Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Light Microscopy
2.3. Immunohistochemistry
2.4. Semiquantitative Analysis
2.5. Quantification of Cell Numbers
3. Results
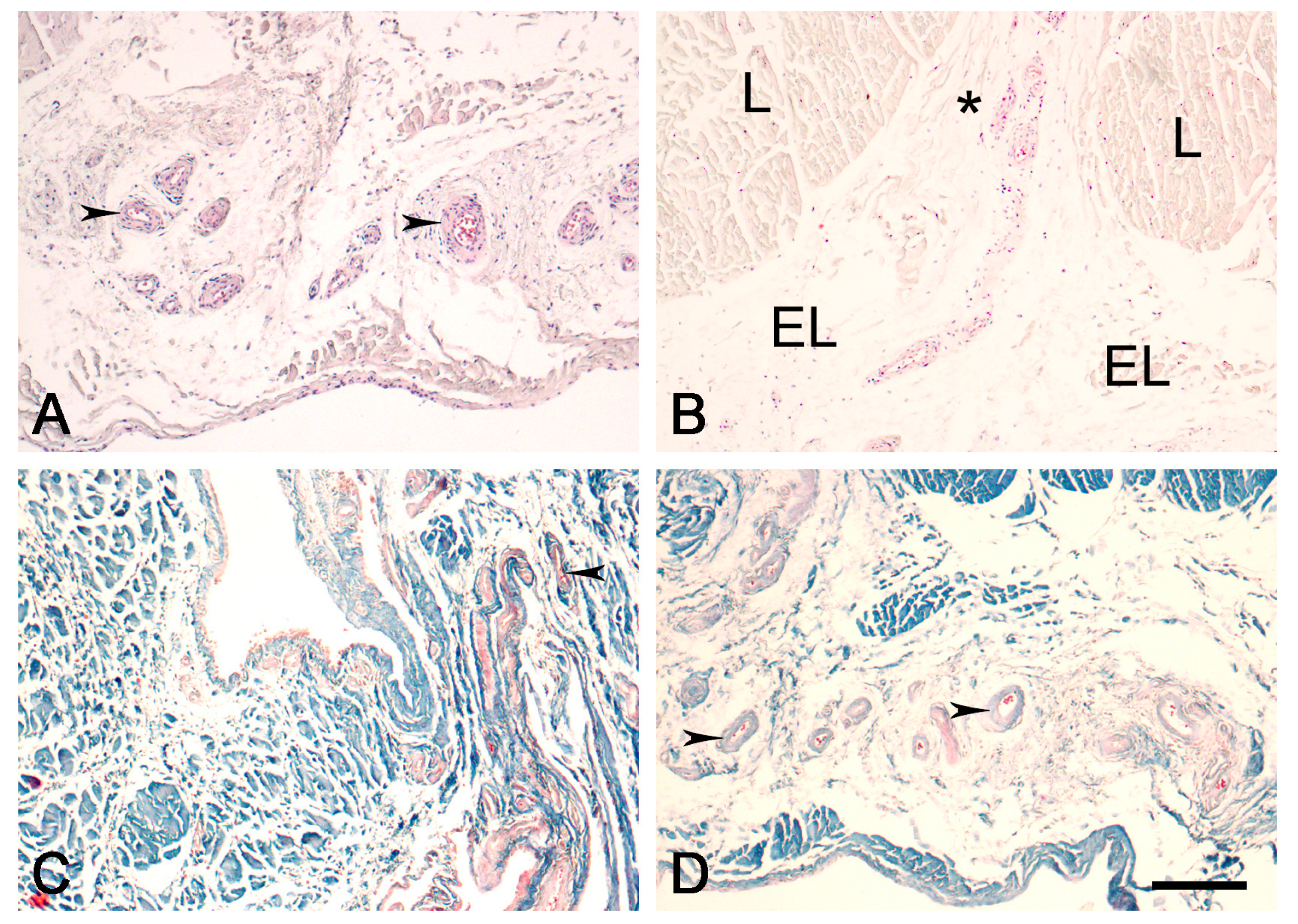
3.1. Light Microscopy
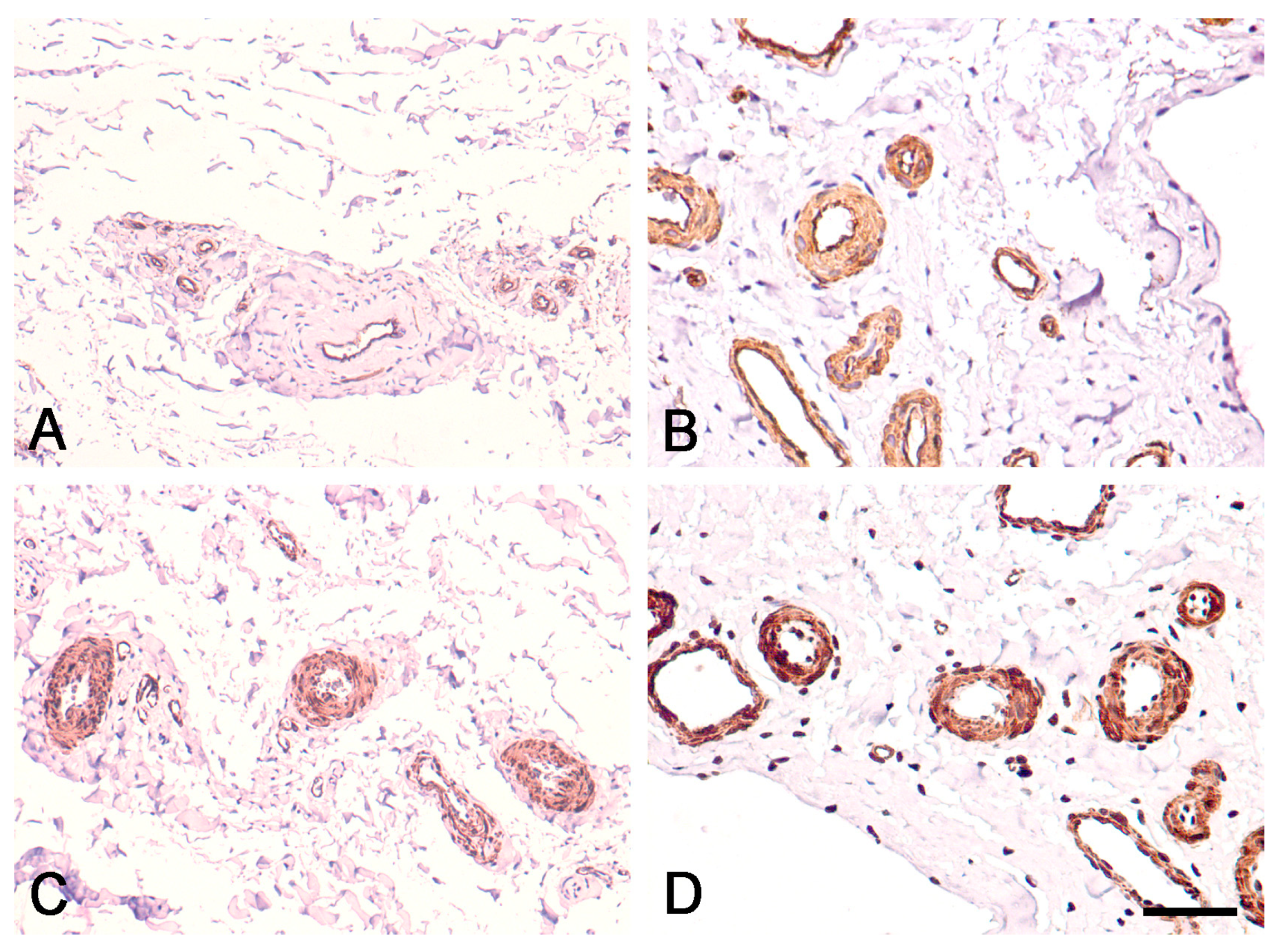
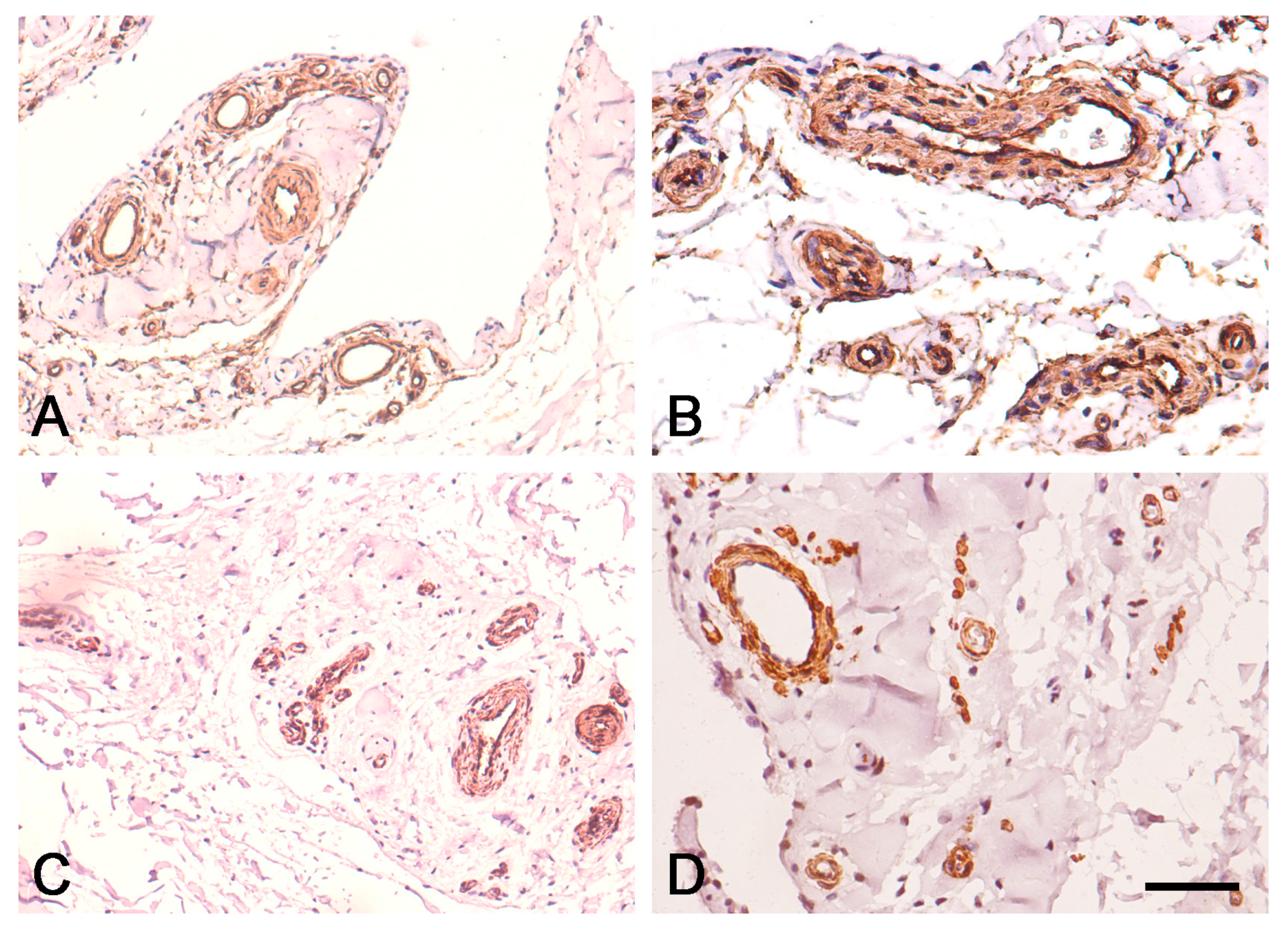
3.2. Expression of CD34 and α-SMA in Proximal and Distal ACL EL

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheth, M.R.; Tapasvi, S.R.; Patil, S.S. Primary repair of tibial-sided avulsion of the anterior cruciate ligament. Arthrosc. Tech. 2016, 5, e901–e906. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Fleming, B.C. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J. Orthop. Res. 2013, 31, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Feagin, J.A., Jr.; Curl, W.W. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am. J. Sports Med. 1976, 4, 95–100. [Google Scholar] [CrossRef] [PubMed]
- van der List, J.P.; Vermeijden, H.D.; Sierevelt, I.N.; Rademakers, M.V.; Falke, M.L.M.; Helmerhorst, G.T.T.; Hoogeslag, R.A.G.; van der Wal, W.A.; van Noort, A.; Kerkhoffs, G.M.M.J. Repair versus reconstruction for proximal anterior cruciate ligament tears: A study protocol for a prospective multicenter randomized controlled trial. BMC Musculoskelet. Disord. 2021, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Oiestad, B.E.; Holm, I.; Aune, A.K.; Gunderson, R.; Myklebust, G.; Engebretsen, L.; Fosdahl, M.A.; Risberg, M.A. Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: A prospective study with 10 to 15 years of follow-up. Am. J. Sports Med. 2010, 38, 2201–2210. [Google Scholar] [CrossRef]
- DiFelice, G.S.; Villegas, C.; Taylor, S.A. Anterior cruciate ligament preservation: Early results of a novel arthroscopic technique for suture anchor primary anterior cruciate ligament repair. Arthroscopy 2015, 31, 2162–2171. [Google Scholar] [CrossRef]
- van der List, J.P.; Vermeijden, H.D.; Sierevelt, I.N.; DiFelice, G.S.; van Noort, A.; Kerkhoffs, G.M.M.J. Arthroscopic primary repair of proximal anterior cruciate ligament tears seems safe but higher level of evidence is needed: A systematic review and meta-analysis of recent literature. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1946–1957. [Google Scholar] [CrossRef]
- Vermeijden, H.D.; Yang, X.A.; van der List, J.P.; Difelice, G.S. Role of age on success of arthroscopic primary repair of proximal anterior cruciate ligament tears. Arthroscopy 2020, 37, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Hopper, G.P.; Aithie, J.M.S.; Jenkins, J.M.; Wilson, W.T.; Mackay, G.M. Satisfactory patient-reported outcomes at 5 years following primary repair with suture tape augmentation for proximal anterior cruciate ligament tears. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 253–259. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Kotov, G.; Iliev, A.; Kinov, P.; Angelova, J.; Landzhov, B. Comparison between Operative and Non-Operative Treatment of the Medial Collateral Ligament: Histological and Ultrastructural Findings during Early Healing in the Epiligament Tissue in a Rat Knee Model. Cells Tis. Organs. 2018, 206, 165–182. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Kotov, G.; Iliev, A.; Slavchev, S.; Ovtscharoff, W.; Landzhov, B. A comparative study of the epiligament of the medial collateral and the anterior cruciate ligament in the human knee. Immunohistochemical analysis of collagen type I and V and procollagen type III. Ann. Anat. 2019, 224, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.P.; Tubbs, R.S.; Olewnik, Ł.; Zielinska, N.; Telang, M.; Ananiev, J.; Dimitrova, I.N.; Slavchev, S.A.; Yordanov, Y.; LaPrade, R.F.; et al. A comparative study of the epiligament of the medial collateral and anterior cruciate ligaments in the human knee: Immunohistochemical analysis of CD34, α-smooth muscle actin and vascular endothelial growth factor in relation to epiligament theory. Knee 2022, 39, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.P.; Landzhov, B.; Kotov, G.; Slavchev, S.A.; Iliev, A. Matrix metalloproteinase-2 and -9 expression in the epiligament of the medial collateral and anterior cruciate ligament in human knees: A comparative study. Cureus 2018, 10, e3550. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.P.; Telang, M.; Landzhov, B.; Olewnik, Ł.; Slavchev, S.A.; LaPrade, R.F.; Ruzik, K.; Tubbs, R.S. The novel epiligament theory: Differences in healing failure between the medial collateral and anterior cruciate ligaments. J. Exp. Orthop. 2022, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Howson, K.M.; Aplin, A.C.; Gelati, M.; Alessandri, G.; Parati, E.A.; Nicosia, R.F. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am. J. Physiol. Cell Physiol. 2005, 289, C1396–C1407. [Google Scholar] [CrossRef] [PubMed]
- Tavian, M.; Zheng, B.; Oberlin, E.; Crisan, M.; Sun, B.; Huard, J.; Peault, B. The vascular wall as a source of stem cells. Ann. N. Y. Acad. Sci. 2005, 1044, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Jo, S.; Lee, Y.L.; Park, H.; Song, J.S.; Sung, I.H.; Kim, T.H. Anterior cruciate ligament remnant cells have different potentials for cell differentiation based on their location. Sci. Rep. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Hinz, B.; Celetta, G.; Tomasek, J.J.; Gabbiani, G.; Chaponnier, C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol. Biol. Cell. 2001, 12, 2730–2741. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Menetrey, J.; Laumonier, T.; Garavaglia, G.; Hoffmeyer, P.; Fritschy, D.; Gabbiani, G.; Bochaton-Piallat, M.L. α-Smooth muscle actin and TGF-β receptor I expression in the healing rabbit medial collateral and anterior cruciate ligaments. Injury 2011, 42, 735–741. [Google Scholar] [CrossRef]
- Kanaya, A.; Deie, M.; Adachi, N.; Nishimori, M.; Yanada, S.; Ochi, M. Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy 2007, 23, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Singh, V.; Takeda, S.; Ogeng’o, J.; Kim, H.J.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Standardized statement for the ethical use of human cadaveric tissues in anatomy research papers: Recommendations from Anatomical Journal Editors-in-Chief. Clin. Anat. 2022, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://www.R-project.org/ (accessed on 25 May 2023).
- Posit Team. RStudio: Integrated Development Environment for R [Internet] (2023). Boston, MA: RStudio, PBC. Available online: http://www.rstudio.com/ (accessed on 25 May 2023).
- Wickham, H. Elegant graphics for data analysis. Media 2009, 35, 211. [Google Scholar]
- Matsumoto, T.; Ingham, S.M.; Mifune, Y.; Osawa, A.; Logar, A.; Usas, A.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Isolation and characterization of human anterior cruciate ligament-derived vascular stem cells. Stem Cells Dev. 2012, 21, 859–872. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kubo, S.; Sasaki, K.; Kawakami, Y.; Oka, S.; Sasaki, H.; Takayama, K.; Tei, K.; Matsushita, T.; Mifune, Y.; et al. Acceleration of tendon-bone healing of anterior cruciate ligament graft using autologous ruptured tissue. Am. J. Sports Med. 2012, 40, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Mifune, Y.; Matsumoto, T.; Ota, S.; Nishimori, M.; Usas, A.; Kopf, S.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012, 21, 1651–1665. [Google Scholar] [CrossRef]
- Mifune, Y.; Matsumoto, T.; Takayama, K.; Terada, S.; Sekiya, N.; Kuroda, R.; Kurosaka, M.; Fu, F.H.; Huard, J. Tendon graft revitalization using adult anterior cruciate ligament (ACL)-derived CD34+ cell sheets for ACL reconstruction. Biomaterials 2013, 34, 5476–5487. [Google Scholar] [CrossRef]
- Nakano, N.; Matsumoto, T.; Takayama, K.; Matsushita, T.; Araki, D.; Uefuji, A.; Nagai, K.; Zhang, S.; Inokuchi, T.; Nishida, K.; et al. Age-dependent healing potential of anterior cruciate ligament remnant-derived cells. Am. J. Sports Med. 2015, 43, 700–708. [Google Scholar] [CrossRef]
- Kirizuki, S.; Matsumoto, T.; Ueha, T.; Uefuji, A.; Inokuchi, T.; Takayama, K.; Hashimoto, S.; Hayashi, S.; Matsushita, T.; Kuroda, R. The influence of ruptured scar pattern on the healing potential of anterior cruciate ligament remnant cells. Am. J. Sports Med. 2018, 46, 1382–1388. [Google Scholar] [CrossRef]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. J. Bone Joint Surg. Am. 2000, 82, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Spector, M. Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: The presence of alpha-smooth muscle actin-positive cells. J. Orthop. Res. 1999, 17, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fishkin, Z.; Miller, D.; Ritter, C.; Ziv, I. Changes in human knee ligament stiffness secondary to osteoarthritis. J. Orthop. Res. 2002, 20, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Seo, G.S.; Gale, D.; Totterman, S.; Gale, M.E.; Felson, D.T. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005, 52, 794–799. [Google Scholar] [CrossRef]
- Nur, H.; Aytekin, A.; Gilgil, E. Medial collateral ligament bursitis in a patient with knee osteoarthritis. J. Back Musculoskelet. Rehabil. 2018, 31, 589–591. [Google Scholar] [CrossRef]
- Iwanaga, J.; Singh, V.; Ohtsuka, A.; Hwang, Y.; Kim, H.J.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clin. Anat. 2021, 34, 2–4. [Google Scholar] [CrossRef]
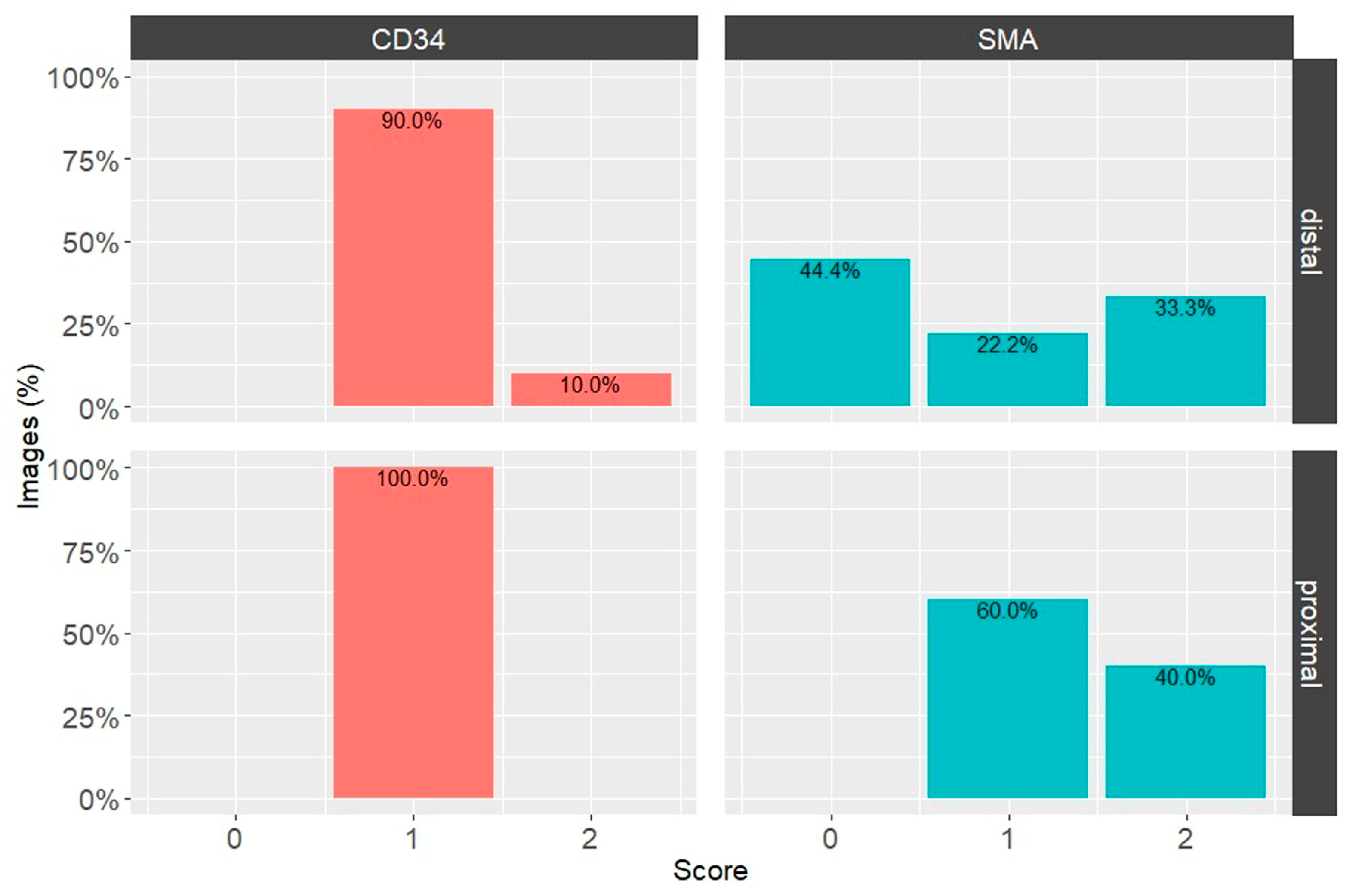

| Proximal Third | Distal Third | IHC Marker |
|---|---|---|
| High Positive (3+) (3.8%) | High Positive (3+) (4.0%) | CD34 |
| Positive (2+) (9.4%) | Positive (2+) (9.7%) | |
| Low Positive (1+) (78.9%) | Low Positive (1+) (55.0%) | |
| High Positive (3+) (7.4%) | High Positive (3+) (3.7%) | α-SMA |
| Positive (2+) (22.5%) | Positive (2+) (27.1%) | |
| Low Positive (1+) (69.3%) | Low Positive (1+) (20.2%) | |
| Negative (0) (0.8%) | Negative (0) (49.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, G.P.; Yordanov, Y.; Olewnik, Ł.; Tubbs, R.S.; LaPrade, R.F.; Ananiev, J.; Slavchev, S.A.; Dimitrova, I.N.; Gaydarski, L.; Landzhov, B. Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory. Biomedicines 2024, 12, 156. https://doi.org/10.3390/biomedicines12010156
Georgiev GP, Yordanov Y, Olewnik Ł, Tubbs RS, LaPrade RF, Ananiev J, Slavchev SA, Dimitrova IN, Gaydarski L, Landzhov B. Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory. Biomedicines. 2024; 12(1):156. https://doi.org/10.3390/biomedicines12010156
Chicago/Turabian StyleGeorgiev, Georgi P., Yordan Yordanov, Łukasz Olewnik, Richard Shane Tubbs, Robert F. LaPrade, Julian Ananiev, Svetoslav A. Slavchev, Iva N. Dimitrova, Lyubomir Gaydarski, and Boycho Landzhov. 2024. "Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory" Biomedicines 12, no. 1: 156. https://doi.org/10.3390/biomedicines12010156
APA StyleGeorgiev, G. P., Yordanov, Y., Olewnik, Ł., Tubbs, R. S., LaPrade, R. F., Ananiev, J., Slavchev, S. A., Dimitrova, I. N., Gaydarski, L., & Landzhov, B. (2024). Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory. Biomedicines, 12(1), 156. https://doi.org/10.3390/biomedicines12010156







