The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective
Abstract
1. Introduction
2. Bacterial Infections
3. Viral Infections
4. Fungal Infections
5. Parasitic Infections
6. Sepsis
7. D-Dopachrome Tautomerase
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Assunção-Miranda, I.; Amaral, F.A.; Bozza, F.A.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of Macrophage Migration Inhibitory Factor to the Pathogenesis of Dengue Virus Infection. FASEB J. 2010, 24, 218–228. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and Mortality of Hospital- and ICU-Treated Sepsis: Results from an Updated and Expanded Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in Sepsis and Septic Shock in Europe, North America and Australia between 2009 and 2019- Results from a Systematic Review and Meta-Analysis. Crit. Care 2020, 24, 239. [Google Scholar] [CrossRef]
- Yatim, K.M.; Lakkis, F.G. A Brief Journey through the Immune System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Fortingo, N.; Melnyk, S.; Sutton, S.H.; Watsky, M.A.; Bollag, W.B. Innate Immune System Activation, Inflammation and Corneal Wound Healing. Int. J. Mol. Sci. 2022, 23, 14933. [Google Scholar] [CrossRef]
- Kudrin, A.; Ray, D. Cunning Factor: Macrophage Migration Inhibitory Factor as a Redox-Regulated Target. Immunol. Cell Biol. 2008, 86, 232–238. [Google Scholar] [CrossRef] [PubMed]
- David, J.R. Delayed Hypersensitivity in Vitro: Its Mediation by Cell-Free Substances Formed by Lymphoid Cell-Antigen Interaction. Proc. Natl. Acad. Sci. USA 1966, 56, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bloom, B.R.; Bennett, B. Mechanism of a Reaction in Vitro Associated with Delayed-Type Hypersensitivity. Science 1966, 153, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Roger, T. Macrophage Migration Inhibitory Factor: A Regulator of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 791–800. [Google Scholar] [CrossRef]
- Bernhagen, J.; Calandra, T.; Mitchell, R.A.; Martin, S.B.; Tracey, K.J.; Voelter, W.; Manogue, K.R.; Cerami, A.; Bucala, R. MIF Is a Pituitary-Derived Cytokine That Potentiates Lethal Endotoxaemia. Nature 1993, 365, 756–759. [Google Scholar] [CrossRef]
- Calandra, T.; Echtenacher, B.; Roy, D.L.; Pugin, J.; Metz, C.N.; Hültner, L.; Heumann, D.; Männel, D.; Bucala, R.; Glauser, M.P. Protection from Septic Shock by Neutralization of Macrophage Migration Inhibitory Factor. Nat. Med. 2000, 6, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xiao, Z.; Dekker, F.J.; Poelarends, G.J.; Melgert, B.N. Macrophage Migration Inhibitory Factor Family Proteins Are Multitasking Cytokines in Tissue Injury. Cell Mol. Life Sci. 2022, 79, 105. [Google Scholar] [CrossRef]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF Is a Noncognate Ligand of CXC Chemokine Receptors in Inflammatory and Atherogenic Cell Recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF Signal Transduction Initiated by Binding to CD74. J. Exp. Med. 2003, 197, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 Is the Signaling Component of the Macrophage Migration Inhibitory Factor-CD74 Receptor Complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef]
- Farr, L.; Ghosh, S.; Moonah, S. Role of MIF Cytokine/CD74 Receptor Pathway in Protecting Against Injury and Promoting Repair. Front. Immunol. 2020, 11, 1273. [Google Scholar] [CrossRef] [PubMed]
- Alampour-Rajabi, S.; El Bounkari, O.; Rot, A.; Müller-Newen, G.; Bachelerie, F.; Gawaz, M.; Weber, C.; Schober, A.; Bernhagen, J. MIF Interacts with CXCR7 to Promote Receptor Internalization, ERK1/2 and ZAP-70 Signaling, and Lymphocyte Chemotaxis. FASEB J. 2015, 29, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Pantouris, G.; Khurana, L.; Ma, A.; Skeens, E.; Reiss, K.; Batista, V.S.; Lisi, G.P.; Lolis, E.J. Regulation of MIF Enzymatic Activity by an Allosteric Site at the Central Solvent Channel. Cell Chem. Biol. 2020, 27, 740–750.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Wen, K.; Zhu, L.-L.; Lv, S.-K.; Cao, Q.; Li, Q.; Deng, L.; Chen, T.; Wang, X.; Deng, K.-Y.; et al. Tautomerase Activity-Lacking of the Macrophage Migration Inhibitory Factor Alleviates the Inflammation and Insulin Tolerance in High Fat Diet-Induced Obese Mice. Front. Endocrinol. 2020, 11, 134. [Google Scholar] [CrossRef] [PubMed]
- Sumaiya, K.; Selvambika, P.; Natarajaseenivasan, K. Anti-Macrophage Migration Inhibitory Factor (MIF) Activity of Ibudilast: A Repurposing Drug Attenuates the Pathophysiology of Leptospirosis. Microb. Pathog. 2022, 173, 105786. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kam, T.-I.; Peng, H.; Chou, S.-C.; Mehrabani-Tabari, A.A.; Song, J.-J.; Yin, X.; Karuppagounder, S.S.; Umanah, G.K.; Rao, A.V.S.; et al. PAAN/MIF Nuclease Inhibition Prevents Neurodegeneration in Parkinson’s Disease. Cell 2022, 185, 1943–1959.e21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, R.; Umanah, G.K.; Park, H.; Nambiar, K.; Eacker, S.M.; Kim, B.; Bao, L.; Harraz, M.M.; Chang, C.; et al. A Nuclease That Mediates Cell Death Induced by DNA Damage and Poly(ADP-Ribose) Polymerase-1. Science 2016, 354, aad6872. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shu, T.; Zhao, H.; Sun, Y.; Xu, W.; Tu, G. Knockdown of Macrophage Migration Inhibitory Factor (MIF), a Novel Target to Protect Neurons from Parthanatos Induced by Simulated Post-Spinal Cord Injury Oxidative Stress. Biochem. Biophys. Res. Commun. 2020, 523, 719–725. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Ke, Y.; Zeng, X.; Gao, J.; Ba, X.; Wang, R. The Key Players of Parthanatos: Opportunities for Targeting Multiple Levels in the Therapy of Parthanatos-Based Pathogenesis. Cell Mol. Life Sci. 2022, 79, 60. [Google Scholar] [CrossRef]
- Machado, F.D.; Gehlen, M.; Caron, V.S.; Mousquer, G.T.; Bello, G.L.; Anton, C.; Bernardi, R.M.; Freitas, A.A.; Unis, G.; Costa, E.R.D.; et al. Macrophage Migration Inhibitory Factor - 794 CATT5-8 Microsatellite Polymorphism and Susceptibility of Tuberculosis. Infection 2021, 49, 457–461. [Google Scholar] [CrossRef]
- Zhang, C.; Ramsey, C.; Berical, A.; Yu, L.; Leng, L.; McGinnis, K.A.; Song, Y.; Michael, H.; McCormack, M.C.; Allore, H.; et al. A Functional Macrophage Migration Inhibitory Factor Promoter Polymorphism Is Associated with Reduced Diffusing Capacity. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L400–L405. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Shenoi, S.; Singh, R.; Wang, M.; Patel, V.; Das, R.; Hiramen, K.; Moosa, Y.; Eksteen, F.; Moll, A.P.; et al. Low Expression Macrophage Migration Inhibitory Factor (MIF) Alleles and Tuberculosis in HIV Infected South Africans. Cytokine X 2019, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, T.H.; Fischer, P.; Backhaus, C.; Bergmann, I.; Brandt, E.F.; Heinrichs, D.; Koenen, M.T.; Schneider, K.M.; Eggermann, T.; Kurth, I.; et al. Genetic Variants in the Promoter Region of the Macrophage Migration Inhibitory Factor Are Associated with the Severity of Hepatitis C Virus-Induced Liver Fibrosis. Int. J. Mol. Sci. 2019, 20, 3753. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, A.; De Baetselier, P.; Roelants, K.; De Trez, C.; Magez, S.; Van Ginderachter, J.A.; Raes, G.; Bucala, R.; Stijlemans, B. The Non-Mammalian MIF Superfamily. Immunobiology 2017, 222, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Schindler, L.; Dickerhof, N.; Hampton, M.B.; Bernhagen, J. Post-Translational Regulation of Macrophage Migration Inhibitory Factor: Basis for Functional Fine-Tuning. Redox Biol. 2018, 15, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bernhagen, J.; Mitchell, R.A.; Calandra, T.; Voelter, W.; Cerami, A.; Bucala, R. Purification, Bioactivity, and Secondary Structure Analysis of Mouse and Human Macrophage Migration Inhibitory Factor (MIF). Biochemistry 1994, 33, 14144–14155. [Google Scholar] [CrossRef]
- Bozza, M.; Satoskar, A.R.; Lin, G.; Lu, B.; Humbles, A.A.; Gerard, C.; David, J.R. Targeted Disruption of Migration Inhibitory Factor Gene Reveals Its Critical Role in Sepsis. J. Exp. Med. 1999, 189, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.A.; Liao, H.; Chesney, J.; Fingerle-Rowson, G.; Baugh, J.; David, J.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF) Sustains Macrophage Proinflammatory Function by Inhibiting P53: Regulatory Role in the Innate Immune Response. Proc. Natl. Acad. Sci. USA 2002, 99, 345–350. [Google Scholar] [CrossRef]
- Flores, M.; Saavedra, R.; Bautista, R.; Viedma, R.; Tenorio, E.P.; Leng, L.; Sánchez, Y.; Juárez, I.; Satoskar, A.A.; Shenoy, A.S.; et al. Macrophage Migration Inhibitory Factor (MIF) Is Critical for the Host Resistance against Toxoplasma Gondii. FASEB J. 2008, 22, 3661–3671. [Google Scholar] [CrossRef]
- Merk, M.; Zierow, S.; Leng, L.; Das, R.; Du, X.; Schulte, W.; Fan, J.; Lue, H.; Chen, Y.; Xiong, H.; et al. The D-Dopachrome Tautomerase (DDT) Gene Product Is a Cytokine and Functional Homolog of Macrophage Migration Inhibitory Factor (MIF). Proc. Natl. Acad. Sci. USA 2011, 108, E577–E585. [Google Scholar] [CrossRef]
- Das, R.; Koo, M.-S.; Kim, B.H.; Jacob, S.T.; Subbian, S.; Yao, J.; Leng, L.; Levy, R.; Murchison, C.; Burman, W.J.; et al. Macrophage Migration Inhibitory Factor (MIF) Is a Critical Mediator of the Innate Immune Response to Mycobacterium Tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E2997–E3006. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Tilstam, P.V.; Hwang, S.S.; Simons, D.; Schulte, W.; Leng, L.; Sauler, M.; Ganse, B.; Averdunk, L.; Kopp, R.; et al. D-Dopachrome Tautomerase in Adipose Tissue Inflammation and Wound Repair. J. Cell Mol. Med. 2017, 21, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Megas, I.-F.; Simons, D.; Kim, B.-S.; Stoppe, C.; Piatkowski, A.; Fikatas, P.; Fuchs, P.C.; Bastiaanse, J.; Pallua, N.; Bernhagen, J.; et al. Macrophage Migration Inhibitory Factor-An Innovative Indicator for Free Flap Ischemia after Microsurgical Reconstruction. Healthcare 2021, 9, 616. [Google Scholar] [CrossRef] [PubMed]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF) as a Therapeutic Target for Rheumatoid Arthritis and Systemic Lupus Erythematosus. Expert. Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xie, Z.; Sun, G.; Chen, L.; Qi, D.; Zhang, H.; Xiong, J.; Furey, A.; Rahman, P.; Lei, G.; et al. Macrophage Migration Inhibitory Factor May Play a Protective Role in Osteoarthritis. Arthritis Res. Ther. 2021, 23, 59. [Google Scholar] [CrossRef] [PubMed]
- Allam, V.S.R.R.; Pavlidis, S.; Liu, G.; Kermani, N.Z.; Simpson, J.; To, J.; Donnelly, S.; Guo, Y.-K.; Hansbro, P.M.; Phipps, S.; et al. Macrophage Migration Inhibitory Factor Promotes Glucocorticoid Resistance of Neutrophilic Inflammation in a Murine Model of Severe Asthma. Thorax 2022, 78, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yan, S.; Wu, J.; Zhang, Z.; Li, X.; Liu, Z.; Ma, X.; Zhou, L.; Zhang, L.; Feng, M.; et al. Serum Macrophage Migration Inhibitory Factor as a Potential Biomarker to Evaluate Therapeutic Response in Patients with Allergic Asthma: An Exploratory Study. J. Zhejiang Univ. Sci. B 2021, 22, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Richard, V.; Kindt, N.; Saussez, S. Macrophage Migration Inhibitory Factor Involvement in Breast Cancer (Review). Int. J. Oncol. 2015, 47, 1627–1633. [Google Scholar] [CrossRef]
- Wirtz, T.H.; Saal, A.; Bergmann, I.; Fischer, P.; Heinrichs, D.; Brandt, E.F.; Koenen, M.T.; Djudjaj, S.; Schneider, K.M.; Boor, P.; et al. Macrophage Migration Inhibitory Factor Exerts Pro-Proliferative and Anti-Apoptotic Effects via CD74 in Murine Hepatocellular Carcinoma. Br. J. Pharmacol. 2021, 178, 4452–4467. [Google Scholar] [CrossRef]
- Balogh, K.N.; Templeton, D.J.; Cross, J.V. Macrophage Migration Inhibitory Factor Protects Cancer Cells from Immunogenic Cell Death and Impairs Anti-Tumor Immune Responses. PLoS ONE 2018, 13, e0197702. [Google Scholar] [CrossRef]
- Grieb, G.; Simons, D.; Piatkowski, A.; Bernhagen, J.; Steffens, G.; Pallua, N. Macrophage Migration Inhibitory Factor-A Potential Diagnostic Tool in Severe Burn Injuries? Burns 2010, 36, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Grieb, G.; Simons, D.; Eckert, L.; Hemmrich, M.; Steffens, G.; Bernhagen, J.; Pallua, N. Levels of Macrophage Migration Inhibitory Factor and Glucocorticoids in Chronic Wound Patients and Their Potential Interactions with Impaired Wound Endothelial Progenitor Cell Migration. Wound Repair. Regen. 2012, 20, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Breuer, B.; Arnke, K.; Ruhl, T.; Hofer, T.; Simons, D.; Knobe, M.; Ganse, B.; Guidi, M.; Beier, J.P.; et al. The Effect of the Macrophage Migration Inhibitory Factor (MIF) on Excisional Wound Healing In Vivo. J. Plast. Surg. Hand Surg. 2020, 54, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-Q.; Tang, Z.; Huang, R.; Qu, W.-F.; Fang, Y.; Yang, R.; Tao, C.-Y.; Gao, J.; Wu, X.-L.; Sun, H.-X.; et al. CD36+ Cancer-Associated Fibroblasts Provide Immunosuppressive Microenvironment for Hepatocellular Carcinoma via Secretion of Macrophage Migration Inhibitory Factor. Cell Discov. 2023, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Cai, W.; Yang, J.; Fu, X.; Putha, L.; Xia, Q.; Windsor, J.A.; Phillips, A.R.; Tyndall, J.D.A.; Du, D.; et al. Targeting Macrophage Migration Inhibitory Factor in Acute Pancreatitis and Pancreatic Cancer. Front. Pharmacol. 2021, 12, 638950. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Feng, Z.; Tao, S.; Gao, J.; Lin, Y.; Wei, X.; Zheng, B.; Huang, B.; Zheng, Z.; Zhang, X.; et al. Destabilization of Macrophage Migration Inhibitory Factor by 4-IPP Reduces NF-κB/P-TEFb Complex-Mediated c-Myb Transcription to Suppress Osteosarcoma Tumourigenesis. Clin. Transl. Med. 2022, 12, e652. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Bustos, M.A.; Cho, S.-N.; Roszik, J.; Ryu, S.; Lopez, V.M.; Burks, J.K.; Lee, J.E.; Grimm, E.A.; Hoon, D.S.B.; et al. Interplay between Soluble CD74 and Macrophage-Migration Inhibitory Factor Drives Tumor Growth and Influences Patient Survival in Melanoma. Cell Death Dis. 2022, 13, 117. [Google Scholar] [CrossRef]
- Satoskar, A.R.; Bozza, M.; Rodriguez Sosa, M.; Lin, G.; David, J.R. Migration-Inhibitory Factor Gene-Deficient Mice Are Susceptible to Cutaneous Leishmania Major Infection. Infect. Immun. 2001, 69, 906–911. [Google Scholar] [CrossRef]
- Koebernick, H.; Grode, L.; David, J.R.; Rohde, W.; Rolph, M.S.; Mittrücker, H.-W.; Kaufmann, S.H.E. Macrophage Migration Inhibitory Factor (MIF) Plays a Pivotal Role in Immunity against Salmonella Typhimurium. Proc. Natl. Acad. Sci. USA 2002, 99, 13681–13686. [Google Scholar] [CrossRef]
- Reyes, J.L.; Terrazas, L.I.; Espinoza, B.; Cruz-Robles, D.; Soto, V.; Rivera-Montoya, I.; Gómez-García, L.; Snider, H.; Satoskar, A.R.; Rodríguez-Sosa, M. Macrophage Migration Inhibitory Factor Contributes to Host Defense against Acute Trypanosoma Cruzi Infection. Infect. Immun. 2006, 74, 3170–3179. [Google Scholar] [CrossRef]
- Tilstam, P.V.; Schulte, W.; Holowka, T.; Kim, B.-S.; Nouws, J.; Sauler, M.; Piecychna, M.; Pantouris, G.; Lolis, E.; Leng, L.; et al. MIF but Not MIF-2 Recruits Inflammatory Macrophages in an Experimental Polymicrobial Sepsis Model. J. Clin. Investig. 2021, 131, e127171. [Google Scholar] [CrossRef] [PubMed]
- Trifone, C.; Salido, J.; Ruiz, M.J.; Leng, L.; Quiroga, M.F.; Salomón, H.; Bucala, R.; Ghiglione, Y.; Turk, G. Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4+ T Cells. Front. Immunol. 2018, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Baugh, J.A.; Donnelly, S.C. Macrophage Migration Inhibitory Factor: A Neuroendocrine Modulator of Chronic Inflammation. J. Endocrinol. 2003, 179, 15–23. [Google Scholar] [CrossRef] [PubMed]
- García-Arellano, S.; Hernández-Palma, L.A.; Bucala, R.; Hernández-Bello, J.; De la Cruz-Mosso, U.; García-Iglesias, T.; Cerpa-Cruz, S.; Padilla-Gutiérrez, J.R.; Valle, Y.; Soñanez-Organis, J.G.; et al. Th1/Th17 Cytokine Profile Is Induced by Macrophage Migration Inhibitory Factor in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Curr. Mol. Med. 2018, 18, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Trifone, C.; Baquero, L.; Czernikier, A.; Benencio, P.; Leng, L.; Laufer, N.; Quiroga, M.F.; Bucala, R.; Ghiglione, Y.; Turk, G. Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection. Viruses 2022, 14, 2218. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Wong, D.W.L.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving Complexity of MIF Signaling. Cell Signal 2019, 57, 76–88. [Google Scholar] [CrossRef]
- Oddo, M.; Calandra, T.; Bucala, R.; Meylan, P.R.A. Macrophage Migration Inhibitory Factor Reduces the Growth of Virulent Mycobacterium Tuberculosis in Human Macrophages. Infect. Immun. 2005, 73, 3783–3786. [Google Scholar] [CrossRef]
- Shang, Z.B.; Wang, J.; Kuai, S.G.; Zhang, Y.Y.; Ou, Q.F.; Pei, H.; Huang, L.H. Serum Macrophage Migration Inhibitory Factor as a Biomarker of Active Pulmonary Tuberculosis. Ann. Lab. Med. 2018, 38, 9–16. [Google Scholar] [CrossRef]
- Sumaiya, K.; Akino Mercy, C.S.; Muralitharan, G.; Hajinur Hirad, A.; Alarfaj, A.A.; Natarajaseenivasan, K. Assessment of Serum Macrophage Migration Inhibitory Factor (MIF) as an Early Diagnostic Marker of Leptospirosis. Front. Cell Infect. Microbiol. 2021, 11, 781476. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, T.; Xu, Q.; Dong, Q.; Luo, Y.; Gao, L.; Pan, Y. Expression Profile of Macrophage Migration Inhibitory Factor in Periodontitis. Arch. Oral. Biol. 2021, 122, 105003. [Google Scholar] [CrossRef]
- Savva, A.; Brouwer, M.C.; Roger, T.; Valls Serón, M.; Le Roy, D.; Ferwerda, B.; van der Ende, A.; Bochud, P.-Y.; van de Beek, D.; Calandra, T. Functional Polymorphisms of Macrophage Migration Inhibitory Factor as Predictors of Morbidity and Mortality of Pneumococcal Meningitis. Proc. Natl. Acad. Sci. USA 2016, 113, 3597–3602. [Google Scholar] [CrossRef]
- Kloek, A.T.; Seron, M.V.; Schmand, B.; Tanck, M.W.T.; van der Ende, A.; Brouwer, M.C.; van de Beek, D. Individual Responsiveness of Macrophage Migration Inhibitory Factor Predicts Long-Term Cognitive Impairment after Bacterial Meningitis. Acta Neuropathol. Commun. 2021, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Mukherjee, A.; Abhyankar, M.M.; Leng, L.; Bucala, R.; Sharma, D.; Madan, R. Neutralization of Macrophage Migration Inhibitory Factor Improves Host Survival after Clostridium Difficile Infection. Anaerobe 2018, 53, 56–63. [Google Scholar] [CrossRef]
- Adamali, H.; Armstrong, M.E.; McLaughlin, A.M.; Cooke, G.; McKone, E.; Costello, C.M.; Gallagher, C.G.; Leng, L.; Baugh, J.A.; Fingerle-Rowson, G.; et al. Macrophage Migration Inhibitory Factor Enzymatic Activity, Lung Inflammation, and Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 162–169. [Google Scholar] [CrossRef]
- Doroudian, M.; O’Neill, A.; O’Reilly, C.; Tynan, A.; Mawhinney, L.; McElroy, A.; Webster, S.S.; MacLoughlin, R.; Volkov, Y.; E Armstrong, M.; et al. Aerosolized Drug-Loaded Nanoparticles Targeting Migration Inhibitory Factors Inhibit Pseudomonas Aeruginosa-Induced Inflammation and Biofilm Formation. Nanomedicine 2020, 15, 2933–2953. [Google Scholar] [CrossRef] [PubMed]
- Panstruga, R.; Donnelly, S.C.; Bernhagen, J. A Cross-Kingdom View on the Immunomodulatory Role of MIF/D-DT Proteins in Mammalian and Plant Pseudomonas Infections. Immunology 2022, 166, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Lei, H.-Y.; Liu, C.-C.; Shiesh, S.-C.; Chen, S.-H.; Liu, H.-S.; Lin, Y.-S.; Wang, S.-T.; Shyu, H.-W.; Yeh, T.-M. Correlation of Serum Levels of Macrophage Migration Inhibitory Factor with Disease Severity and Clinical Outcome in Dengue Patients. Am. J. Trop. Med. Hyg. 2006, 74, 142–147. [Google Scholar] [CrossRef]
- Chen, H.-R.; Chao, C.-H.; Liu, C.-C.; Ho, T.-S.; Tsai, H.-P.; Perng, G.-C.; Lin, Y.-S.; Wang, J.-R.; Yeh, T.-M. Macrophage Migration Inhibitory Factor Is Critical for Dengue NS1-Induced Endothelial Glycocalyx Degradation and Hyperpermeability. PLoS Pathog. 2018, 14, e1007033. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chao, C.-H.; Yeh, T.-M. Roles of Macrophage Migration Inhibitory Factor in Dengue Pathogenesis: From Pathogenic Factor to Therapeutic Target. Microorganisms 2020, 8, 891. [Google Scholar] [CrossRef]
- Sorour, N.E.; Hamed, A.M.; Tabl, H.A.-E.M.; Ahmed, A.A.-E.A. Assessment of Macrophage Migration Inhibitory Factor in Patients with Verruca Vulgaris. Clin. Cosmet. Investig. Dermatol. 2019, 12, 591–595. [Google Scholar] [CrossRef]
- Smith, C.A.; Tyrell, D.J.; Kulkarni, U.A.; Wood, S.; Leng, L.; Zemans, R.L.; Bucala, R.; Goldstein, D.R. Macrophage Migration Inhibitory Factor Enhances Influenza-Associated Mortality in Mice. JCI Insight 2019, 4, e128034. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.F.; Muraro, S.P.; Santos, L.D.; Monteiro, A.P.T.; da Silva, A.G.; de Souza, A.P.D.; Stein, R.T.; Bozza, P.T.; Porto, B.N. Macrophage Migration Inhibitory Factor (MIF) Controls Cytokine Release during Respiratory Syncytial Virus Infection in Macrophages. Inflamm. Res. 2019, 68, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Bae, S.-J.; Kim, Y.; Park, B.-J.; Choi, J.-W.; Kwon, J.; Cha, G.-H.; Yoo, H.J.; Jo, E.-K.; et al. NADPH Oxidase 4 Is Required for the Generation of Macrophage Migration Inhibitory Factor and Host Defense against Toxoplasma Gondii Infection. Sci. Rep. 2017, 7, 6361. [Google Scholar] [CrossRef]
- Dheir, H.; Yaylaci, S.; Sipahi, S.; Genc, A.C.; Cekic, D.; Tuncer, F.B.; Cokluk, E.; Kocayigit, H.; Genc, A.B.; Salihi, S.; et al. Does Macrophage Migration Inhibitory Factor Predict the Prognosis of COVID-19 Disease? J. Infect. Dev. Ctries. 2021, 15, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, A.; Kerget, B.; Kerget, F.; Aşkın, S. Evaluation of the Relationship between Macrophage Migration Inhibitory Factor Level and Clinical Course in Patients with COVID-19 Pneumonia. J. Med. Virol. 2021, 93, 6519–6524. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, I.; Mirkov, I.; Kataranovski, M.; Glamoclija, J.; Stosic-Grujicic, S. A Role for Macrophage Migration Inhibitory Factor in Protective Immunity against Aspergillus Fumigatus. Immunobiology 2011, 216, 1018–1027. [Google Scholar] [CrossRef]
- Mirkov, I.; Belij, S.; Kataranovski, M.; Zolotarevski, L.; Glamoclija, J.; Stojanovic, I.; Stosic-Grujicic, S. The Relevance of the Migration Inhibitory Factor (MIF) for Peripheral Tissue Response in Murine Sublethal Systemic Aspergillus Fumigatus Infection. Med. Mycol. 2012, 50, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Nicolo, C.; Le Roy, D.; Reymond, M.K.; Roger, T.; Calandra, T. Macrophage Migration Inhibitory Factor Plays an Important Role in the Host Innate Immune Defenses against Candida Infection. Int. J. Infect. Dis. 2006, 10, S46–S47. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, L.-T.; Wang, Q.; Lin, J.; Jiang, N.; Li, C.; Zhao, G.-Q. Expression of Macrophage Migration Inhibitory Factor in Aspergillus Fumigatus Keratitis. Int. J. Ophthalmol. 2019, 12, 711–716. [Google Scholar] [CrossRef]
- Salazar-Castañón, V.H.; Juárez-Avelar, I.; Legorreta-Herrera, M.; Rodriguez-Sosa, M. Macrophage Migration Inhibitory Factor Contributes to Immunopathogenesis during Plasmodium Yoelii 17XL Infection. Front. Cell Infect. Microbiol. 2022, 12, 968422. [Google Scholar] [CrossRef]
- Damle, S.R.; Martin, R.K.; Cross, J.V.; Conrad, D.H. Macrophage Migration Inhibitory Factor Deficiency Enhances Immune Response to Nippostrongylus Brasiliensis. Mucosal Immunol. 2017, 10, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y. GATA3: A Master of Many Trades in Immune Regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef]
- Ghosh, S.; Padalia, J.; Ngobeni, R.; Abendroth, J.; Farr, L.; Shirley, D.-A.; Edwards, T.; Moonah, S. Targeting Parasite-Produced Macrophage Migration Inhibitory Factor as an Antivirulence Strategy With Antibiotic-Antibody Combination to Reduce Tissue Damage. J. Infect. Dis. 2020, 221, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Holowka, T.; Song, Y.; Zierow, S.; Leng, L.; Chen, Y.; Xiong, H.; Griffith, J.; Nouraie, M.; Thuma, P.E.; et al. A Plasmodium-Encoded Cytokine Suppresses T-Cell Immunity during Malaria. Proc. Natl. Acad. Sci. USA 2012, 109, E2117–E2126. [Google Scholar] [CrossRef] [PubMed]
- Baeza Garcia, A.; Siu, E.; Sun, T.; Exler, V.; Brito, L.; Hekele, A.; Otten, G.; Augustijn, K.; Janse, C.J.; Ulmer, J.B.; et al. Neutralization of the Plasmodium-Encoded MIF Ortholog Confers Protective Immunity against Malaria Infection. Nat. Commun. 2018, 9, 2714. [Google Scholar] [CrossRef]
- Moonah, S.N.; Abhyankar, M.M.; Haque, R.; Petri, W.A. The Macrophage Migration Inhibitory Factor Homolog of Entamoeba Histolytica Binds to and Immunomodulates Host Macrophages. Infect. Immun. 2014, 82, 3523–3530. [Google Scholar] [CrossRef]
- Ngobeni, R.; Abhyankar, M.M.; Jiang, N.M.; Farr, L.A.; Samie, A.; Haque, R.; Moonah, S.N. Entamoeba Histolytica-Encoded Homolog of Macrophage Migration Inhibitory Factor Contributes to Mucosal Inflammation during Amebic Colitis. J. Infect. Dis. 2017, 215, 1294–1302. [Google Scholar] [CrossRef]
- Ghosh, S.; Leaton, L.A.; Farr, L.; Barfield, A.; Moonah, S. Interaction between Parasite-Encoded JAB1/CSN5 and Macrophage Migration Inhibitory Factor Proteins Attenuates Its Proinflammatory Function. Sci. Rep. 2018, 8, 10241. [Google Scholar] [CrossRef]
- Twu, O.; Dessí, D.; Vu, A.; Mercer, F.; Stevens, G.C.; de Miguel, N.; Rappelli, P.; Cocco, A.R.; Clubb, R.T.; Fiori, P.L.; et al. Trichomonas Vaginalis Homolog of Macrophage Migration Inhibitory Factor Induces Prostate Cell Growth, Invasiveness, and Inflammatory Responses. Proc. Natl. Acad. Sci. USA 2014, 111, 8179–8184. [Google Scholar] [CrossRef]
- Sommerville, C.; Richardson, J.M.; Williams, R.A.M.; Mottram, J.C.; Roberts, C.W.; Alexander, J.; Henriquez, F.L. Biochemical and Immunological Characterization of Toxoplasma Gondii Macrophage Migration Inhibitory Factor. J. Biol. Chem. 2013, 288, 12733–12741. [Google Scholar] [CrossRef]
- Holowka, T.; Castilho, T.M.; Garcia, A.B.; Sun, T.; McMahon-Pratt, D.; Bucala, R. Leishmania-Encoded Orthologs of Macrophage Migration Inhibitory Factor Regulate Host Immunity to Promote Parasite Persistence. FASEB J. 2016, 30, 2249–2265. [Google Scholar] [CrossRef] [PubMed]
- Buchko, G.W.; Abendroth, J.; Robinson, H.; Zhang, Y.; Hewitt, S.N.; Edwards, T.E.; Van Voorhis, W.C.; Myler, P.J. Crystal Structure of a Macrophage Migration Inhibitory Factor from Giardia Lamblia. J. Struct. Funct. Genomics 2013, 14, 47–57. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Twu, O.; Johnson, P.J. Trichomonas Vaginalis Macrophage Migration Inhibitory Factor Mediates Parasite Survival during Nutrient Stress. mBio 2018, 9, e00910-18. [Google Scholar] [CrossRef]
- Liu, K.; Wen, H.; Cai, H.; Wu, M.; An, R.; Chu, D.; Yu, L.; Shen, J.; Chen, L.; Du, J. Protective Effect Against Toxoplasmosis in BALB/c Mice Vaccinated With Toxoplasma Gondii Macrophage Migration Inhibitory Factor. Front. Microbiol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Gabarin, R.S.; Li, M.; Zimmel, P.A.; Marshall, J.C.; Li, Y.; Zhang, H. Intracellular and Extracellular Lipopolysaccharide Signaling in Sepsis: Avenues for Novel Therapeutic Strategies. J. Innate Immun. 2021, 13, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M. Endotoxins and Other Sepsis Triggers. Contrib. Nephrol. 2010, 167, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Grieb, G.; Merk, M.; Bernhagen, J.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF): A Promising Biomarker. Drug News Perspect. 2010, 23, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.G.; Ganea, D.; Gamero, A.M. Cecal Ligation Puncture Procedure. J. Vis. Exp. 2011, 51, 2860. [Google Scholar] [CrossRef]
- Cohen, J.; Abraham, E. Microbiologic Findings and Correlations with Serum Tumor Necrosis Factor-Alpha in Patients with Severe Sepsis and Septic Shock. J. Infect. Dis. 1999, 180, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Beishuizen, A.; Thijs, L.G.; Haanen, C.; Vermes, I. Macrophage Migration Inhibitory Factor and Hypothalamo-Pituitary-Adrenal Function during Critical Illness. J. Clin. Endocrinol. Metab. 2001, 86, 2811–2816. [Google Scholar] [CrossRef][Green Version]
- Bozza, F.A.; Gomes, R.N.; Japiassú, A.M.; Soares, M.; Castro-Faria-Neto, H.C.; Bozza, P.T.; Bozza, M.T. Macrophage Migration Inhibitory Factor Levels Correlate with Fatal Outcome in Sepsis. Shock. 2004, 22, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Emonts, M.; Sweep, F.C.G.J.; Grebenchtchikov, N.; Geurts-Moespot, A.; Knaup, M.; Chanson, A.L.; Erard, V.; Renner, P.; Hermans, P.W.M.; Hazelzet, J.A.; et al. Association between High Levels of Blood Macrophage Migration Inhibitory Factor, Inappropriate Adrenal Response, and Early Death in Patients with Severe Sepsis. Clin. Infect. Dis. 2007, 44, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Flores, C.; Fan-Ma, S.; Miller, E.J.; Moitra, J.; Moreno, L.; Wadgaonkar, R.; Simon, B.; Brower, R.; Sevransky, J.; et al. Macrophage Migration Inhibitory Factor in Acute Lung Injury: Expression, Biomarker, and Associations. Transl. Res. 2007, 150, 18–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Payen, D.; Lukaszewicz, A.-C.; Legrand, M.; Gayat, E.; Faivre, V.; Megarbane, B.; Azoulay, E.; Fieux, F.; Charron, D.; Loiseau, P.; et al. A Multicentre Study of Acute Kidney Injury in Severe Sepsis and Septic Shock: Association with Inflammatory Phenotype and HLA Genotype. PLoS ONE 2012, 7, e35838. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Tsuruta, R.; Fujita, M.; Kaneko, T.; Kasaoka, S.; Maekawa, T. Serum Macrophage Migration Inhibitory Factor Reflects Adrenal Function in the Hypothalamo-Pituitary-Adrenal Axis of Septic Patients: An Observational Study. BMC Infect. Dis. 2009, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.E.; Novender, U.; Schroeder, S.; Pietsch, T.; von Spiegel, T.; Putensen, C.; Hoeft, A.; Stüber, F. Plasma Levels of Macrophage Migration Inhibitory Factor Are Elevated in Patients with Severe Sepsis. Intensive Care Med. 2001, 27, 1412–1415. [Google Scholar] [CrossRef]
- Toldi, J.; Nemeth, D.; Hegyi, P.; Molnar, Z.; Solymar, M.; Farkas, N.; Alizadeh, H.; Rumbus, Z.; Pakai, E.; Garami, A. Macrophage Migration Inhibitory Factor as a Diagnostic and Predictive Biomarker in Sepsis: Meta-Analysis of Clinical Trials. Sci. Rep. 2021, 11, 8051. [Google Scholar] [CrossRef]
- Tohyama, S.; Onodera, S.; Tohyama, H.; Yasuda, K.; Nishihira, J.; Mizue, Y.; Hamasaka, A.; Abe, R.; Koyama, Y. A Novel DNA Vaccine-Targeting Macrophage Migration Inhibitory Factor Improves the Survival of Mice with Sepsis. Gene Ther. 2008, 15, 1513–1522. [Google Scholar] [CrossRef][Green Version]
- Al-Abed, Y.; Dabideen, D.; Aljabari, B.; Valster, A.; Messmer, D.; Ochani, M.; Tanovic, M.; Ochani, K.; Bacher, M.; Nicoletti, F.; et al. ISO-1 Binding to the Tautomerase Active Site of MIF Inhibits Its pro-Inflammatory Activity and Increases Survival in Severe Sepsis. J. Biol. Chem. 2005, 280, 36541–36544. [Google Scholar] [CrossRef]
- Zan, C.; Yang, B.; Brandhofer, M.; El Bounkari, O.; Bernhagen, J. D-Dopachrome Tautomerase in Cardiovascular and Inflammatory Diseases-A New Kid on the Block or Just Another MIF? FASEB J. 2022, 36, e22601. [Google Scholar] [CrossRef]
- Li, H.; He, B.; Zhang, X.; Hao, H.; Yang, T.; Sun, C.; Song, H.; Wang, Y.; Zhou, Y.; Zhu, Z.; et al. D-Dopachrome Tautomerase Drives Astroglial Inflammation via NF-κB Signaling Following Spinal Cord Injury. Cell Biosci. 2022, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Illescas, O.; Pacheco-Fernández, T.; Laclette, J.P.; Rodriguez, T.; Rodriguez-Sosa, M. Immune Modulation by the Macrophage Migration Inhibitory Factor (MIF) Family: D-Dopachrome Tautomerase (DDT) Is Not (Always) a Backup System. Cytokine 2020, 133, 155121. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.; Du, W.; Gupta, Y.; Yeh, I.-J.; Montano, M.; Magi-Galuzzi, C.; Welford, S.M. Dysregulated D-Dopachrome Tautomerase, a Hypoxia-Inducible Factor-Dependent Gene, Cooperates with Macrophage Migration Inhibitory Factor in Renal Tumorigenesis. J. Biol. Chem. 2014, 289, 3713–3723. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Zierow, S.; Syed, M.; Bucala, R.; Bhandari, V.; Lolis, E.J. Targeting Distinct Tautomerase Sites of D-DT and MIF with a Single Molecule for Inhibition of Neutrophil Lung Recruitment. FASEB J. 2014, 28, 4961–4971. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Osipyan, A.; Song, S.; Chen, D.; Schut, R.A.; van Merkerk, R.; van der Wouden, P.E.; Cool, R.H.; Quax, W.J.; Melgert, B.N.; et al. Thieno[2,3-d]Pyrimidine-2,4(1H,3H)-Dione Derivative Inhibits d-Dopachrome Tautomerase Activity and Suppresses the Proliferation of Non-Small Cell Lung Cancer Cells. J. Med. Chem. 2022, 65, 2059–2077. [Google Scholar] [CrossRef] [PubMed]
- Caltabiano, R.; De Pasquale, R.; Piombino, E.; Campo, G.; Nicoletti, F.; Cavalli, E.; Mangano, K.; Fagone, P. Macrophage Migration Inhibitory Factor (MIF) and Its Homologue d-Dopachrome Tautomerase (DDT) Inversely Correlate with Inflammation in Discoid Lupus Erythematosus. Molecules 2021, 26, 184. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Di Marco, R.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Upregulated Expression of Macrophage Migration Inhibitory Factor, Its Analogue D-Dopachrome Tautomerase, and the CD44 Receptor in Peripheral CD4 T Cells from Clinically Isolated Syndrome Patients with Rapid Conversion to Clinical Defined Multiple Sclerosis. Medicina 2019, 55, 667. [Google Scholar] [CrossRef]
- Yoshihisa, Y.; Rehman, M.U.; Andoh, T.; Tabuchi, Y.; Makino, T.; Shimizu, T. Overexpression of D-Dopachrome Tautomerase Increases Ultraviolet B Irradiation-Induced Skin Tumorigenesis in Mice. FASEB J. 2021, 35, e21671. [Google Scholar] [CrossRef]
- Alaskarov, A.; Barel, S.; Bakavayev, S.; Kahn, J.; Israelson, A. MIF Homolog D-Dopachrome Tautomerase (D-DT/MIF-2) Does Not Inhibit Accumulation and Toxicity of Misfolded SOD1. Sci. Rep. 2022, 12, 9570. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, Y.; Chen, C.; Li, H.; He, B.; Yang, T.; Sun, C.; Hao, H.; Zhang, X.; Wang, Y.; et al. D-Dopachrome Tautomerase Activates COX2/PGE2 Pathway of Astrocytes to Mediate Inflammation Following Spinal Cord Injury. J. Neuroinflammation 2021, 18, 130. [Google Scholar] [CrossRef]
- Ma, Y.; Su, K.N.; Pfau, D.; Rao, V.S.; Wu, X.; Hu, X.; Leng, L.; Du, X.; Piecychna, M.; Bedi, K.; et al. Cardiomyocyte D-Dopachrome Tautomerase Protects against Heart Failure. JCI Insight 2019, 4, e128900. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, B.; Habibie, H.; van den Bor, J.; Smit, M.J.; Gosens, R.; Wu, X.; Brandsma, C.-A.; Cool, R.H.; Haisma, H.J.; et al. D-Dopachrome Tautomerase Contributes to Lung Epithelial Repair via Atypical Chemokine Receptor 3-Dependent Akt Signaling. EBioMedicine 2021, 68, 103412. [Google Scholar] [CrossRef] [PubMed]
- Baron-Stefaniak, J.; Schiefer, J.; Lichtenegger, P.; Miller, E.J.; Berlakovich, G.A.; Faybik, P.; Baron, D.M. D-Dopachrome Tautomerase Predicts Outcome but Not the Development of Acute Kidney Injury after Orthotopic Liver Transplantation. HPB 2019, 21, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Voss, S.; Krüger, S.; Scherschel, K.; Warnke, S.; Schwarzl, M.; Schrage, B.; Girdauskas, E.; Meyer, C.; Blankenberg, S.; Westermann, D.; et al. Macrophage Migration Inhibitory Factor (MIF) Expression Increases during Myocardial Infarction and Supports Pro-Inflammatory Signaling in Cardiac Fibroblasts. Biomolecules 2019, 9, 38. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Welsh, J.; Cui, X.; Suffredini, A.F.; Eichacker, P.Q. Increased Body Mass Index and Adjusted Mortality in ICU Patients with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. Crit. Care 2016, 20, 181. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Chen, Q.; Liu, C.; Huang, C.; Fang, X. The Role of Increased Body Mass Index in Outcomes of Sepsis: A Systematic Review and Meta-Analysis. BMC Anesthesiol. 2017, 17, 118. [Google Scholar] [CrossRef]
- Kim, B.-S.; Tilstam, P.V.; Arnke, K.; Leng, L.; Ruhl, T.; Piecychna, M.; Schulte, W.; Sauler, M.; Frueh, F.S.; Storti, G.; et al. Differential Regulation of Macrophage Activation by the MIF Cytokine Superfamily Members MIF and MIF-2 in Adipose Tissue during Endotoxemia. FASEB J. 2020, 34, 4219–4233. [Google Scholar] [CrossRef]
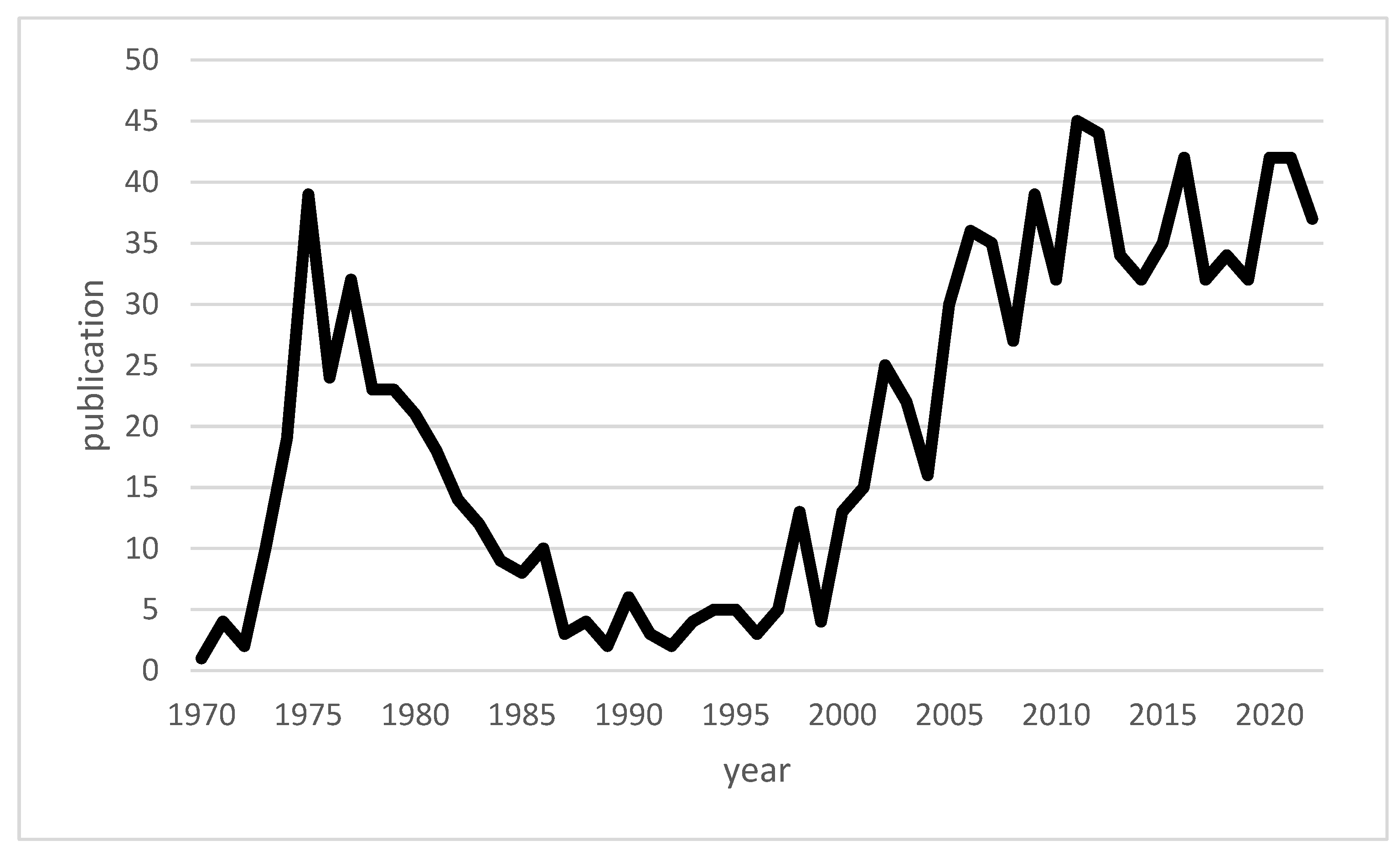


| Authors | Year of Publication | Title | Type | Topic |
|---|---|---|---|---|
| Bernhagen et al. [15] | 1993 | MIF is a pituitary-derived cytokine that potentiates lethal endotoxemia | In vitro and in vivo | MIF in sepsis |
| Bozza et al. [37] | 1999 | Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis | In vivo | MIF in sepsis |
| Calandra et al. [16] | 2000 | Protection from septic shock by neutralization of macrophage migration inhibitory factor | In vitro and in vivo | MIF in sepsis |
| Mitchell et al. [38] | 2002 | Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response | In vitro and in vivo | MIF in apoptosis |
| Bernhagen et al. [18] | 2007 | MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment | In vitro and in vivo | MIF in inflammation and atherosclerosis |
| Flores et al. [39] | 2008 | Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii | In vitro and in vivo | MIF in Toxoplasma gondii |
| Merk et al. [40] | 2011 | The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor | In vitro and in vivo | D-DT as a homolog of MIF |
| Das et al. [41] | 2013 | Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis | In vitro and in vivo | MIF in Mycobacterium Tuberculosis |
| Kim et al. [42] | 2020 | D-dopachrome tautomerase in adipose tissue inflammation and wound repair | In vitro and in vivo | MIF and D-DT in adipose tissue during endotoxemia |
| Microorganism | Gram Stain | Author(s) | MIF Effect |
|---|---|---|---|
| Mycobacterium tuberculosis | Positive | Oddo et al. [67], Das et al. [41], | Protective |
| Streptococcus pneumoniae | Positive | Savva et al. [71], Kloek et al. [72] | Detrimental |
| Clostridium difficile | Positive | Jose et al. [73] | Detrimental |
| Salmonella typhimurium | Negative | Koebernick et al. [59] | Protective |
| Pseudomonas aeruginosa | Negative | Adamali et al. [74], Doroudian et al. [75] | Detrimental |
| Viral Pathogen | Author(s) | MIF Effect |
|---|---|---|
| Dengue Virus | Assunção-Miranda et al. [1] Chen et al. [78], Lai et al. [79] | Deleterious (Facilitation of replication, vascular leakage, immune modulation) |
| Human immunodeficiency virus type I (HIV) | Trifone et al. [62,65] | Deleterious (Cytokine induction, CD4+ T cell predisposition, increased Th17-like cell profile) |
| Influenza A Virus (IAV) | Smith et al. [81] | Deleterious (Increased viral load, inflammation, and mortality) |
| Respiratory Syncytial Virus (RSV) | de Souza et al. [82] | Protective (Increased viral clearance) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breidung, D.; Megas, I.-F.; Freytag, D.L.; Bernhagen, J.; Grieb, G. The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective. Biomedicines 2024, 12, 2. https://doi.org/10.3390/biomedicines12010002
Breidung D, Megas I-F, Freytag DL, Bernhagen J, Grieb G. The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective. Biomedicines. 2024; 12(1):2. https://doi.org/10.3390/biomedicines12010002
Chicago/Turabian StyleBreidung, David, Ioannis-Fivos Megas, David Lysander Freytag, Jürgen Bernhagen, and Gerrit Grieb. 2024. "The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective" Biomedicines 12, no. 1: 2. https://doi.org/10.3390/biomedicines12010002
APA StyleBreidung, D., Megas, I.-F., Freytag, D. L., Bernhagen, J., & Grieb, G. (2024). The Role of Macrophage Migration Inhibitory Factor (MIF) and D-Dopachrome Tautomerase (D-DT/MIF-2) in Infections: A Clinical Perspective. Biomedicines, 12(1), 2. https://doi.org/10.3390/biomedicines12010002








