In chronic pain populations and healthy individuals, the cold pressor test as a conditioning stimulus has demonstrated favorable intra-session reliability (ICC = 0.77 and ICC = 0.64, respectively) [31].
2.5. Psychological Variables
Psychological variables were evaluated through self-administered questionnaires, with a researcher present throughout the process to address any inquiries from participants. Various validated scales were employed to assess emotional and cognitive aspects, as detailed below.
2.5.1. Depression
Depressive symptoms were assessed using the Spanish version of the Beck Depression Inventory—Second Edition (BDI-II). Each of the 21 items ranges from 0 to 3, with 63 points being the highest score, where 0 to 13 means minimal depression, 14 to 19 means mild depression, 20 to 28 indicates moderate depression, and 29 points or more indicates severe depression. This validated questionnaire is extensively employed in chronic pain populations and has demonstrated strong reliability (ICC = 0.73–0.86) [32,33].
2.5.2. Anxiety
Anxiety levels were rated using the validated Spanish iteration of the Hospital Anxiety and Depression Scale (HADS), specifically focusing on the anxiety subscale. This subset comprises seven items, each scored from 0 to 3. A cumulative score exceeding 10 points indicates the presence of anxiety, while a score ranging from 8 to 10 denotes a borderline case and a score below 8 indicates an absence of significant anxiety. The reliability of this test was found to be excellent (ICC = 0.85) [34,35].
2.5.3. Pain Catastrophizing
The Pain Catastrophizing Scale (PCS) in its Spanish version was employed to evaluate cognitive distortions related to pain catastrophizing. This is a self-administered questionnaire consisting of 13 items with a score ranging from 0 “Not at all” to 4 “All the time”. It encompasses three dimensions: helplessness (questions from 1 to 5 and 12, regarding the person’s belief in their influence on their pain); magnification (corresponds to questions 6, 7 and 13 and refers to the exaggeration of the threatening properties of the painful stimulus); rumination (corresponds to questions 8 to 11 and refers to the fact that the patient is unable to stop thinking about pain, being unable to get away from the idea). A total score is obtained (ranging from 0 to 52), so a higher score means greater pain catastrophizing. This validated questionnaire holds substantial prominence in the scientific literature and is widely utilized. Notably, the PCS demonstrates excellent reliability specifically among patients with FM, showing an ICC value of 0.94 [36].
2.5.4. Fear-Related Movement
The assessment of cognitive distortions related to fear of movement (kinesiophobia) was conducted using the Spanish version of the Tampa Kinesiophobia Scale (TSK-11). It is an 11-item scale, each with 4 possible answers, where “totally disagree” obtains 1 point and “totally agree” obtains 4 points. Therefore, the total score ranges from 11 to 44 points, a high score being indicative of greater fear of movement/injury, that is, high levels of fear of movement. This scale has been extensively employed among individuals enduring chronic pain, including those with FM, exhibiting commendable psychometric properties with an ICC of 0.85 [37,38].
2.6. Statistical Analysis
The data analysis employed the Statistics Package for Social Science (SPSS 25.00, IBM Chicago, IL, USA) with a 95% confidence interval (95% CI), considering p-values below 0.05 as statistically significant. To compare differences among nominal variables (e.g., profession or marital status), the Chi-square test was utilized across groups, each consisting of twenty-two participants. Normality tests, specifically the Shapiro–Wilk and Kolmogorov–Smirnov tests, were conducted, revealing no statistically significant differences indicating an abnormal distribution within the data sets. For the comparison of continuous variables between groups, the Student’s t-test for independent samples was applied as the statistical test. Subsequently, Cohen’s d was calculated to assess the effect size of the study variables, categorized as small (0.20–0.49), medium (0.50–0.79) or large (>0.8) according to Cohen’s criteria. The correlation between psychological variables (depression, anxiety, catastrophizing and fear-related movement) and psychophysiological measures (CPM, PPT, FIQ and NPRS) was examined using the Pearson correlation coefficient. A correlation coefficient >0.60 indicated a strong correlation; a coefficient between 0.30 and 0.60 indicated a moderate correlation and a coefficient <0.30 indicated a low correlation. A significance level of p < 0.05 was set for all statistical tests conducted during the analysis.
3. Results
3.1. Clinical Status of FM Patients and Pain-Free Controls
Twenty-two participants were pain-free women controls (with a mean age of 48.55 ± 8.19 years), and twenty-two patients were women diagnosed with FM (52.05 ± 8.35 years). According to the FIQ, patients had on average mildly severe symptoms and severe function deficits with 86.49 ± 3.62. The mean pain intensity was 6.05/10 (SD ± 1.88). There were no statistically significant differences between healthy subjects and patients according to the sociodemographic variables shown in Table 1.

Table 1.
Descriptive statistics in demographic measures (n = 44).
3.2. Mechanical Hyperalgesia and Conditioned Pain Modulation
Patients with FM presented a reduced PPT, which is indicative of mechanical hyperalgesia (t = 6.5; p < 0.001; d = 0.53), as well as showing lower values in the CPM (t = 7.8; p < 0.001; d = 0.64), which indicates alterations in the functioning of the descending pain inhibitory system. Data are shown in Table 2.

Table 2.
Between-group comparison in psychophysiological measures.
3.3. Anxiety, Depression, Fear-Related Movement and Pain Catastrophism
The independent sample Student’s t-test revealed significant inter-group differences. Patients with FM had higher scores on the HADS anxiety subscale (t = 4.3; p < 0.001; higher scores on the BDI-II (t = 18.25; p < 0.0001, d = 5.8); d = 2.9), higher scores on the PCS (t = 16.1; p < 0.01; d = 3.8) and higher scores on the TSK-11(t = 7.4; p < 0.01; d = 7.01) compared to the control group. Data are shown in Table 3.

Table 3.
Between-group comparison in anxiety, depression, fear-related movement and pain catastrophizing psychological variables.
3.4. Correlation Analysis
After examining the bivariate relationships between somatosensory and psychological variables, statistically significant correlations were found. Results are shown in Table 4.

Table 4.
Correlation coefficients between somatosensory and psychological variables.
4. Discussion
The main objective of this research was to explore somatosensory and psychological alterations in women with FM. The secondary objective was to carry out a secondary analysis to correlate the different variables studied and delve into the influences between them.
Women with FM displayed mechanical hyperalgesia and reduced capacity to modulate pain compared to the control group, which is characteristic of populations experiencing chronic pain. Moreover, females with FM display adverse emotional symptoms such as anxiety and depression alongside cognitive distortions related to pain catastrophizing and fear of movement when compared to healthy controls. Depression symptoms exhibited a moderate correlation with pain modulation capacity and a strong correlation with emotional symptoms of anxiety and cognitive distortion related to fear of movement. Anxiety demonstrated a strong correlation with depression, as well as with cognitive distortions regarding pain catastrophizing and fear-related movement. Pain catastrophizing cognitions exhibited a moderate negative correlation with pain intensity (NPRS) and disability (FIQ), whereas they displayed a strong correlation with emotional symptoms of anxiety and cognitive distortions related to fear of movement. Furthermore, fear-related cognitive distortions showed a moderate correlation with altered functioning of pain inhibitory systems, as well as a strong correlation with the presence of negative emotional symptoms of depression and anxiety and pain-related catastrophic cognitions.
4.1. Correlations between Somatosensory and Psychological Variables
Patients with FM experience moderate to severe levels of pain intensity and disability. According to Tabach Apraiz et al., the average level of pain intensity is 7.29 [39]. Regarding the validation study of the FIQ, the mean scores may vary depending on the authors (Rivera and González’s study scored 52, whereas Monterde et al. set it at 70.8) [27,40].
In our study, through a secondary correlation analysis, we observed that patients exhibiting higher levels of catastrophizing subsequently reported lower levels of pain intensity and disability. Being that this finding is likely to be surprising, the prior scientific literature warns about potential biases among FM patients in self-administered questionnaires, particularly those undergoing legal processes, as highlighted in the study by Capilla Ramirez et al. [41]. Previous scientific evidence demonstrates that patients with FM in ongoing legal litigation are more consistent in their responses regarding different disability and psychological questionnaires or general health status. Conversely, as shown in the validation study of the FIQ in the Spanish population, those who do not have pending legal litigation do not show such a marked consistency in their responses between questionnaires [40]. Our study shows that catastrophizing pain exaggeration traits does not always correlate with other scales measuring pain intensity or disability in patients with FM. Furthermore, no correlation has been found between high scores on the HADS anxiety questionnaire, higher pain intensity levels (NPRS) and mechanical hyperalgesia (PTT). Previous scientific evidence suggests that patients with anxiety symptoms present greater activation of the medial prefrontal cortex, which is involved in the emotional aspects of pain while they are under a painful stimulus. Nonetheless, our study does not report higher NPRS scores in baseline situations where there was not a painful stimulation, nor when measurements were made with the PPT [42]. These findings suggest that even though patients with FM present high anxiety levels, these symptoms do not influence the detection of mechanical stimuli nor the pain levels reported (NPRS) in rest situations.
In different populations suffering from chronic pain, the CPM test shows reduced activity of descending inhibitory systems [43]. In addition, reduced CPM is also associated with pain in more body regions [44], as could be the case of patients with FM. Owing to the influence of the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC) on the emotional response to pain and pain behaviors, it is hypothesized that psychological factors such as depression or pain catastrophizing can influence the ability to modulate pain [45,46,47]. Conflicting results have been reported regarding the correlation between psychological factors and CPM, both in healthy subjects and in those suffering from chronic pain [48,49]. There is evidence of alterations in the CPM in patients with FM [19], although the scientific literature is scarce when it comes to how psychological variables can influence the ability to modulate pain measured with the CPM paradigm [50]. According to our findings, depression and fear-related movement moderately correlate with reduced functioning of inhibitory systems. In our work, the cold thermal stimulus was used to trigger the conditioning stimulus. It must be considered that there are other methods used to provoke the conditioning stimulus in the CPM test, such as thermal heat stimuli, electrical stimuli or ischemic stimuli. Future studies should evaluate how the psychological variables of patients with FM influence the CPM test performed with different conditioning stimuli.
4.2. Correlations between Different Psychological Variables
Previous scientific evidence has found psychological alterations in patients with FM, although the relationship between them lacks certainty. Increasing knowledge of this relationship can help make clinical decisions in each case. Our findings regarding emotional psychological variables (anxiety and depression) align with the prior scientific literature, as demonstrated in the recent study by Henao-Perez et al. [51] One of the remarkable features of our work involves conducting a secondary analysis to delve into the correlation between psychological variables. Our results provide evidence of a strong correlation between anxiety and depression, indicating their frequent co-occurrence in FM patients. Additionally, a moderate correlation was found between heightened anxiety levels and cognitive distortions related to pain catastrophizing or fear of movement. Depressive symptoms exhibited a stronger correlation with fear of movement but not with pain catastrophizing cognitions.
With regard to cognitive psychological variables, our findings also align with the prior scientific literature, revealing cognitive distortions of pain catastrophizing and kinesiophobia in patients with FM. Furthermore, in the secondary correlation analysis, our results agree with those introduced by Koçyigit et al. [9], who found a correlation between kinesiophobia, depression and functional disability (FIQ). In our current study, we observed a correlation between kinesiophobia and other psychological variables (anxiety, depression and catastrophizing), but not with disability. Finally, regarding pain catastrophizing, our results are consistent with the prior scientific literature, indicating elevated levels of pain catastrophizing among women with FM. In this regard, the previous scientific literature has also linked pain catastrophizing with lower levels of physical activity and increased fatigue [52].
4.3. Limitations
The primary limitation refers to the absence of blinding among evaluators conducting measurements, resulting in knowledge of participants’ group allocations. Secondly, this study did not assess the medications administered to patients, potentially impacting the outcomes. Lastly, this study encountered a reduced sample size. Future studies that have a larger sample size will study the influences between the variables through linear regression analysis.
4.4. Applications for Clinical Practice
The findings of this investigation allow us to better understand the correlation between psychological symptoms and somatosensory variables, as well as the correlations among various psychological factors in FM patients. Thereby, it is suggested that patients with FM who have high depressive symptoms or fear of movement are likely to lack the ability to modulate pain, which must be taken into account when prescribing their treatment. For instance, it is known that physical exercise activates the descending pain inhibitory system. However, when prescribed to patients with FM, their fear of movement can play an important role; being gradually exposed to physical exercise is required to carry out their treatment.
4.5. Future Lines of Research
We suggest conducting future longitudinal studies periodically assessing changes in somatosensory and psychological variables in women with FM. This approach would enable a more accurate identification of the interaction between these variables during the course of the disease. Also, future longitudinal studies that subclassify patients with FM and identify the best treatments according to the variables affected in each case are encouraged. Finally, in accordance with the line of research of our research group, it seems appropriate to carry out studies where implicit motor imagery is used in the early stages as a pain treatment technique in patients with FM, since, according to our previous studies, implicit motor imagery is related to the dysfunction of their pain inhibitory systems [53,54]. Consequently, as its implementation does not evoke painful sensations, nor is it influenced by the psychological aspects in patients with FM, we hypothesize that it could enhance their pain coping and subsequently, secondarily influence psychological symptom improvement.
5. Conclusions
Depression and fear-related movement are two psychological variables that can influence pain modulation in patients with fibromyalgia. The correlations between the different psychological variables must be taken into account in the clinical setting.
Author Contributions
Conceptualization, V.R.-A., A.G.-C. and F.G.-E.; methodology, V.R.-A., A.G.-C., F.G.-E. and J.F.-C.; data curation, V.R.-A., A.G.-C., J.F.-C. and F.G.-E.; writing—original draft preparation, V.R.-A., A.G.-C., F.G.-E. and A.Z.-d.C.; writing—review and editing, V.R.-A., A.G.-C., F.G.-E., J.F.-C., G.B.-K. and A.Z.-d.C.; supervision, A.G.-C. and F.G.-E.; project administration, V.R.-A., A.G.-C. and F.G.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethical Review Board of the Rey Juan Carlos University (2605202012920) in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
AFYNSYFACRO (fibromyalgia association in Móstoles, Spain).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia Prevalence, Somatic Symptom Reporting, and the Dimensionality of Polysymptomatic Distress: Results From a Survey of the General Population. Arthritis Care Res. 2013, 65, 777–785. [Google Scholar] [CrossRef]
- Silverman, S.L.; Harnett, J.; Zlateva, G.; Mardekian, J. Identifying Fibromyalgia-Associated Symptoms and Conditions from a Clinical Perspective: A Step toward Evaluating Healthcare Resource Utilization in Fibromyalgia. Pain Pract. 2010, 10, 520–529. [Google Scholar] [CrossRef]
- Finan, P.H.; Zautra, A.J.; Davis, M.C.; Lemery-Chalfant, K.; Covault, J.; Tennen, H. COMT Moderates the Relation of Daily Maladaptive Coping and Pain in Fibromyalgia. Pain 2011, 152, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Regal Ramos, R.J. Epidemiological Characteristics of Patients Evaluated with Fibromyalgia in the Assessment of Disability Unit of Madrid. Semergen 2017, 43, 28–33. [Google Scholar] [CrossRef]
- Lee, J.; Protsenko, E.; Lazaridou, A.; Franceschelli, O.; Ellingsen, D.M.; Mawla, I.; Isenburg, K.; Berry, M.P.; Galenkamp, L.; Loggia, M.L.; et al. Encoding of Self-Referential Pain Catastrophizing in the Posterior Cingulate Cortex in Fibromyalgia. Arthritis Rheumatol. 2018, 70, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Bernik, M.; Sampaio, T.P.A.; Gandarela, L. Fibromyalgia Comorbid with Anxiety Disorders and Depression: Combined Medical and Psychological Treatment. Curr. Pain Headache Rep. 2013, 17, 358. [Google Scholar] [CrossRef]
- Giesecke, T.; Williams, D.A.; Harris, R.E.; Cupps, T.R.; Tian, X.; Tian, T.X.; Gracely, R.H.; Clauw, D.J. Subgrouping of Fibromyalgia Patients on the Basis of Pressure-Pain Thresholds and Psychological Factors. Arthritis Rheum. 2003, 48, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Leon-Llamas, J.L.; Murillo-Garcia, A.; Villafaina, S.; Domínguez-Muñoz, F.J.; Morenas, J.; Gusi, N. Relationship between Kinesiophobia and Mobility, Impact of the Disease, and Fear of Falling in Women with and without Fibromyalgia: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 8257. [Google Scholar] [CrossRef]
- Koçyiğit, B.F.; Akaltun, M.S. Kinesiophobia Levels in Fibromyalgia Syndrome and the Relationship Between Pain, Disease Activity, Depression. Arch. Rheumatol. 2020, 35, 214–219. [Google Scholar] [CrossRef]
- Haider, S.; Janowski, A.J.; Lesnak, J.B.; Hayashi, K.; Dailey, D.L.; Chimenti, R.; Frey-Law, L.A.; Sluka, K.A.; Berardi, G. A Comparison of Pain, Fatigue, and Function between Post-COVID-19 Condition, Fibromyalgia, and Chronic Fatigue Syndrome: A Survey Study. Pain 2022, 164, 385–401. [Google Scholar] [CrossRef]
- Lazaridou, A.; Paschali, M.; Vilsmark, E.S.; Wilkins, T.; Napadow, V.; Edwards, R. The Impact of COVID-19 Pandemic on Mental and Physical Wellbeing in Women with Fibromyalgia: A Longitudinal Mixed-Methods Study. BMC Women’s Health 2022, 22, 267. [Google Scholar] [CrossRef] [PubMed]
- Gyorfi, M.; Rupp, A.; Abd-Elsayed, A. Fibromyalgia Pathophysiology. Biomedicines 2022, 10, 3070. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.; Collado, A.; Solà, R.; Antonelli, F.; Torres, X.; Salgueiro, M.; Quiles, C.; Bostock, H. Hyperexcitable C Nociceptors in Fibromyalgia. Ann. Neurol. 2014, 75, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Desmeules, J.A.; Cedraschi, C.; Rapiti, E.; Baumgartner, E.; Finckh, A.; Cohen, P.; Dayer, P.; Vischer, T.L. Neurophysiologic Evidence for a Central Sensitization in Patients with Fibromyalgia. Arthritis Rheum. 2003, 48, 1420–1429. [Google Scholar] [CrossRef]
- Gracely, R.H.; Ambrose, K.R. Neuroimaging of Fibromyalgia. Best Pract. Res. Clin. Rheumatol. 2011, 25, 271–284. [Google Scholar] [CrossRef]
- Kaplan, C.M.; Schrepf, A.; Vatansever, D.; Larkin, T.E.; Mawla, I.; Ichesco, E.; Kochlefl, L.; Harte, S.E.; Clauw, D.J.; Mashour, G.A.; et al. Functional and Neurochemical Disruptions of Brain Hub Topology in Chronic Pain. Pain 2019, 160, 973–983. [Google Scholar] [CrossRef]
- Staud, R.; Domingo, M. Evidence for Abnormal Pain Processing in Fibromyalgia Syndrome. Pain Med. 2001, 2, 208–215. [Google Scholar] [CrossRef][Green Version]
- Harris, R.E.; Clauw, D.J.; Scott, D.J.; McLean, S.A.; Gracely, R.H.; Zubieta, J.-K. Decreased Central Mu-Opioid Receptor Availability in Fibromyalgia. J. Neurosci. 2007, 27, 10000–10006. [Google Scholar] [CrossRef]
- O’Brien, A.T.; Deitos, A.; Triñanes Pego, Y.; Fregni, F.; Carrillo-de-la-Peña, M.T. Defective Endogenous Pain Modulation in Fibromyalgia: A Meta-Analysis of Temporal Summation and Conditioned Pain Modulation Paradigms. J. Pain 2018, 19, 819–836. [Google Scholar] [CrossRef]
- Nir, R.-R.; Yarnitsky, D. Conditioned Pain Modulation. Curr. Opin. Support. Palliat. Care 2015, 9, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Firouzian, S.; Osborne, N.R.; Cheng, J.C.; Kim, J.A.; Bosma, R.L.; Hemington, K.S.; Rogachov, A.; Davis, K.D. Individual Variability and Sex Differences in Conditioned Pain Modulation and the Impact of Resilience, and Conditioning Stimulus Pain Unpleasantness and Salience. Pain 2020, 161, 1847–1860. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Petersen, K.K.; Mørch, C.D.; Imai, Y.; Arendt-Nielsen, L. Assessment of CPM Reliability: Quantification of the within-Subject Reliability of 10 Different Protocols. Scand. J. Pain 2018, 18, 729–737. [Google Scholar] [CrossRef]
- Meade, E.; Garvey, M. The Role of Neuro-Immune Interaction in Chronic Pain Conditions; Functional Somatic Syndrome, Neurogenic Inflammation, and Peripheral Neuropathy. Int. J. Mol. Sci. 2022, 23, 8574. [Google Scholar] [CrossRef]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of Four Pain Intensity Rating Scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Euasobhon, P.; Atisook, R.; Bumrungchatudom, K.; Zinboonyahgoon, N.; Saisavoey, N.; Jensen, M.P. The Reliability and Responsivity of Pain Intensity Scales in Individuals with Chronic Pain. Pain 2022, 163, e1184–e1191. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, B.; García-Fructuoso, F.; Belenguer, R.; Alegre, C.; Moreno-Muelas, J.V.; Hernández, J.L.; Pina, T.; González-Gay, M.Á. The Spanish Version of the 2010 American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia: Reliability and Validity Assessment. Clin. Exp. Rheumatol. 2016, 34, S55–S58. [Google Scholar] [PubMed]
- Rivera, J.; González, T. The Fibromyalgia Impact Questionnaire: A Validated Spanish Version to Assess the Health Status in Women with Fibromyalgia. Clin. Exp. Rheumatol. 2004, 22, 554–560. [Google Scholar] [PubMed]
- Walton, D.M.; Macdermid, J.C.; Nielson, W.; Teasell, R.W.; Chiasson, M.; Brown, L. Reliability, Standard Error, and Minimum Detectable Change of Clinical Pressure Pain Threshold Testing in People with and without Acute Neck Pain. J. Orthop. Sports Phys. Ther. 2011, 41, 644–650. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Kolber, M.J.; Mokha, G.M.; Hanney, W.J. Concurrent Validation of a Pressure Pain Threshold Scale for Individuals with Myofascial Pain Syndrome and Fibromyalgia. J. Man. Manip. Ther. 2018, 26, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.L.; Downes, L. Reliability of Clinical Pressure-Pain Algometric Measurements Obtained on Consecutive Days. Phys. Ther. 1998, 78, 160–169. [Google Scholar] [CrossRef]
- Nuwailati, R.; Bobos, P.; Drangsholt, M.; Curatolo, M. Reliability of Conditioned Pain Modulation in Healthy Individuals and Chronic Pain Patients: A Systematic Review and Meta-Analysis. Scand. J. Pain 2022, 22, 262–278. [Google Scholar] [CrossRef]
- Sanz, J. Adaptación Española Del Inventario Para La Depresión de Beck-II (BDI-II): 3. Propiedades Psicométricas En Pacientes Con Trastornos Psicológicos Spanish Adaptation of the Beck Depression Inventory-II (BDI-II): 3. Psychometric Features in Patiens with Psychological Disorders. Clínica y Salud 2005, 16, 121–142. [Google Scholar]
- Wiebe, J.S.; Penley, J.A. A Psychometric Comparison of the Beck Depression Inventory-II in English and Spanish. Psychol. Assess. 2005, 17, 481–485. [Google Scholar] [CrossRef]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A Validation Study of the Hospital Anxiety and Depression Scale (HADS) in a Spanish Population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ryde-Brandt, B. Anxiety and Depression in Mothers of Children with Psychotic Disorders and Mental Retardation. Br. J. Psychiatry 1990, 156, 118–121. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de La Versión Española de La Escala de La Catastrofización Ante El Dolor (Pain Catastrophizing Scale) En La Fibromialgia. Med. Clin. 2008, 131, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Larsson, C.; Ekvall Hansson, E.; Sundquist, K.; Jakobsson, U. Kinesiophobia and Its Relation to Pain Characteristics and Cognitive Affective Variables in Older Adults with Chronic Pain. BMC Geriatr. 2016, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.M.E.S.; Franco, K.F.M.; Miyamoto, G.C.; Franco, Y.R.D.S.; Cabral, C.M.N. Analysis of the Measurement Properties of the Brazilian-Portuguese Version of the Tampa Scale for Kinesiophobia-11 in Patients with Fibromyalgia. Braz. J. Phys. Ther. 2021, 25, 168. [Google Scholar] [CrossRef] [PubMed]
- Tabach Apraiz, A.D.; Oyanadel Maldonado, M.L.; Gutierrez Espinoza, H.; Bueno Buker, D. Correlación Entre Oportunidad Diagnóstica y Severidad Del Dolor En Pacientes Con Fibromialgia Que Ingresan a La Unidad de Dolor Crónico No Oncológico En Hospital Clínico San Borja Arriarán. Rev. Soc. Española Dolor 2019, 26, 331–336. [Google Scholar] [CrossRef][Green Version]
- Monterde, S.; Salvat, I.; Montull, S.; Fernández-Ballart, J. Validación de La Versión Española Del Fibromyalgia Impact Questionnaire. Rev. Española Reumatol. 2004, 31, 507–513. [Google Scholar]
- Capilla Ramírez, P.; González Ordi, H.; Santamaría Fernández, P.; Pérez Nieto, M.Á.; Casado Morales, M.I. Fibromialgia: ¿exageración o Simulación? Clin. Salud 2013, 24, 185–195. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Ludlow, D.H.; Knierim, K.; Hanelin, J.; Ramachandran, T.; Glover, G.C.; Mackey, S.C. Neural Correlates of Individual Differences in Pain-Related Fear and Anxiety. Pain 2006, 120, 69–77. [Google Scholar] [CrossRef]
- Lewis, G.N.; Rice, D.A.; McNair, P.J. Conditioned Pain Modulation in Populations with Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain 2012, 13, 936–944. [Google Scholar] [CrossRef]
- Vaegter, H.B.; Graven-Nielsen, T. Pain Modulatory Phenotypes Differentiate Subgroups with Different Clinical and Experimental Pain Sensitivity. Pain 2016, 157, 1480–1488. [Google Scholar] [CrossRef]
- Goffaux, P.; Redmond, W.J.; Rainville, P.; Marchand, S. Descending Analgesia–When the Spine Echoes What the Brain Expects. Pain 2007, 130, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P. A Neuropsychological Model of Pain: Research and Clinical Implications. J. Pain 2010, 11, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Salomons, T.V.; Johnstone, T.; Backonja, M.-M.; Shackman, A.J.; Davidson, R.J. Individual Differences in the Effects of Perceived Controllability on Pain Perception: Critical Role of the Prefrontal Cortex. J. Cogn. Neurosci. 2007, 19, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Chakiath, R.J.; Siddall, P.J.; Kellow, J.E.; Hush, J.M.; Jones, M.P.; Costa, D.S.J.; Wrigley, P.J. Conditioned Pain Modulation (CPM) Is Reduced in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of CPM and the Role of Psychological Factors. J. Clin. Gastroenterol. 2019, 53, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Nahman-Averbuch, H.; Nir, R.R.; Sprecher, E.; Yarnitsky, D. Psychological Factors and Conditioned Pain Modulation: A Meta-Analysis. Clin. J. Pain 2016, 32, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Paul-Savoie, E.; Marchand, S.; Morin, M.; Bourgault, P.; Brissette, N.; Rattanavong, V.; Cloutier, C.; Bissonnette, A.; Potvin, S. Is the Deficit in Pain Inhibition in Fibromyalgia Influenced by Sleep Impairments? Open Rheumatol. J. 2012, 6, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Henao-Pérez, M.; López-Medina, D.C.; Arboleda, A.; Bedoya Monsalve, S.; Zea, J.A. Patients With Fibromyalgia, Depression, and/or Anxiety and Sex Differences. Am. J. Men’s Health 2022, 16, 155798832211103. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, I.; Velasco, L.; Gutiérrez, L.; Écija, C.; Catalá, P.; Peñacoba, C. Symptoms in Women with Fibromyalgia after Performing Physical Activity: The Role of Pain Catastrophizing and Disease Impact. Clin. Rheumatol. 2023, 42, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-Aguado, V.; Gil-Crujera, A.; Fernández-Carnero, J.; Cuenca-Martínez, F.; Gómez Esquer, F. Limb Laterality Discrimination, Evoked Sensations and Somatosensory Behavior in Fibromyalgia Syndrome: A Cross-Sectional Study. Appl. Sci. 2022, 12, 7495. [Google Scholar] [CrossRef]
- Riquelme-Aguado, V.; Gil-Crujera, A.; Fernández-Carnero, J.; Cuenca-Martínez, F.; Klett, G.B.; Esquer, F.G. The Influence of Emotional and Cognitive Factors on Limb Laterality Discrimination in Women with Fibromyalgia Syndrome: A Cross-Sectional Study Secondary Analysis. Appl. Sci. 2023, 13, 1894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).