Chronic Pelvic Pain, Vulvar Pain Disorders, and Proteomics Profiles: New Discoveries, New Hopes
Abstract
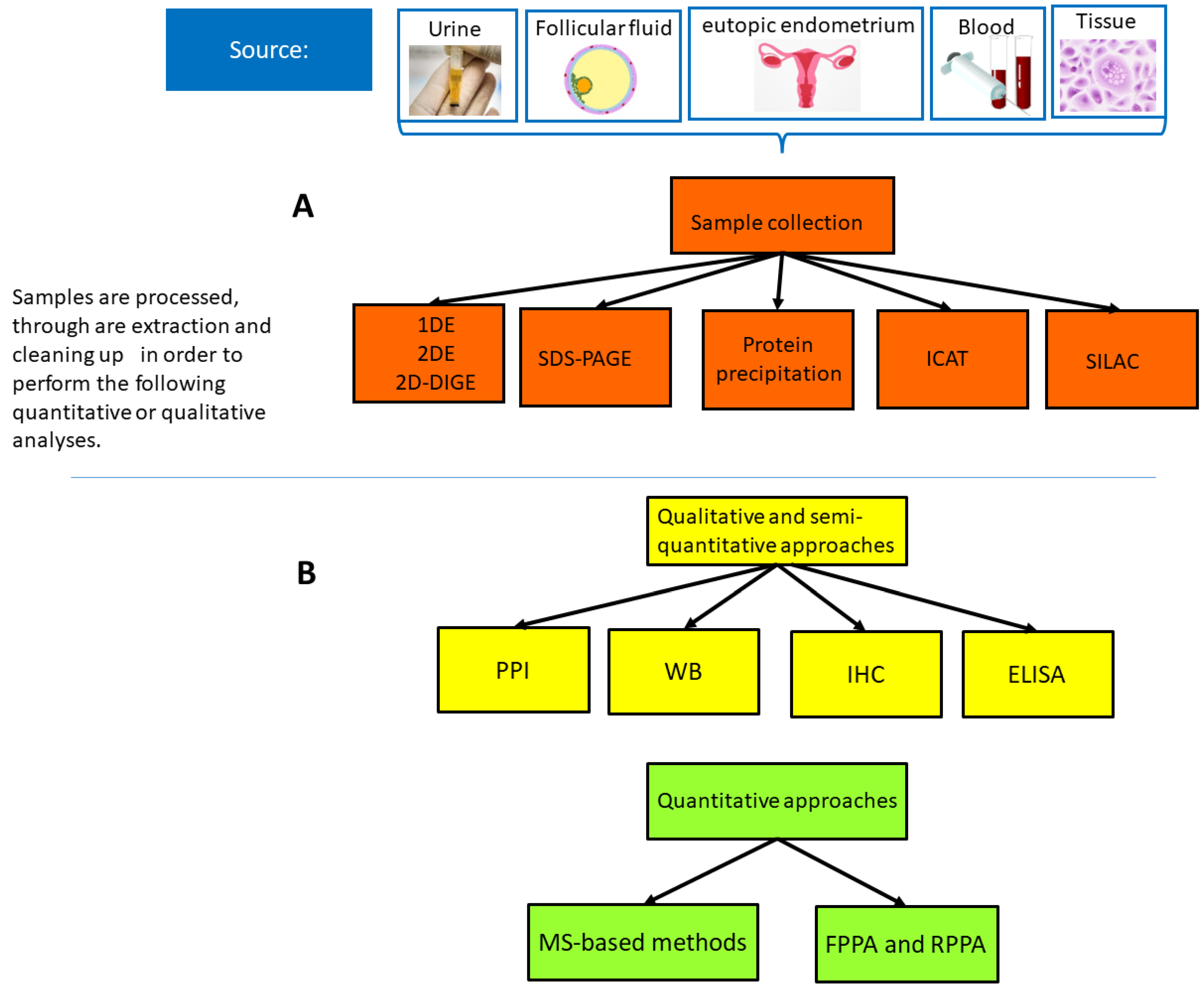
:1. Introduction
2. Proteomics Methods
3. Proteomics-Based Investigation of Dysregulated Proteins Involved in Endometriosis, Adenomyosis and Interstitial Cystitis/Bladder Pain Syndrome (IC/PBS), and Vulvodynia
3.1. Vulvodynia
3.2. Endometriosis
3.3. Adenomyosis
3.4. Interstitial Cystitis/Bladder Pain Syndrome (IC/PBS)
| Pain Conditions | Site | Gene and Protein Abundances | Type of Abundance | Proteomics Methods |
|---|---|---|---|---|
| Vulvodynia | vaginal/vestibular areas [35] | Annexin A1, interleukin 1 receptor antagonist, protein S100A9, immunoglobulin G κ chain | up | gel electrophoresis and mass spectrometry |
| vaginal fluid [36] | sphingolipid | up | UPLC-MS/MS | |
| Endometriosis | endometrium [42,44] | Genes: C7, CFH, FZD7, LY96, PDLIM3, PTGIS, and WISP2 Proteins: LY96, PDLIM3, PTGIS, and WISP2 [Bae] MIF (Zhang 2015) | up | microarray |
| Plasma [41,45] | proteins related to angiogenesis/cell migration; inflammatory cytokines | up | multiplex aptamer-based proteomics discovery | |
| Peritoneal fluid [41,46] | proteoforms of 1-antitrypsin and S100-A8, transferrin, 1b-glycoprotein and aptoglobin; inflammatory cytokines | up | 2D-PAGE combined with LC-MS/MS | |
| Endometrial fluid [33] | signal transduction and cytoskeletal structure | up | 2D-PAGE) | |
| eutopic endometrium [52] | P450 | hybridization method | ||
| eutopic endometrium [50] | molecular chaperones, proteins involved in protein and DNA formation/breakdown, and secreted proteins | up | 2D-PAGE mass spectroscopic | |
| Serum and eutopic endometrium [54] | proteins of cytoskeletons or involved in the regulation of the cell cycle, signal transduction, or immunological function | up | 2D-PAGE | |
| eutopic endometrium [47] | proteins involved in apoptosis, immune reaction, glycolytic pathway, cell structure, and transcription | up | Immunoblot and immunohistochemical analyses | |
| eutopic endometrium [49] | structural proteins (vimentin, actins), stress response (Peroxiredoxins, HSP B1, HSP70, HSP90) or signaling (14-3-3 proteins, annexins), protein-folding and protein-turnover, immunity, energy production, signal transduction, RNA biogenesis, protein biosynthesis, and nuclear proteins | up | 2D-PAGE Western blot, and MS | |
| eutopic endometrium [51] | Protein involved in the PI3K/AKT signaling pathway and focal adhesion (the laminin family) | up | LC-MS-MS analysis | |
| members of the S100 protein family | down | LC-MS-MS analysis | ||
| eutopic endometrium [62] | Humoral immune response pathways, antimicrobial humoral response, and the regulation of the nitric oxide biosynthetic process | up | Tandem mass tags combined with multidimensional liquid chromatography and mass spectrometry analyses | |
| necroptotic process, regulation of necrotic cell death | down | Tandem mass tags combined with multidimensional liquid chromatography and mass spectrometry analyses | ||
| Follicular fluid [61] | Immunoglobulin heavy constant gamma 2 (IGHG2), glia-derived nexin (GDN), and Inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3) | up | LFQP and PRM | |
| corticosteroid-binding globulin (CBG), angiotensinogen (AGT), and Fetuin-B (FETUB) | down | LFQP and PRM | ||
| Adenomyosis | Adenomyotic tissue [65] | cytoskeleton proteins, HSP and | up | 2D_PAGE MALDI-TOF |
| serum [66] | immune response, the inflammatory response, and cell adhesion | up. | iTRAQ | |
| Ectopic endometrium [67] | annexin A2 correlated with markers of epithelial-to-mesenchymal transition enhanced the proangiogenic capacity of adenomyotic endometrial cells through the HIF-1/VEGF-A pathway | up | polyacrylamide gel electrophoresis/MS | |
| tissue and blood [68] | regulation of cell morphogenesis’ and ‘cytoskeletal organization (HSP90A, STIP1 and TAGLN-2) | up | TEM, LC-MS | |
| Interstitial cystitis/bladder pain syndrome | Bladder biopsy [73] | inflammatory setting (T and B cells markers); endoplasmic reticulum stressed proteins | up | nHPLC-MS/MS |
| Bladder biopsy [73] | cellular adhesive proteins, cell proliferation/wound healing Rap1-related proteins | down | nHPLC-MS/MS | |
| ubiquitination | down | |||
| NDA tissue [74] | protein; muscarin M2, purinergic P2 × 1, P2 × 2, and histamine receptors | up | multiple antigen bead assay | |
| bladder submucosa [74] | urothelial markers, focal lymphoid aggregates | down | multiple antigen bead assay | |
| bladder submucosa [74] | cytokines, chemokines, and enhanced immunoreactivity for muscarinic M2, purinergic P2 × 1, P2 × 2, and histamine H1 receptors | up | multiple antigen bead assay | |
| Urine samples [76] | tyramine and 2-oxoglutarate phenylacetylglutamin | up | MS | |
| Urine [77] | CXCL-8 and bladder mast cell counts | up | tryptase stain | |
| One glycosylated nonapeptide, antiproliferative | - | thymidine incorporation assay |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gin, G.T. Female Pelvic Conditions: Chronic Pelvic Pain. FP Essent. 2022, 515, 11–19. [Google Scholar] [PubMed]
- Speer, L.M.; Mushkbar, S.; Erbele, T. Chronic Pelvic Pain in Women. Am. Fam. Physician 2016, 93, 380–387. [Google Scholar] [PubMed]
- Schlaeger, J.M.; Glayzer, J.E.; Villegas-Downs, M.; Li, H.; Glayzer, E.J.; He, Y.; Tkayama, M.; Yajima, H.; Takakura, N.; Kobak, W.H.; et al. Evaluation and Treatment of Vulvodynia: State of the Science. J. Midwifery Womens Health 2023, 68, 9–34. [Google Scholar] [CrossRef]
- Lamvu, G.; Carrillo, J.; Ouyang, C.; Rapkin, A. Chronic Pelvic Pain in Women: A Review. JAMA 2021, 325, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Colaco, M.; Koslov, D.S.; Keys, T.; Evans, R.J.; Badlani, G.H.; Andersson, K.-E.; Walker, S.J. Correlation of gene expression with bladder capacity in interstitial cystitis/bladder pain syndrome. J. Urol. 2014, 192, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Killinger, K.; Tyagi, V.; Nirmal, J.; Chancellor, M.; Peters, K.M. Urinary chemokines as non invasive predictors of ulcerative interstitial cystitis. J. Urol. 2012, 187, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Keerthikumar, S. An Introduction to Proteome Bioinformatics. In Proteome Bioinformatics; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1549, pp. 1–3. [Google Scholar]
- Schmidt, A.; Forne, I.; Imhof, A. Bioinformatic analysis of proteomics data. BMC Syst. Biol. 2014, 8, S3. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; McCarthy, M.I.; Schwenk, J.M. Genetics meets proteomics: Perspectives for large population-based studies. Nat. Rev. Genet. 2021, 22, 19–37. [Google Scholar] [CrossRef]
- Vignaroli, F.; Mele, A.; Tondo, G.; De Giorgis, V.; Manfredi, M.; Comi, C.; Mazzini, L.; De Marchi, F. The Need for Biomarkers in the ALS–FTD Spectrum: A Clinical Point of View on the Role of Proteomics. Proteomes 2023, 11, 1. [Google Scholar] [CrossRef]
- Amrani, S.A.; Jabri, Z.A.; Zaabi, A.A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Gurke, R.; Bendes, A.; Bowes, J.; Koehm, M.; Twyman, R.M.; Barton, A.; Elewaut, D.; Goodyear, C.; Hahnefeld, L.; Hillenbrand, R.; et al. Omics and Multi-Omics Analysis for the Early Identification and Improved Outcome of Patients with Psoriatic Arthritis. Biomedicines 2022, 10, 2387. [Google Scholar] [CrossRef]
- Neagu, A.; Jayathirtha, M.; Whitham, D.; Mutsengi, P.; Sullivan, I.; Petre, B.A.; Darie, C.C. Proteomics-Based Identification of Dysregulated Proteins in Breast Cancer. Proteomes 2022, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lih, T.M.; Pan, J.; Höti, N.; Dong, M.; Cao, L.; Hu, Y.; Cho, K.-C.; Chen, S.-Y.; Eguez, R.V.; et al. Proteomic signatures of 16 major types of human cancer reveal universal and cancer-type-specific proteins for the identification of potential therapeutic targets. J. Hematol. Oncol. 2020, 13, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Li, B.; Zhan, X.; Schluter, H.; Jungblut, P.R.; Coorssen, J.R. Innovating the Concept and Practice of Two-Dimensional Gel Electrophoresis in the Analysis of Proteomes at the Proteoform Level. Proteomes 2019, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Boellner, S.; Becker, K.-F. Reverse phase protein arrays-quantitative assessment of multiple biomarkers in biopsies for clinical use. Microarrays 2015, 4, 98–114. [Google Scholar] [CrossRef]
- Duraiyan, J.; Govindarajan, R.; Kaliyappan, K.; Palanisamy, M. Applications of immunohistochemistry. J. Pharm. Bioallied Sci. 2012, 4, 307–309. [Google Scholar]
- Ősz, Á.; Lánczky, A.; Győrffy, B. Survival analysis in breast cancer using proteomic data from four independent datasets. Sci. Rep. 2021, 11, 16787. [Google Scholar] [CrossRef]
- Yagnik, G.; Liu, Z.; Rothschild, K.J.; Lim, M.J. Highly Multiplexed Immunohistochemical MALDI-MS Imaging of Biomarkers in Tissues. J. Am. Soc. Mass Spectrom. 2021, 32, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, F.; Fang, D.; Chen, Y. Targeted Proteomics Enables Simultaneous Quantification of Folate Receptor Isoforms and Potential Isoform-based Diagnosis in Breast Cancer. Sci. Rep. 2015, 5, 16733. [Google Scholar] [CrossRef]
- Im, K.; Mareninov, S.; Diaz, M.F.P.; Yong, W.H. An Introduction to Performing Immunofluorescence Staining. Methods Mol. Biol. 2019, 1897, 299–311. [Google Scholar]
- Darie-Ion, L.; Whitham, D.; Jayathirtha, M.; Rai, Y.; Neagu, A.-N.; Darie, C.C.; Petre, B.A. Applications of MALDI-MS/MS-Based Proteomics in Biomedical Research. Molecules 2022, 27, 6196. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.V.; Millikin, R.J.; Miller, R.M.; Anderson, L.C.; Fellers, R.T.; Ge, Y.; Kelleher, N.L.; LeDuc, R.D.; Liu, X.; Payne, S.H. Identification and Quantification of Proteoforms by Mass Spectrometry. Proteom. 2019, 19, e1800361. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.V.; Millikin, R.J.; Shortreed, M.R.; Scalf, M.; Smith, L.M. Improving Proteoform Identifications in Complex Systems Through Integration of Bottom-Up and Top-Down Data. J. Proteome Res. 2020, 19, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kelleher, N.L. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Kelleher, N.L. Proteoforms as the next proteomics currency. Science 2018, 359, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhan, X. Mass spectrometry analysis of phosphotyrosine-containing proteins. Mass Spectrom. Rev. 2023, 14, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Raed Abdullah Alharbi, R.A. Proteomics approach and techniques in identification of reliable biomarkers for diseases. Saudi J. Biol. Sci. 2020, 27, 968–974. [Google Scholar] [CrossRef]
- Wang, X.; Fan, D.; Yang, Y.; Gimple, R.C.; Zhou, S. Integrative multi-omics approaches to explore immune cell functions: Challenges and opportunities. iScience 2023, 26, 106359. [Google Scholar] [CrossRef]
- Akopians, A.L.; Rapkin, A.J. Vulvodynia: The Role of Inflammation in the Etiology of Localized Provoked Pain of the Vulvar Vestibule (Vestibulodynia). Semin. Reprod. Med. 2015, 33, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ventolini, G.; Barhan, S.M. Vulvodynia. Dermatol. Online J. 2008, 14, 1–10. [Google Scholar] [CrossRef]
- Arnold, L.D.; Bachmann, G.A.; Rosen, R.; Rhoads, G.G. Assessment of vulvodynia symptoms in a sample of US women: A prevalence survey with a nested case control study. Am. J. Obstet. Gynecol. 2007, 196, 128-e1. [Google Scholar] [CrossRef] [PubMed]
- Sadownik, L.A. Etiology, diagnosis, and clinical management of vulvodynia. Int. J. Women’s Health 2014, 6, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, J.; Goldstein, A.T.; Stockdale, C.K.; Bergeron, S.; Pukall, C.; Zolnoun, D.; Coady, D. 2015 ISSVD, ISSWSH and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. Obstet. Gynecol. 2016, 127, 745–751. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, C.; Umstead, T.; Shearer, D.; Weisz, J.; Phelps, D.S.; Floros, J. A Pilot Proteomic Study of Vestibular Fluid from Patients with Vulvodynia. J. Low. Genit. Tract Dis. 2022, 26, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Labus, J.S.; Mayer, E.A.; Aagaard, K.; Stains, J.; Broniowska, K.; Rapkin, A. Reduced concentrations of vaginal metabolites involved in steroid hormone biosynthesis are associated with increased vulvar vestibular pain and vaginal muscle tenderness in provoked vestibulodynia: An exploratory metabolomics study. Mol. Pain 2021, 17, 17448069211041853. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Di Tucci, C.; Di Feliciantonio, M.; Galati, G.; Marchetti, C.; Perniola, G.; Pecorini, F.; Benedetti Panici, P. Management of endometriosis from diagnosis to treatment: Roadmap for the future. Minerva Ginecol. 2019, 71, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Muzii, L.; Galati, G.; Di Tucci, C.; Di Feliciantonio, M.; Perniola, G.; Di Donato, V.; Benedetti Panici, P.; Vignali, M. Medical treatment of ovarian endometriomas: A prospective evaluation of the effect of dienogest on ovarian reserve, cyst diameter, and associated pain. Gynecol. Endocrinol. 2020, 36, 81–83. [Google Scholar] [CrossRef]
- Ab, S.; Srivastava, P.; Shivaji, S. Understanding the pathogenesis of endometriosis through proteomics: Recent advances and future prospects. Proteom. Clin. Appl. 2014, 8, 86–98. [Google Scholar] [CrossRef]
- Janša, V.; Klančič, T.; Pušić, M.; Klein, M.; Vrtačnik Bokal, E.; Ban Frangež, H.; Rižner, T.L. Proteomic analysis of peritoneal fluid identified COMP and TGFBI as new candidate biomarkers for endometriosis. Sci. Rep. 2021, 11, 20870. [Google Scholar] [CrossRef]
- Kapoor, R.; Stratopoulou, C.A.; Dolmans, M.-M. Pathogenesis of endometriosis: New insights into prospective therapies. Int. J. Mol. Sci. 2021, 22, 11700. [Google Scholar] [CrossRef]
- Zhang, X.; Mu, L. Association between macrophage migration inhibitory factor in the endometrium and estrogen in endometriosis. Exp. Ther. Med. 2015, 10, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Akoum, A.; Khoufache, K. Macrophage migration inhibitory factor: A key cytokine for endometriosis. Med. Sci. 2015, 31, 824–825. [Google Scholar]
- Bae, S.-J.; Jo, Y.; Cho, M.K.; Jin, J.-S.; Kim, J.-Y.; Shim, J.; Kim, Y.H.; Park, J.-K.; Ryu, D.; Lee, H.J.; et al. Identification and analysis of novel endometriosis biomarkers via integrative bioinformatics. Front. Endocrinol. 2022, 13, 942368. [Google Scholar] [CrossRef] [PubMed]
- Sasamoto, N.; Ngo, L.; Vitonis, A.F.; Dillon, S.T.; Missmer, S.A.; Libermann, T.A.; Terry, K.L. Circulating proteomic profiles associated with endometriosis in adolescents and young adults. Hum. Reprod. 2022, 37, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Gillott, D.J.; Remorgida, V.; Anserini, P.; Ragni, N.; Grudzinskas, J.G. Proteomic analysis of peritoneal fluid in fertile and infertile women with endometriosis. J. Reprod. Med. Obstet. Gynecol. 2009, 54, 32–40. [Google Scholar]
- ten Have, S.; Fraser, I.; Markham, R.; Lam, A.; Matsumoto, I. Proteomic analysis of protein expression in the eutopic endometrium of women with endometriosis. Proteom. Clin. Appl. 2007, 1, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Ametzazurra, A.; Matorras, R.; García-Velasco, J.A.; Prieto, B.; Simón, L.; Martínez, A.; Nagore, D. Endometrial fluid is a specific and non-invasive biological sample for protein biomarker identification in endometriosis. Hum. Reprod. 2009, 24, 954–965. [Google Scholar] [CrossRef]
- Rai, P.; Kota, V.; Deendayal, M.; Shivaji, S. Differential proteome profiling of eutopic endometrium from women with endometriosis to understand etiology of endometriosis. J. Proteome Res. 2010, 9, 4407–4419. [Google Scholar] [CrossRef]
- Fowler, P.A.; Tattum, J.; Bhattacharya, S.; Klonisch, T.; Hombach-Klonisch, S.; Gazvani, R.; Lea, R.G.; Miller, I.; Simpson, W.G.; Cash, P. An investigation of the effects of endometriosis on the proteome of human eutopic endometrium: A heterogeneous tissue with a complex disease. Proteomics 2007, 7, 130–142. [Google Scholar] [CrossRef]
- Méar, L.; Com, E.; Fathallah, K.; Guillot, L.; Lavigne, R.; Guével, B.; Fauconnier, A.; Vialard, F.; Pineau, C. The eutopic endometrium proteome in endometriosis reveals candidate markers and molecular mechanisms of physiopathology. Diagnostics 2022, 12, 419. [Google Scholar] [CrossRef]
- Noble, L.S.; Takayama, K.; Zeitoun, K.M.; Putman, J.M.; Johns, D.A.; Hinshelwood, M.M.; Agarwal, V.R.; Zhao, Y.; Carr, B.R.; Bulun, S.E. Prostaglandin E2 stimulates aromatase expression in endometriosis- derived stromal cells. J. Clin. Endocrinol. Metab. 1997, 82, 600–606. [Google Scholar] [CrossRef]
- Bulun, S.E.; Zeitoun, K.M.; Takayama, K.; Sasano, H. Molecular basis for treating endometriosis with aromatase inhibitors. Hum. Reprod. Update 2000, 6, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Niu, Y.; Feng, J.; Guo, H.; Ye, X.; Cui, H. Use of proteomic analysis of endometriosis to identify different protein expression in patients with endometriosis versus normal controls. Fertil. Steril. 2006, 86, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Wataba, K.; Saito, T.; Fukunaka, K.; Ashihara, K.; Nishimura, M.; Kudo, R. Over-expression of heat shock proteins in carcinogenic endometrium. Int. J. Cancer 2001, 91, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.X.; Derks, J.B.; Zhang, Q.; Nathanielsz, P.W. Changes in heat shock protein-90 and -70 messenger ribonucleic acid in uterine tissues of the ewe in relation to parturition and regulation by estradiol and progesterone. Endocrinology 1996, 137, 5685–5693. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B. The hsp90-based chaperone system: Involvement in signal transduction from a variety of hormone and growth factor receptors. Proc. Soc. Exp. Biol. Med. 1998, 217, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef]
- Bastian, B.C. Annexins in cancer and autoimmune diseases. Cell Mol. Life Sci. 1997, 53, 554–556. [Google Scholar] [CrossRef]
- Cao, X.-L.; Song, J.-Y.; Sun, Z.-G. Quantitative label-free proteomic analysis of human follicle fluid to identify novel candidate protein biomarker for endometriosis-associated infertility. J. Proteom. 2022, 266, 104680. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Y.; Yan, J.; Yan, L. Deciphering biomarkers of endometriosis by proteomic analysis of eutopic endometrium in infertile patients. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102043. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Campo, S.; Campo, V.; Benagiano, G. Adenomyosis and infertility. Reprod. BioMedicine Online 2012, 24, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lang, J.; Wang, X.; Wu, S. Comparative proteomic analysis of human adenomyosis using two-dimensional gel electrophoresis and mass spectrometry. Fertil. Steril. 2008, 89, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyu, L.; Weiyuan, Z.; Ping, J.; Anxia, W.; Liane, Z. Comparative serum proteomic analysis of adenomyosis using the isobaric tags for relative and absolute quantitation technique. Fertil. Steril. 2013, 100, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yi, T.; Liu, R.; Bian, C.; Qi, X.; He, X.; Wang, K.; Li, J.; Zhao, X.; Huang, C.; et al. Proteomics identification of annexin A2 as a key mediator in the metastasis and proangiogenesis of endometrial cells in human adenomyosis. Mol. Cell. Proteom. 2012, 11, M112-017988. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhou, L.; Qiao, H.; Wang, Y.; Xiao, Y.; Fang, L.; Yang, B.; Wang, Z. Comparative proteomics identify HSP90A, STIP1 and TAGLN-2 in serum extracellular vesicles as potential circulating biomarkers for human adenomyosis. Exp. Ther. Med. 2022, 23, 374. [Google Scholar] [CrossRef]
- Peeker, R.F.M. Toward a precise definition of interstitial cystitis: Further evidence of differences in classic and nonulcer disease. J. Urol. 2002, 167, 2470–2472. [Google Scholar] [CrossRef]
- Sant, G.R. Etiology, pathogenesis, and diagnosis of interstitial cystitis. Rev. Urol. 2002, 4, 9–15. [Google Scholar]
- Gamper, M.; Regauer, S.; Welter, J.; Eberhard, J.; Viereck, V. Are mast cells still good biomarkers for bladder pain syndrome/interstitial cystitis? J. Urol. 2015, 193, 1994–2000. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Lee, U.J.; Jellison, F.C.; Tan, N.; Patel, M.; Raman, S.S.; Rodriguez, L.V. MRI suggests increased tonicity of the levator ani in women with interstitial cystitis/bladder pain syndrome. Int. Urogynecol. J. 2016, 27, 77–83. [Google Scholar] [CrossRef]
- Ward, E.P.; Bartolone, S.; Chancellor, M.; Peters, K.; Lamb, E. Proteomic analysis of bladder biopsies from interstitial cystitis/bladder pain syndrome patients with and without Hunner’s lesions reveals differences in expression of inflammatory and structural proteins. BMC Urol. 2020, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.T.; Yoshimura, N.; Tyagi, V.; Jacobs, B.; Leng, W.; Tyagi, P. Mapping the cytokine profile of painful bladder syndrome/interstitial cystitis in human bladder and urine specimens. World J. Urol. 2013, 31, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.; Schulte-Baukloh, H.; Stolzenburg, J.-U.; Speroni di Fenizio, P.; Horn, L.-C.; Rüffert, H.; Hartenstein, S.; Burger, M.; Schulze, M.; Schwalenberg, T. Individual receptor profiling as a novel tool to support diagnosis of bladder pain syndrome/interstitial cystitis (BPS/IC). World J. Urol. 2012, 30, 693–700. [Google Scholar] [CrossRef]
- Argade, S.; Chermansky, C.; Tyagi, P. Biomarkers for interstitial cystitis/painful bladder syndrome. Women’s Health 2016, 12, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.R.; Tomaszewski, J.E.; Kunselman, A.R. Urine markers do not predict biopsy findings or presence of bladder ulcers in interstitial cystitis/painful bladder syndrome. J. Urol. 2008, 179, 1850–1856. [Google Scholar] [CrossRef]
- Gou, R.; Zhu, L.; Zheng, M.; Guo, Q.; Hu, Y.; Li, X.; Liu, J.; Lin, B. Annexin A8 can serve as potential prognostic biomarker and therapeutic target for ovarian cancer: Based on the comprehensive analysis of Annexins. J. Transl. Med. 2019, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Lei, Y.; Cong, C.; Tan, D.; Zhou, X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer. Mol. Med. Rep. 2019, 19, 4529–4535. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Tong, Y.; Chen, Y.; Chen, Y. Sunitinib Reduced the Migration of Ectopic Endometrial Cells via p-VEGFR-PI3K-AKT-YBX1-Snail Signaling Pathway. Anal. Cell. Pathol. 2022, 2022, 6042518. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Das, V.; Agarwal, A. PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: An update. Gynecol. Endocrinol. 2012, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Fukushima, Y.; Yoshida, K.; Sakakibara, H.; Tsubata, N.; Yoshie, M.; Kojima, J.; Nishi, H.; Tamura, K. Endometrial epithelial-mesenchymal transition (EMT) by menstruation-related inflammatory factors during hypoxia. Mol. Hum. Reprod. 2021, 27, gaab036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Jhang, J.-F.; Kuo, H.-C. Can We Use Urinary Cytokine/Chemokine Analysis in Discriminating Ulcer-Type Interstitial Cystitis/Bladder Pain Syndrome? Diagnostics 2022, 12, 1093. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lu, J.-H.; Chuang, S.-M.; Chueh, K.-S.; Juan, T.-J.; Liu, Y.-C.; Juan, Y.-S. Urinary Biomarkers in Interstitial Cystitis/Bladder Pain Syndrome and Its Impact on Therapeutic Outcome. Diagnostics 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Tucci, C.; Muzii, L. Chronic Pelvic Pain, Vulvar Pain Disorders, and Proteomics Profiles: New Discoveries, New Hopes. Biomedicines 2024, 12, 1. https://doi.org/10.3390/biomedicines12010001
Di Tucci C, Muzii L. Chronic Pelvic Pain, Vulvar Pain Disorders, and Proteomics Profiles: New Discoveries, New Hopes. Biomedicines. 2024; 12(1):1. https://doi.org/10.3390/biomedicines12010001
Chicago/Turabian StyleDi Tucci, Chiara, and Ludovico Muzii. 2024. "Chronic Pelvic Pain, Vulvar Pain Disorders, and Proteomics Profiles: New Discoveries, New Hopes" Biomedicines 12, no. 1: 1. https://doi.org/10.3390/biomedicines12010001





