A Platform for Testing the Biocompatibility of Implants: Silicone Induces a Proinflammatory Response in a 3D Skin Equivalent
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of 3D Skin and Implanted 3D Skin Equivalents
2.2. Histochemistry and Immunofluorescence
2.3. 3D Skin Equivalent Response Quantification
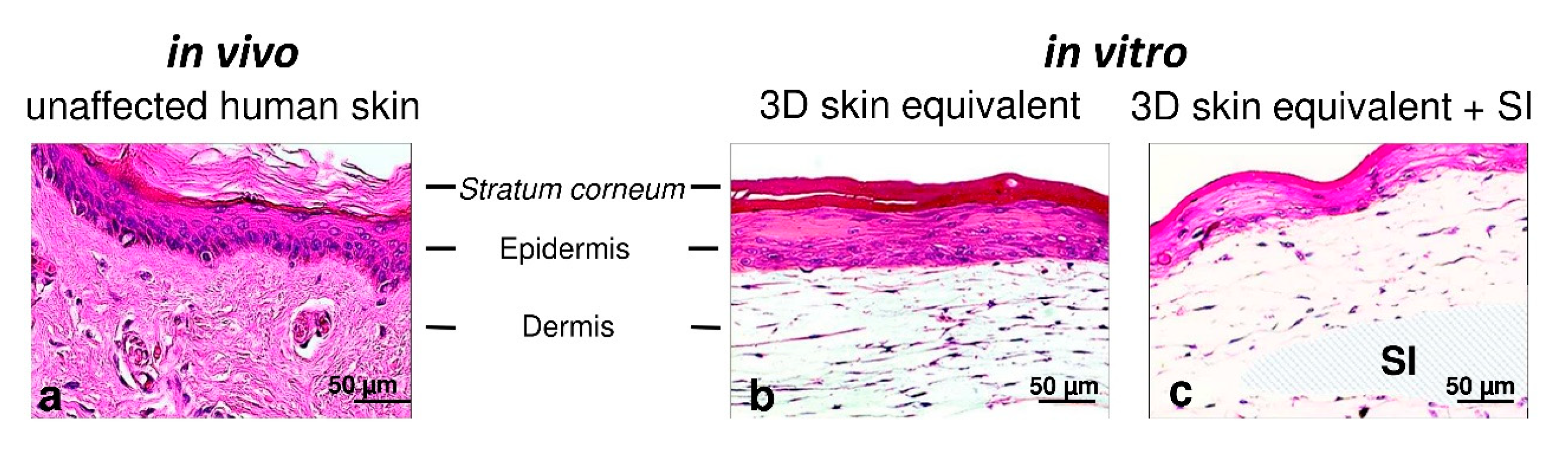
3. Results
3.1. Establishment of 3D Skin Equivalents with and without Silicone Implants
3.2. Validation of Layered Architecture in Newly Developed 3D Skin Equivalent
3.3. Cell Contacts
3.4. Cytotoxicity/Cytokine Response/Inflammatory Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pound, P.; Ebrahim, S.; Sandercock, P.; Bracken, M.B.; Roberts, I. Where is the evidence that animal research benefits humans? BMJ 2004, 328, 514–517. [Google Scholar] [CrossRef]
- Meigs, L.; Smirnova, L.; Rovida, C.; Leist, M.; Hartung, T. Animal testing and its alternatives—The most important omics is economics. ALTEX 2018, 35, 275–305. [Google Scholar] [CrossRef]
- Swaters, D.; van Veen, A.; van Meurs, W.; Turner, J.E.; Ritskes-Hoitinga, M. A History of Regulatory Animal Testing: What Can We Learn? Altern. Lab. Anim. 2022, 50, 322–329. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Kohda, K.; Li, X.; Soga, N.; Nagura, R.; Duerna, T.; Nakajima, S.; Nakagawa, I.; Ito, M.; Ikeuchi, A. An In Vitro Mixed Infection Model with Commensal and Pathogenic Staphylococci for the Exploration of Interspecific Interactions and Their Impacts on Skin Physiology. Front. Cell Infect. Microbiol. 2021, 11, 712360. [Google Scholar] [CrossRef]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin Toxicity Assessment of Silver Nanoparticles in a 3D Epidermal Model Compared to 2D Keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar] [CrossRef]
- Silva-Pedrosa, R.; Salgado, A.J.; Ferreira, P.E. Revolutionizing Disease Modeling: The Emergence of Organoids in Cellular Systems. Cells 2023, 12, 930. [Google Scholar] [CrossRef]
- Law, A.M.K.; de la Fuente, L.R.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.-C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef]
- Wu, B.C.; Haney, E.F.; Akhoundsadegh, N.; Pletzer, D.; Trimble, M.J.; Adriaans, A.E.; Nibbering, P.H.; Hancock, R.E.W. Human organoid biofilm model for assessing antibiofilm activity of novel agents. NPJ Biofilms Microbiomes 2021, 7, 8. [Google Scholar] [CrossRef]
- Popov, L.; Kovalski, J.; Grandi, G.; Bagnoli, F.; Amieva, M.R. Three-Dimensional Human Skin Models to Understand Staphylococcus aureus Skin Colonization and Infection. Front. Immunol. 2014, 5, 41. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Yaszemski, M.; Lemons, J.E. (Eds.) Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Helal, B. The use of silicone rubber spacers in flexor tendon surgery. Hand 1973, 5, 85–90. [Google Scholar] [CrossRef]
- Rehart, S.; Kerschbaumer, F. Endoprothetik an der Hand. Orthopade 2003, 32, 779–783. [Google Scholar] [CrossRef]
- Besmens, I.S.; Giesen, T.; Guidi, M.; Calcagni, M. Der Gelenkersatz mit einem Silikonimplantat bei der Primärversorgung offener Defektverletzungen eines Fingergelenkes. Handchir. Mikrochir. Plast. Chir. 2021, 53, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34 (Suppl. S1), S82–S84. [Google Scholar] [CrossRef]
- Yamamoto, S.; Takeuchi, S. Silicone oil and fluorosilicone. Semin. Ophthalmol. 2000, 15, 15–24. [Google Scholar] [CrossRef]
- Sahan, B.; Ciftci, F. Trephination and silicone tube intubation in the treatment of canalicular obstruction. Int. Ophthalmol. 2023, 43, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G.; Koran, A.; Yu, R. Elastomers for maxillofacial applications. Biomaterials 1980, 1, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Walser, E.M. Venous access ports: Indications, implantation technique, follow-up, and complications. Cardiovasc. Intervent. Radiol. 2012, 35, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Bolle, E.C.L.; Verderosa, A.D.; Dhouib, R.; Parker, T.J.; Fraser, J.F.; Dargaville, T.R.; Totsika, M. An in vitro Reconstructed Human Skin Equivalent Model to Study the Role of Skin Integration Around Percutaneous Devices against Bacterial Infection. Front. Microbiol. 2020, 11, 670. [Google Scholar] [CrossRef]
- Kim, I.-S. Augmentation Rhinoplasty Using Silicone Implants. Facial Plast. Surg. Clin. N. Am. 2018, 26, 285–293. [Google Scholar] [CrossRef]
- Heggers, J.P.P.; Kossovsky, N.M.; Parsons, R.W.M.; Robson, M.C.M.; Pelley, R.P.M.; Raine, T.J.M. Biocompatibility of silicone implants. Ann. Plast. Surg. 1983, 11, 38–45. [Google Scholar] [CrossRef]
- Achilles, R.B.; DE Souza, D.V.; Quintana, H.T.; Malinverni, A.C.M.; DE Moura, C.F.G.; Branda, G.W.; Renno, A.C.M.; Ribeiro, D.A. Cytotoxicity, Inflammatory Activity, and Angiogenesis Are Induced by Different Silicone Implants. In Vivo 2022, 36, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Colaris, M.J.L.; Ruhl, T.; Beier, J.P. Effects of Silicone Breast Implants on Human Cell Types In Vitro: A Closer Look on Host and Implant. Aesthetic Plast. Surg. 2022, 46, 2208–2217. [Google Scholar] [CrossRef]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Use of International Standard ISO 10993-1, “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process”: Guidance for Industry and Food and Drug Administration Staff. 2023. Available online: https://www.fda.gov/media/142959/download (accessed on 16 January 2024).
- Bolle, E.C.L.; Bartnikowski, N.; Haridas, P.; Parker, T.J.; Fraser, J.F.; Gregory, S.D.; Dargaville, T.R. Improving skin integration around long-term percutaneous devices using fibrous scaffolds in a reconstructed human skin equivalent model. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, I.B.; Kraemer, S.; Steinert, M.; Langer, S.; Stock, P.; Kurow, O. Novel 3D organotypic co-culture model of pleura. PLoS ONE 2022, 17, e0276978. [Google Scholar] [CrossRef]
- Kurow, O.; Nuwayhid, R.; Stock, P.; Steinert, M.; Langer, S.; Krämer, S.; Metelmann, I.B. Organotypic 3D Co-Culture of Human Pleura as a Novel In Vitro Model of Staphylococcus aureus Infection and Biofilm Development. Bioengineering 2023, 10, 537. [Google Scholar] [CrossRef]
- Moon, S.; Kim, D.H.; Shin, J.U. In Vitro Models Mimicking Immune Response in the Skin. Yonsei Med. J. 2021, 62, 969–980. [Google Scholar] [CrossRef]
- Morgner, B.; Tittelbach, J.; Wiegand, C. Induction of psoriasis- and atopic dermatitis-like phenotypes in 3D skin equivalents with a fibroblast-derived matrix. Sci. Rep. 2023, 13, 1807. [Google Scholar] [CrossRef]
- Barker, C.L.; McHale, M.T.; Gillies, A.K.; Waller, J.; Pearce, D.M.; Osborne, J.; Hutchinson, P.E.; Smith, G.M.; Pringle, J.H. The development and characterization of an in vitro model of psoriasis. J. Investig. Dermatol. 2004, 123, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Williams, I.R.; Kupper, T.S. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): Analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J. Investig. Dermatol. 1995, 105, 635–643. [Google Scholar] [CrossRef]
- Morizane, S.; Mukai, T.; Sunagawa, K.; Tachibana, K.; Kawakami, Y.; Ouchida, M. “Input/output cytokines” in epidermal keratinocytes and the involvement in inflammatory skin diseases. Front. Immunol. 2023, 14, 1239598. [Google Scholar] [CrossRef]
- Conti, P.; DiGioacchino, M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001, 22, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Lu, D.; Liu, X.; Chen, L. MCP-1 produced by keratinocytes is associated with leucocyte recruitment during elicitation of nickel-induced occupational allergic contact dermatitis. Toxicol. Ind. Health 2018, 34, 36–43. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Ruge, F.; Harding, K.G. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Exp. Ther. Med. 2012, 3, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sundnes, O.; Pietka, W.; Loos, T.; Sponheim, J.; Rankin, A.L.; Pflanz, S.; Bertelsen, V.; Sitek, J.C.; Hol, J.; Haraldsen, G.; et al. Epidermal Expression and Regulation of Interleukin-33 during Homeostasis and Inflammation: Strong Species Differences. J. Investig. Dermatol. 2015, 135, 1771–1780. [Google Scholar] [CrossRef]
- Nair, R.R.; Hsu, J.; Jacob, J.T.; Pineda, C.M.; Hobbs, R.P.; Coulombe, P.A. A role for keratin 17 during DNA damage response and tumor initiation. Proc. Natl. Acad. Sci. USA 2021, 118, e2020150118. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, M.; Zhang, L.-J. Keratin 6, 16 and 17-Critical Barrier Alarmin Molecules in Skin Wounds and Psoriasis. Cells 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Zietlow, J.; Köller, M.; Esenwein, S.A.; Halfmann, H.; Awakowicz, P.; Steinau, H.U. Enhanced cell adhesion to silicone implant material through plasma surface modification. J. Mater. Sci. Mater. Med. 2009, 20, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Tandara, A.A.; Mustoe, T.A. The role of the epidermis in the control of scarring: Evidence for mechanism of action for silicone gel. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 1219–1225. [Google Scholar] [CrossRef]




| Source | Dilution | |
|---|---|---|
| Primary Antibody | ||
| Filaggrin | Abcam plc, Cambridge, UK | 1:400 |
| Involucrin | Abcam, UK | 1:200 |
| Cytokeratin 1 | Abcam, UK | 1:200 |
| Keratin 17 | Cell Signaling Technology, Danvers, MA, USA | 1:400 |
| E-Cadherin | Proteintech Europe, Manchester UK | 1:250 |
| ZO-1 | Proteintech, UK | 1:1000 |
| Secondary Antibodies | ||
| Goat anti-rabbit Alexa Fluor 488 | Cell Signaling Technology, Danvers, MA, USA | 1:1000 |
| Goat anti-rabbit Alexa Fluor 555 | Cell Signaling Technology, USA | 1:1000 |
| Goat anti-rabbit Alexa Fluor 647 | Invitrogen, Themo Fisher Scientific, Waltham, MA, USA | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuwayhid, R.; Schulz, T.; Siemers, F.; Schreiter, J.; Kobbe, P.; Hofmann, G.; Langer, S.; Kurow, O. A Platform for Testing the Biocompatibility of Implants: Silicone Induces a Proinflammatory Response in a 3D Skin Equivalent. Biomedicines 2024, 12, 224. https://doi.org/10.3390/biomedicines12010224
Nuwayhid R, Schulz T, Siemers F, Schreiter J, Kobbe P, Hofmann G, Langer S, Kurow O. A Platform for Testing the Biocompatibility of Implants: Silicone Induces a Proinflammatory Response in a 3D Skin Equivalent. Biomedicines. 2024; 12(1):224. https://doi.org/10.3390/biomedicines12010224
Chicago/Turabian StyleNuwayhid, Rima, Torsten Schulz, Frank Siemers, Jeannine Schreiter, Philipp Kobbe, Gunther Hofmann, Stefan Langer, and Olga Kurow. 2024. "A Platform for Testing the Biocompatibility of Implants: Silicone Induces a Proinflammatory Response in a 3D Skin Equivalent" Biomedicines 12, no. 1: 224. https://doi.org/10.3390/biomedicines12010224
APA StyleNuwayhid, R., Schulz, T., Siemers, F., Schreiter, J., Kobbe, P., Hofmann, G., Langer, S., & Kurow, O. (2024). A Platform for Testing the Biocompatibility of Implants: Silicone Induces a Proinflammatory Response in a 3D Skin Equivalent. Biomedicines, 12(1), 224. https://doi.org/10.3390/biomedicines12010224






