Accelerated Brain Atrophy, Microstructural Decline and Connectopathy in Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging
2.3. Image Analysis
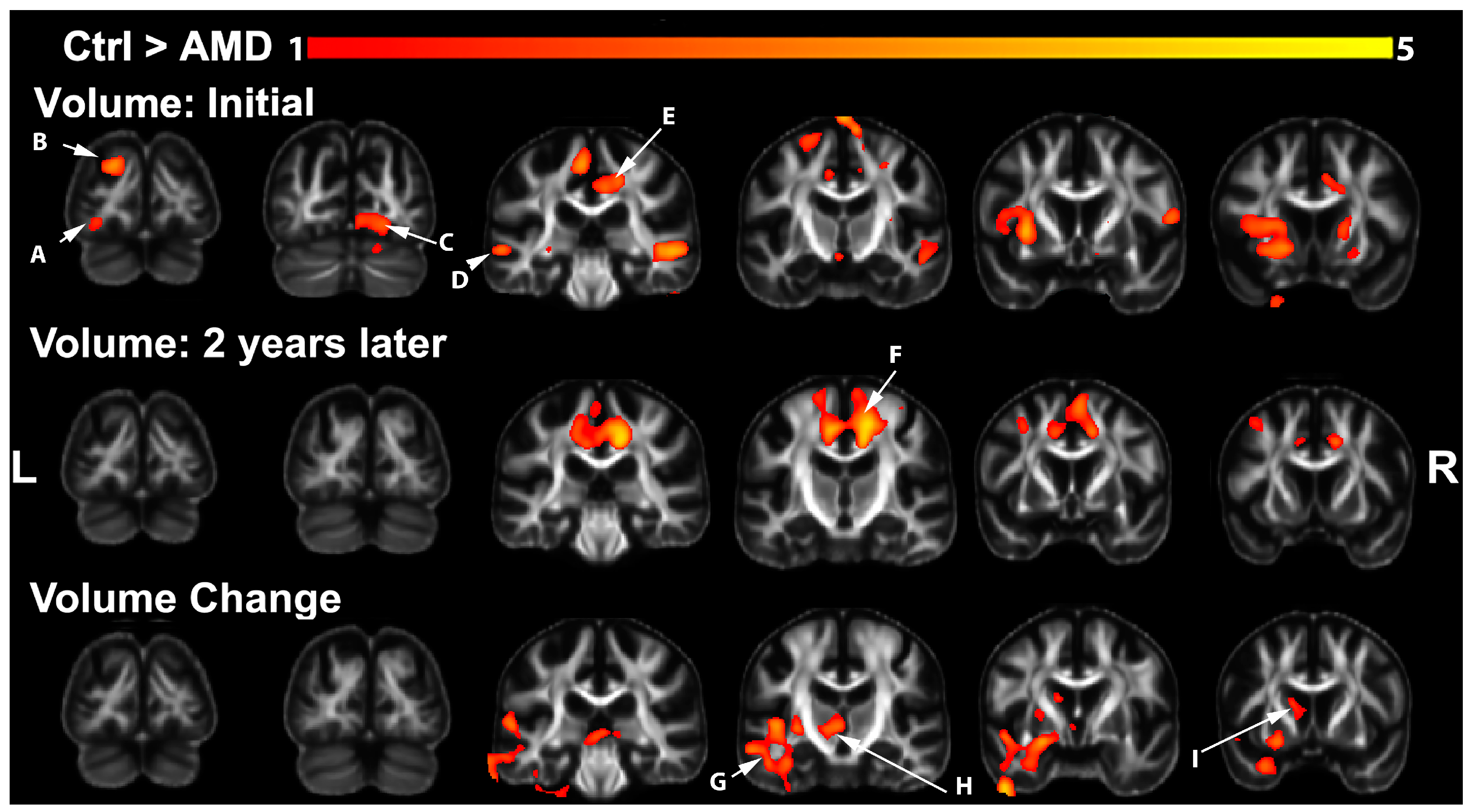
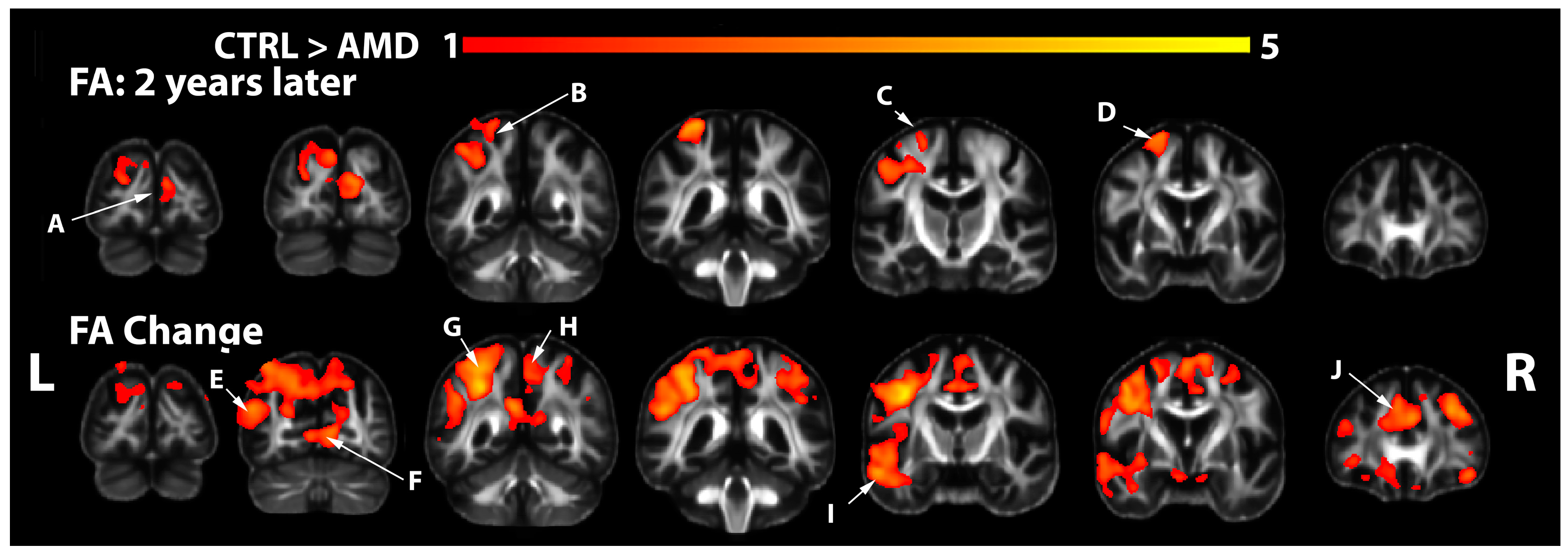
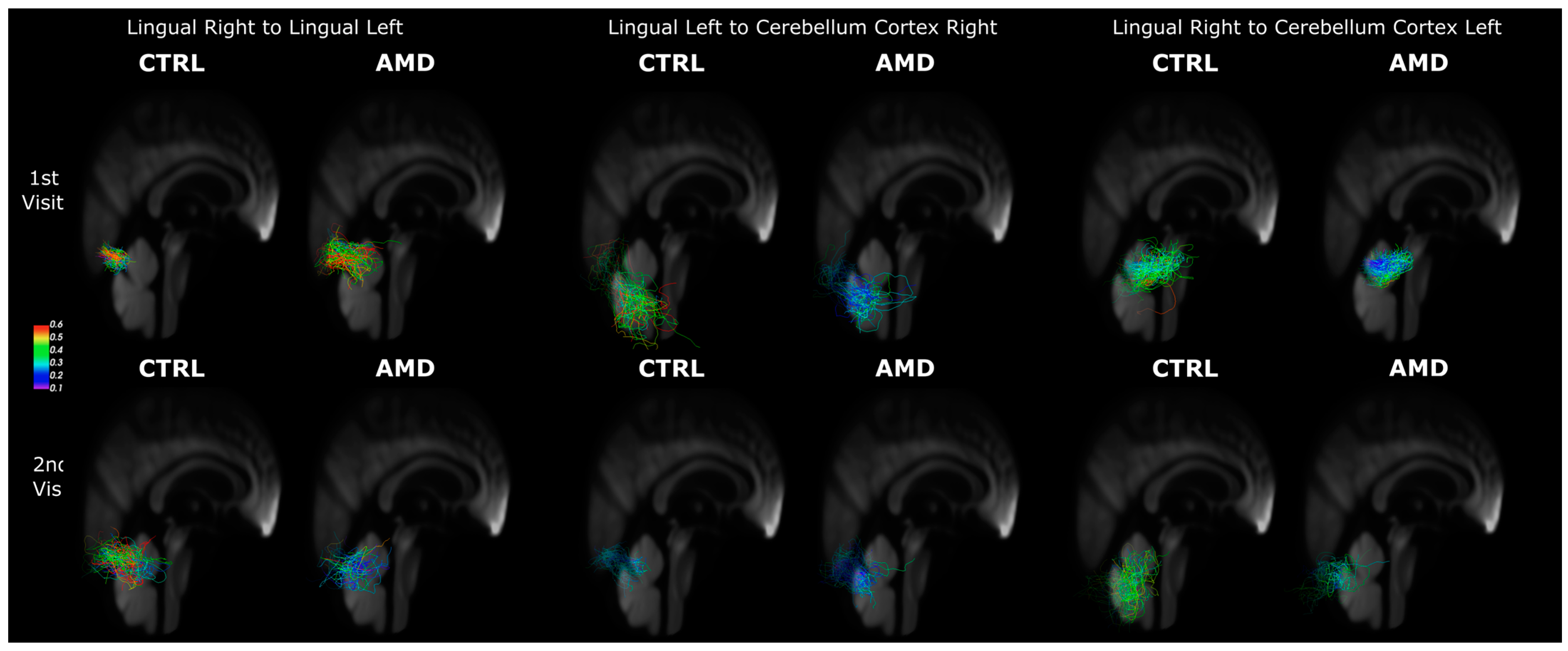
3. Results
3.1. Volumetric Changes in AMD Participants
3.2. FA Changes in AMD Participants
3.3. Connectivity Changes in AMD Participants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, R.; Klein, B.E.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Chakravarthy, U.; Klein, R.; Mitchell, P.; Zlateva, G.; Buggage, R.; Fahrbach, K.; Probst, C.; Sledge, I. The natural history and prognosis of neovascular age-related macular degeneration: A systematic review of the literature and meta-analysis. Ophthalmology 2008, 115, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L., 3rd; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Baker, M.L.; Wang, J.J.; Rogers, S.; Klein, R.; Kuller, L.H.; Larsen, E.K.; Wong, T.Y. Early age-related macular degeneration, cognitive function, and dementia: The Cardiovascular Health Study. Arch. Ophthalmol. 2009, 127, 667–673. [Google Scholar] [CrossRef]
- Hageman, G.S.; Luthert, P.J.; Chong, N.V.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Umeda, S.; Suzuki, M.T.; Okamoto, H.; Ono, F.; Mizota, A.; Terao, K.; Yoshikawa, Y.; Tanaka, Y.; Iwata, T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis). FASEB J. 2005, 19, 1683–1685. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Nieto, F.J.; Moraes, S.A.; Mosley, T.H.; Couper, D.J.; Klein, B.E.; Boland, L.L.; Hubbard, L.D.; Sharrett, A.R. Is early age-related maculopathy related to cognitive function? The Atherosclerosis Risk in Communities Study. Am. J. Ophthalmol. 2002, 134, 828–835. [Google Scholar] [CrossRef]
- Pham, T.Q.; Kifley, A.; Mitchell, P.; Wang, J.J. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology 2006, 52, 353–358. [Google Scholar] [CrossRef]
- Woo, S.J.; Park, K.H.; Ahn, J.; Choe, J.Y.; Jeong, H.; Han, J.W.; Kim, T.H.; Kim, K.W. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology 2012, 119, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.S.; Lee, B.Y.; Kuk, A.K.; Yu, X.T.; Li, S.S.; Li, J.; Guo, Y.; Yin, Y.; Osterbur, D.L.; Yam, J.C.S.; et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: A meta-analysis. Br. J. Ophthalmol. 2019, 103, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.E.; Rankin, M.W.; McBee, W.L. Cognitive impairment in the age-related eye disease study: AREDS report no. 16. Arch. Ophthalmol. 2006, 124, 537–543. [Google Scholar] [PubMed]
- Whitson, H.E.; Ansah, D.; Whitaker, D.; Potter, G.; Cousins, S.W.; MacDonald, H.; Pieper, C.F.; Landerman, L.; Steffens, D.C.; Cohen, H.J. Prevalence and patterns of comorbid cognitive impairment in low vision rehabilitation for macular disease. Arch. Gerontol. Geriatr. 2010, 50, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Madden, D.J.; Cunha, P.; Badea, A.; Davis, S.W.; Potter, G.G.; Lad, E.M.; Cousins, S.W.; Chen, N.-K.; Allen, K. Cerebral white matter connectivity, cognition, and age-related macular degeneration. NeuroImage Clin. 2021, 30, 102594. [Google Scholar] [CrossRef]
- Zhuang, J.; Madden, D.J.; Duong-Fernandez, X.; Chen, N.-k.; Cousins, S.W.; Potter, G.G.; Diaz, M.T.; Whitson, H.E. Language processing in age-related macular degeneration associated with unique functional connectivity signatures in the right hemisphere. Neurobiol. Aging 2018, 63, 65–74. [Google Scholar] [CrossRef]
- Hernowo, A.T.; Prins, D.; Baseler, H.A.; Plank, T.; Gouws, A.D.; Hooymans, J.M.M.; Morland, A.B.; Greenlee, M.W.; Cornelissen, F.W. Morphometric analyses of the visual pathways in macular degeneration. Cortex 2014, 56, 99–110. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Middleton, L.E.; Yaffe, K. Promising strategies for the prevention of dementia. Arch. Neurol. 2009, 66, 1210–1215. [Google Scholar] [CrossRef]
- Wittich, W.; Pichora-Fuller, M.K.; Johnson, A.; Joubert, S.; Kehayia, E.; Bachir, V.; Aubin, G.; Jaiswal, A.; Phillips, N. Effect of Reading Rehabilitation for Age-Related Macular Degeneration on Cognitive Functioning: Protocol for a Nonrandomized Pre-Post Intervention Study. JMIR Res. Protoc. 2021, 10, e19931. [Google Scholar] [CrossRef]
- Hart, N.J.; Koronyo, Y.; Black, K.L.; Koronyo-Hamaoui, M. Ocular indicators of Alzheimer’s: Exploring disease in the retina. Acta Neuropathol. 2016, 132, 767–787. [Google Scholar] [CrossRef]
- Klaver, C.C.; Ott, A.; Hofman, A.; Assink, J.J.; Breteler, M.M.; de Jong, P.T. Is age-related maculopathy associated with Alzheimer’s Disease? The Rotterdam Study. Am. J. Epidemiol. 1999, 150, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Goldacre, R.; Goldacre, M.J. Associations between age-related macular degeneration, Alzheimer disease, and dementia: Record linkage study of hospital admissions. JAMA Ophthalmol. 2014, 132, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.Y.; Wan, L.; Lai, J.N.; Chen, C.S.; Chen, J.J.; Wu, M.Y.; Hu, K.C.; Chiu, L.T.; Tien, P.T.; Lin, H.J. Increased risk of Alzheimer’s disease among patients with age-related macular degeneration: A nationwide population-based study. PLoS ONE 2021, 16, e0250440. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Green, W.R.; Enger, C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993, 100, 1519–1535. [Google Scholar] [CrossRef] [PubMed]
- Sarks, S.H.; Arnold, J.J.; Killingsworth, M.C.; Sarks, J.P. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: A clinicopathological study. Br. J. Ophthalmol. 1999, 83, 358–368. [Google Scholar] [CrossRef]
- Dentchev, T.; Milam, A.H.; Lee, V.M.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.-T.; Ashfaq, A. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef]
- Anderson, D.H.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 2002, 134, 411–431. [Google Scholar] [CrossRef]
- Biron, K.E.; Dickstein, D.L.; Gopaul, R.; Jefferies, W.A. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PLoS ONE 2011, 6, e23789. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.H.; Talaga, K.C.; Rivest, A.J.; Barron, E.; Hageman, G.S.; Johnson, L.V. Characterization of β amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s Aβ-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef] [PubMed]
- Malek, G.; Johnson, L.V.; Mace, B.E.; Saloupis, P.; Schmechel, D.E.; Rickman, D.W.; Toth, C.A.; Sullivan, P.M.; Rickman, C.B. Apolipoprotein E allele-dependent pathogenesis: A model for age-related retinal degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 11900–11905. [Google Scholar] [CrossRef]
- Swaroop, A.; Chew, E.Y.; Rickman, C.B.; Abecasis, G.R. Unraveling a multifactorial late-onset disease: From genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu. Rev. Genom. Hum. Genet. 2009, 10, 19–43. [Google Scholar] [CrossRef]
- Tsai, H.R.; Lo, R.Y.; Liang, K.H.; Chen, T.L.; Huang, H.K.; Wang, J.H.; Lee, Y.C. Risk of Subsequent Dementia or Alzheimer Disease among Patients with Age-Related Macular Degeneration: A Systematic Review and Meta-analysis. Am. J. Ophthalmol. 2023, 247, 161–169. [Google Scholar] [CrossRef]
- Zuo, X.; Zhuang, J.; Chen, N.-k.; Cousins, S.; Cunha, P.; Lad, E.M.; Madden, D.J.; Potter, G.; Whitson, H.E. Relationship between neural functional connectivity and memory performance in age-related macular degeneration. Neurobiol. Aging 2020, 95, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Monge, Z.A.; Madden, D.J. Linking cognitive and visual perceptual decline in healthy aging: The information degradation hypothesis. Neurosci. Biobehav. Rev. 2016, 69, 166–173. [Google Scholar] [CrossRef]
- Powell, D.S.; Oh, E.S.; Reed, N.S.; Lin, F.R.; Deal, J.A. Hearing Loss and Cognition: What We Know and Where We Need to Go. Front. Aging Neurosci. 2022, 13, 769405. [Google Scholar] [CrossRef]
- Deal, J.A.; Power, M.C.; Betz, J.; Reed, N.S.; Albert, M.S.; Gottesman, R.F.; Resnick, S.M.; Sharrett, R.; Jack, C.R.; Lin, F.R. Relationship of hearing impairment with MRI brain volumes and cognitive decline in the Atherosclerosis Risk in Communities study. Alzheimer’s Dement. 2020, 16, e046473. [Google Scholar] [CrossRef]
- Qu, H.; Tang, H.; Pan, J.; Zhao, Y.; Wang, W. Alteration of Cortical and Subcortical Structures in Children with Profound Sensorineural Hearing Loss. Front. Hum. Neurosci. 2020, 14, 565445. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, S.; Thiel, C.M. Neuroanatomical changes associated with age-related hearing loss and listening effort. Brain Struct. Funct. 2020, 225, 2689–2700. [Google Scholar] [CrossRef]
- Plank, T.; Frolo, J.; Brandl-Rühle, S.; Renner, A.B.; Hufendiek, K.; Helbig, H.; Greenlee, M.W. Gray matter alterations in visual cortex of patients with loss of central vision due to hereditary retinal dystrophies. NeuroImage 2011, 56, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Boucard, C.C.; Hernowo, A.T.; Maguire, R.P.; Jansonius, N.M.; Roerdink, J.B.T.M.; Hooymans, J.M.M.; Cornelissen, F.W. Changes in cortical grey matter density associated with long-standing retinal visual field defects. Brain 2009, 132, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Yoshimine, S.; Ogawa, S.; Horiguchi, H.; Terao, M.; Miyazaki, A.; Matsumoto, K.; Tsuneoka, H.; Nakano, T.; Masuda, Y.; Pestilli, F. Age-related macular degeneration affects the optic radiation white matter projecting to locations of retinal damage. Brain Struct. Funct. 2018, 223, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Malania, M.; Konrad, J.; Jägle, H.; Werner, J.S.; Greenlee, M.W. Compromised Integrity of Central Visual Pathways in Patients With Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Takemura, H.; Horiguchi, H.; Terao, M.; Haji, T.; Pestilli, F.; Yeatman, J.D.; Tsuneoka, H.; Wandell, B.A.; Masuda, Y. White matter consequences of retinal receptor and ganglion cell damage. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6976–6986. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.L.; Plank, T.; Greenlee, M.W. Aging and central vision loss: Relationship between the cortical macro-structure and micro-structure. NeuroImage 2020, 212, 116670. [Google Scholar] [CrossRef]
- Markl, M.; Leupold, J. Gradient echo imaging. J. Magn. Reson. Imaging 2012, 35, 1274–1289. [Google Scholar] [CrossRef]
- Anderson, R.J.; Long, C.M.; Calabrese, E.D.; Robertson, S.H.; Johnson, G.A.; Cofer, G.P.; O’Brien, R.J.; Badea, A. Optimizing Diffusion Imaging Protocols for Structural Connectomics in Mouse Models of Neurological Conditions. Front. Phys. 2020, 8, 88. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P.; Concha, L.; Buades, A.; Collins, D.L.; Robles, M. Diffusion weighted image denoising using overcomplete local PCA. PLoS ONE 2013, 8, e73021. [Google Scholar] [CrossRef]
- Garyfallidis, E.; Brett, M.; Amirbekian, B.; Rokem, A.; van der Walt, S.; Descoteaux, M.; Nimmo-Smith, I.; Dipy, C. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Tournier, J.D.; Calamante, F.; Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012, 22, 53–66. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Zhang, S.; Arfanakis, K. Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. Neuroimage 2018, 172, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Cook, J.J.; Delpratt, N.; Nouls, J.C.; Gu, B.; McNamara, J.O.; Avants, B.B.; Johnson, G.A.; Badea, A. Small Animal Multivariate Brain Analysis (SAMBA)—A High Throughput Pipeline with a Validation Framework. Neuroinformatics 2019, 17, 451–472. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nichols, K. False Discovery Rate procedures. In Statistical Parametric Mapping; Friston, K., Ashburner, J., Kiebel, S., Nichols, T., Penny, W., Eds.; Academic Press: London, UK, 2007; pp. 246–252. [Google Scholar]
- Smith, R.E.; Tournier, J.-D.; Calamante, F.; Connelly, A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 2012, 62, 1924–1938. [Google Scholar] [CrossRef]
- Tournier, J.D.; Calamante, F.; Connelly, A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In Proceedings of the International Society for Magnetic Resonance in Medicine, Stockholm, Sweden, 1–7 May 2010. [Google Scholar]
- Qi, X.; Zhang, S.; Arfanakis, K. IIT Human Brain Atlas: Enhancement of T1-weighted Template In: Tissue Probability Maps and Gray Matter Atlas. In Proceedings of the International Society for Magnetic Resonance in Medicine (ISRMRM), Honolulu, HI, USA, 22–24 April 2017. [Google Scholar]
- Zhang, Z.; Allen, G.I.; Zhu, H.; Dunson, D. Tensor network factorizations: Relationships between brain structural connectomes and traits. Neuroimage 2019, 197, 330–343. [Google Scholar] [CrossRef]
- Badea, A.; Wu, W.; Shuff, J.; Wang, M.; Anderson, R.J.; Qi, Y.; Johnson, G.A.; Wilson, J.G.; Koudoro, S.; Garyfallidis, E.; et al. Identifying Vulnerable Brain Networks in Mouse Models of Genetic Risk Factors for Late Onset Alzheimer’s Disease. Front. Neuroinform. 2019, 13, 72. [Google Scholar] [CrossRef]
- Baran, T.M.; Zhang, Z.; Anderson, A.J.; McDermott, K.; Lin, F. Brain structural connectomes indicate shared neural circuitry involved in subjective experience of cognitive and physical fatigue in older adults. Brain Imaging Behav. 2020, 14, 2488–2499. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Dunson, D. Symmetric bilinear regression for signal subgraph estimation. IEEE Trans. Signal Process. 2019, 67, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Garyfallidis, E.; Cote, M.A.; Rheault, F.; Sidhu, J.; Hau, J.; Petit, L.; Fortin, D.; Cunanne, S.; Descoteaux, M. Recognition of white matter bundles using local and global streamline-based registration and clustering. Neuroimage 2018, 170, 283–295. [Google Scholar] [CrossRef]
- Garyfallidis, E.; Brett, M.; Correia, M.M.; Williams, G.B.; Nimmo-Smith, I. QuickBundles, a Method for Tractography Simplification. Front. Neurosci. 2012, 6, 175. [Google Scholar] [CrossRef] [PubMed]
- Portegies, J.M.; Fick, R.H.J.; Sanguinetti, G.R.; Meesters, S.P.; Girard, G.; Duits, R. Improving fiber alignment in HARDI by combining contextual PDE flow with constrained spherical deconvolution. PLoS ONE 2015, 10, e0138122. [Google Scholar] [CrossRef] [PubMed]
- Chandio, B.Q.; Risacher, S.L.; Pestilli, F.; Bullock, D.; Yeh, F.C.; Koudoro, S.; Rokem, A.; Harezlak, J.; Garyfallidis, E. Bundle analytics, a computational framework for investigating the shapes and profiles of brain pathways across populations. Sci. Rep. 2020, 10, 17149. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Palejwala, A.H.; Dadario, N.B.; Young, I.M.; O’Connor, K.; Briggs, R.G.; Conner, A.K.; O’Donoghue, D.L.; Sughrue, M.E. Anatomy and white matter connections of the lingual gyrus and cuneus. World Neurosurg. 2021, 151, e426–e437. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Placentino, A.; Carletti, F.; Landi, P.; Allen, P.; Surguladze, S.; Benedetti, F.; Abbamonte, M.; Gasparotti, R.; Barale, F. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009, 34, 418–432. [Google Scholar]
- Grill-Spector, K.; Malach, R. The human visual cortex. Annu. Rev. Neurosci. 2004, 27, 649–677. [Google Scholar] [CrossRef] [PubMed]
- Nomi, J.S.; Scherfeld, D.; Friederichs, S.; Schäfer, R.; Franz, M.; Wittsack, H.-J.; Azari, N.P.; Missimer, J.; Seitz, R.J. On the neural networks of empathy: A principal component analysis of an fMRI study. Behav. Brain Funct. 2008, 4, 41. [Google Scholar] [CrossRef]
- Lepore, N.; Voss, P.; Lepore, F.; Chou, Y.Y.; Fortin, M.; Gougoux, F.; Lee, A.D.; Brun, C.; Lassonde, M.; Madsen, S.K.; et al. Brain structure changes visualized in early- and late-onset blind subjects. Neuroimage 2010, 49, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Desai, R.H.; Graves, W.W.; Conant, L.L. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex 2009, 19, 2767–2796. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Nyberg, L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Liu, X.; Zhou, Z.; Zhou, J.; Li, H. Reduction of interhemispheric functional brain connectivity in early blindness: A resting-state fMRI study. BioMed Res. Int. 2017, 2017, 6756927. [Google Scholar] [CrossRef]
- Sabbah, N.; Sanda, N.; Authié, C.N.; Mohand-Saïd, S.; Sahel, J.-A.; Habas, C.; Amedi, A.; Safran, A.B. Reorganization of early visual cortex functional connectivity following selective peripheral and central visual loss. Sci. Rep. 2017, 7, 43223. [Google Scholar] [CrossRef] [PubMed]
- Ptito, M.; Schneider, F.C.; Paulson, O.B.; Kupers, R. Alterations of the visual pathways in congenital blindness. Exp. Brain Res. 2008, 187, 41–49. [Google Scholar] [CrossRef]
- Bogousslavsky, J.; Miklossy, J.; Deruaz, J.P.; Assal, G.; Regli, F. Lingual and fusiform gyri in visual processing: A clinico-pathologic study of superior altitudinal hemianopia. J. Neurol. Neurosurg. Psychiatry 1987, 50, 607–614. [Google Scholar] [CrossRef]
- Sims, S.A.; Demirayak, P.; Cedotal, S.; Visscher, K.M. Frontal cortical regions associated with attention connect more strongly to central than peripheral V1. Neuroimage 2021, 238, 118246. [Google Scholar] [CrossRef]
- Hyva, J. Regional distribution of functions in parietal association area 7 of the monkey. Brain Res. 1981, 206, 287–303. [Google Scholar]
- Baseler, H.A.; Gouws, A.; Haak, K.V.; Racey, C.; Crossland, M.D.; Tufail, A.; Rubin, G.S.; Cornelissen, F.W.; Morland, A.B. Large-scale remapping of visual cortex is absent in adult humans with macular degeneration. Nat. Neurosci. 2011, 14, 649–655. [Google Scholar] [CrossRef]
- Burton, H. Visual cortex activity in early and late blind people. J. Neurosci. 2003, 23, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Amedi, A.; Merabet, L.B.; Bermpohl, F.; Pascual-Leone, A. The Occipital Cortex in the Blind:Lessons about Plasticity and Vision. Curr. Dir. Psychol. Sci. 2005, 14, 306–311. [Google Scholar] [CrossRef]
- He, B.J.; Snyder, A.Z.; Vincent, J.L.; Epstein, A.; Shulman, G.L.; Corbetta, M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron 2007, 53, 905–918. [Google Scholar] [CrossRef]
- Capotosto, P.; Babiloni, C.; Romani, G.L.; Corbetta, M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J. Neurosci. 2009, 29, 5863–5872. [Google Scholar] [CrossRef]
- Wurm, M.F.; Caramazza, A. Distinct roles of temporal and frontoparietal cortex in representing actions across vision and language. Nat. Commun. 2019, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Li, H.J.; Li, Q.Y.; Liang, R.B.; Shu, H.Y.; Ge, Q.M.; Liao, X.L.; Pan, Y.C.; Wu, J.L.; Su, T.; et al. Functional Connectivity Hypointensity of Middle Cingulate Gyrus and Thalamus in Age-Related Macular Degeneration Patients: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 2022, 14, 854758. [Google Scholar] [CrossRef]
- Barris, R.W.; Schuman, H.R. Bilateral anterior cingulate gyrus lesions; syndrome of the anterior cingulate gyri. Neurology 1953, 3, 44–52. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E. National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.G. How inferior temporal cortex became a visual area. Cereb Cortex 1994, 4, 455–469. [Google Scholar] [CrossRef]
- Karnath, H.O. New insights into the functions of the superior temporal cortex. Nat. Rev. Neurosci. 2001, 2, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R. Current Controversies on Wernicke’s Area and its Role in Language. Curr. Neurol. Neurosci. Rep. 2017, 17, 58. [Google Scholar] [CrossRef]
- Yi, H.G.; Leonard, M.K.; Chang, E.F. The Encoding of Speech Sounds in the Superior Temporal Gyrus. Neuron 2019, 102, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- du Boisgueheneuc, F.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the left superior frontal gyrus in humans: A lesion study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef]
- Counts, S.E.; Nadeem, M.; Lad, S.P.; Wuu, J.; Mufson, E.J. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J. Neuropathol. Exp. Neurol. 2006, 65, 592–601. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Scheff, M.A.; Mufson, E.J. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 24, 547–557. [Google Scholar] [CrossRef]
- Rita Machado, A.; Carvalho Pereira, A.; Ferreira, F.; Ferreira, S.; Quendera, B.; Silva, E.; Castelo-Branco, M. Structure-function correlations in Retinitis Pigmentosa patients with partially preserved vision: A voxel-based morphometry study. Sci. Rep. 2017, 7, 11411. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Hou, H.; Chen, X.; Zhang, J.; Liu, J.; Guo, Z.; Bai, G. Decreased functional connectivity between the dorsal anterior cingulate cortex and lingual gyrus in Alzheimer’s disease patients with depression. Behav. Brain Res. 2017, 326, 132–138. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; Marcos-Dolado, A.; Yus-Fuertes, M.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Salazar, J.J.; Ramírez, A.I.; Sánchez-Puebla, L.; Fuentes-Ferrer, M.E.; et al. The relationship between retinal layers and brain areas in asymptomatic first-degree relatives of sporadic forms of Alzheimer’s disease: An exploratory analysis. Alzheimer’s Res. Ther. 2022, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Silveri, M.C.; Misciagna, S. Language, memory, and the cerebellum. J. Neurolinguistics 2000, 13, 129–143. [Google Scholar] [CrossRef]
- Mariën, P.; Ackermann, H.; Adamaszek, M.; Barwood, C.H.; Beaton, A.; Desmond, J.; De Witte, E.; Fawcett, A.J.; Hertrich, I.; Küper, M. Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum 2014, 13, 386–410. [Google Scholar] [CrossRef]
- Murdoch, B.E. The cerebellum and language: Historical perspective and review. Cortex 2010, 46, 858–868. [Google Scholar] [CrossRef]
- Doyon, J.; Song, A.W.; Karni, A.; Lalonde, F.; Adams, M.M.; Ungerleider, L.G. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. USA 2002, 99, 1017–1022. [Google Scholar] [CrossRef]

| Connection | Average Number Streamlines Control | Average Number Streamlines AMD | T Values | p Values | FDR-Corrected p-Values | |
|---|---|---|---|---|---|---|
| 1st Visit | Insula R– Rostral Middle Frontal R | 270 | 451 | −5.85 | 1.24 × 10−5 | 0.041 |
| Rostral middle frontal R– Post Central R | 47 | 76 | −4.45 | 2.75 × 10−4 | 0.46 | |
| Parsopercularis L– Lateral Orbito Frontal L | 430 | 270 | 3.59 | 5.37 × 10−4 | 0.89 | |
| Inferior Temporal L– Inferior Temporal L | 1836 | 1377 | 3.57 | 2.04 × 10−3 | 0.89 | |
| 2nd Visit | Lingual R– Lateral Occipital R | 642 | 468 | 3.31 | 3.66 × 10−3 | 0.95 |
| Lateral Orbito Frontal R– Caudal Middle Frontal R | 155 | 69 | 3.25 | 4.22 × 10−3 | 0.95 | |
| Parsopercularis R– Lateral Orbito Frontal R | 332 | 219 | 3.23 | 4.38 × 10−3 | 0.95 | |
| Cuneus R–Superior Parietal L | 57 | 123 | −3.16 | 5.18 × 10−3 | 0.95 |
| Index | Connections | Weight | |
|---|---|---|---|
| (a) 1st Visit | 1 | Lingual Right–Lingual Left | 1063.86 |
| 2 | Fusiform Right–Superior temporal Left | 1019.53 | |
| 3 | Superior frontal Right–Superior frontal Left | 966.39 | |
| 4 | Inferior temporal Right–Superior temporal Left | 924.91 | |
| 5 | Superior temporal Right–Superior temporal Left | 888.21 | |
| 6 | Fusiform Right–Insula Left | 840.45 | |
| 7 | Inferior temporal Right–Insula Left | 823.66 | |
| 8 | Insula Right–Superior temporal Right | 801.33 | |
| 9 | Superior temporal Right–Fusiform Right | 776.92 | |
| 10 | Middle temporal Right–Superior temporal Left | 756.07 | |
| (b) 2nd Visit | 1 | Lingual Right–Cerebellum Cortex Right | 1194.30 |
| 2 | Lingual Right–Cerebellum Cortex Left | 1070.30 | |
| 3 | Lingual Left–Cerebellum Cortex Left | 986.70 | |
| 4 | Lingual Right–Cerebellum Cortex Right | 980.10 | |
| 5 | Fusiform Left–Cerebellum Cortex Right | 978.35 | |
| 6 | Fusiform Right–Cerebellum Cortex Left | 966.35 | |
| 7 | Fusiform Right–Lingual Left | 930.43 | |
| 8 | Lingual Right–Fusiform Left | 929.39 | |
| 9 | Cerebellum Cortex Right–Cerebellum Cortex Left | 925.83 | |
| 10 | Lingual Right–Lingual Left | 923.16 | |
| 9 | Cerebellum Cortex Right–Cerebellum Cortex Left | 925.83 | |
| 10 | Lingual Right–Lingual Left | 923.16 | |
| (c) Change Rate | 1 | Superior Frontal Right–Superior Frontal Left | 4280.35 |
| 2 | Rostral Middle Frontal Right–Superior Frontal Left | 3656.48 | |
| 3 | Lateral Orbitofrontal Right–Medial Orbitofrontal Left | 3218.92 | |
| 4 | Medial Orbitofrontal Right–Lateral Orbitofrontal Left | 2436.21 | |
| 5 | Pre-Central Right–Superior Frontal Left | 2070.61 | |
| 6 | Caudal Middle Frontal Right–Superior Frontal Left | 2057.30 | |
| 7 | Superior Frontal Right–Paracentral Right | 1965.98 | |
| 8 | Medial Orbitofrontal Right–Lateral Orbitofrontal Right | 1915.67 | |
| 9 | Superior Frontal Right–Precentral Left | 1842.59 | |
| 10 | Lateral Orbitofrontal Right–Lateral Orbito-frontal Left | 1674.94 |
| Connections | Group | Mean Length | Std Length | p-Val F-Val Cohen | Mean FA | Std FA | p-Val F-Val Cohen | |
|---|---|---|---|---|---|---|---|---|
| 1st Visit | Lingual Right–Lingual Left | Control | 34 | 1.08 | <0.001 108.2 0.45 | 0.109 | 0.004 | 0.0975 2.9 −0.10 |
| AMD | 28 | 1.04 | 0.119 | 0.004 | ||||
| Lingual Left–Right Cerebellum Cortex | Control | 66.6 | 1.73 | <0.001 52.0 0.45 | 0.141 | 0.005 | 0.05 4.1 0.134 | |
| AMD | 54.7 | 1.74 | 0.127 | 0.005 | ||||
| Lingual Right–Left Cerebellum Cortex | Control | 48.3 | 1.13 | <0.001 15.7 0.17 | 0.115 | 0.004 | 0.40 0.7 −0.06 | |
| AMD | 45.4 | 1.1 | 0.119 | 0.004 | ||||
| 2nd Visit | Lingual Right– Lingual Left | Control | 34.4 | 0.88 | <0.001 290.5 0.68 | 0.105 | 0.006 | 0.27 1.2 −0.11 |
| AMD | 25.9 | 0.82 | 0.114 | 0.006 | ||||
| Lingual Left– Right Cerebellum Cortex | Control | 53.8 | 1.06 | <0.001 130.8 0.35 | 0.130 | 0.005 | 0.37 0.8 0.07 | |
| AMD | 47.5 | 1.02 | 0.124 | 0.005 | ||||
| Lingual Right– Left Cerebellum Cortex | Control | 59.2 | 1.31 | <0.001 1330 1.08 | 0.128 | 0.005 | 0.12 2.6 0.13 |
| Year | COI | Group | Avg Dist | Std Dist | p-Val T-Val Cohen | Avg BUAN | Std BUAN | p-Val F-Val Cohen | Avg Coh | Std Coh | p-Val F-Val Cohen | Avg Len | Std Len | p-Val F-Val Cohen | Avg FA | Std FA | p-Val F-Val Cohen |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Visit | Lingual R–Lingual L | CTRL | 16.3 | 8.48 | 0.02 −2.36 −0.40 | 0.83 | 0.35 | 0.06 1.88 0.32 | 0.36 | 0.04 | 0.34 2.2 0.22 | 37 | 2.1 | 0.05 5.46 −0.34 | 0.22 | 0.01 | 0.28 2.49 −0.2 |
| AMD | 20.0 | 10.04 | 0.71 | 0.41 | 0.28 | 0.04 | 43 | 2.1 | 0.24 | 0.01 | |||||||
| Lingual L–Cereb Cortex R | CTRL | 15.0 | 4.6 | 0.18 1.33 0.20 | 0.95 | 0.1 | 0.02 −2.33 −0.35 | 0.26 | 0.03 | 0.85 0.047 −0.03 | 83.7 | 4.33 | 0.0016 15.1 0.77 | 0.22 | 0.006 | <0.001 40.4 0.63 | |
| AMD | 14.1 | 4.1 | 0.98 | 0.05 | 0.27 | 0.03 | 67.9 | 3.74 | 0.17 | 0.006 | |||||||
| Lingual R–Cereb Cortex L | CTRL | 16.8 | 6.8 | 0.84 −0.20 −0.03 | 0.86 | 0.29 | 0.52 −0.64 −0.096 | 0.30 | 0.03 | 0.89 0.089 0.034 | 58.5 | 2.3 | <0.001 23.7 0.59 | 0.22 | 0.006 | 0.02 9.45 0.31 | |
| AMD | 17.0 | 6.60 | 0.88 | 0.22 | 0.29 | 0.03 | 45.7 | 2.2 | 0.19 | 0.006 | |||||||
| 2nd Visit | Lingual R–Lingual L | CTRL | 17.9 | 8.2 | 0.010 1.68 0.28 | 0.85 | 0.30 | 0.15 −1.43 −0.24 | 0.34 | 0.041 | 0.37 1.12 0.16 | 42.5 | 2.8 | 0.11 3.58 0.33 | 0.21 | 0.008 | 0.007 12.0 0.35 |
| AMD | 15.7 | 7.4 | 0.91 | 0.21 | 0.28 | 0.044 | 36.2 | 2.9 | 0.17 | 0.009 | |||||||
| Lingual L–Cereb Cortex R | CTRL | 14.9 | 5.2 | 0.50 −0.67 −0.10 | 0.94 | 0.14 | 0.52 0.65 0.01 | 0.34 | 0.03 | 0.008 12.1 0.40 | 72.0 | 0.03 | 0.81 0.21 −0.07 | 0.19 | 0.005 | 0.01 11.2 0.39 | |
| AMD | 15.4 | 5.5 | 0.93 | 0.16 | 0.23 | 0.02 | 46.3 | 0.03 | 0.16 | 0.006 | |||||||
| Lingual R–Cereb Cortex L | CTRL | 16.4 | 5.37 | 0.45 −0.76 −0.11 | 0.92 | 0.16 | 0.066 1.85 0.28 | 0.25 | 0.03 | 0.98 0.26 0.09 | 92.1 | 3.4 | 0.002 14.6 0.69 | 0.23 | 0.005 | <0.001 39.8 0.59 | |
| AMD | 17.1 | 6.73 | 0.86 | 0.27 | 0.23 | 0.03 | 71.5 | 4.4 | 0.18 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stout, J.A.; Mahzarnia, A.; Dai, R.; Anderson, R.J.; Cousins, S.; Zhuang, J.; Lad, E.M.; Whitaker, D.B.; Madden, D.J.; Potter, G.G.; et al. Accelerated Brain Atrophy, Microstructural Decline and Connectopathy in Age-Related Macular Degeneration. Biomedicines 2024, 12, 147. https://doi.org/10.3390/biomedicines12010147
Stout JA, Mahzarnia A, Dai R, Anderson RJ, Cousins S, Zhuang J, Lad EM, Whitaker DB, Madden DJ, Potter GG, et al. Accelerated Brain Atrophy, Microstructural Decline and Connectopathy in Age-Related Macular Degeneration. Biomedicines. 2024; 12(1):147. https://doi.org/10.3390/biomedicines12010147
Chicago/Turabian StyleStout, Jacques A., Ali Mahzarnia, Rui Dai, Robert J. Anderson, Scott Cousins, Jie Zhuang, Eleonora M. Lad, Diane B. Whitaker, David J. Madden, Guy G. Potter, and et al. 2024. "Accelerated Brain Atrophy, Microstructural Decline and Connectopathy in Age-Related Macular Degeneration" Biomedicines 12, no. 1: 147. https://doi.org/10.3390/biomedicines12010147





