Potential Interaction between WNT16 and Vitamin D on Bone Qualities in Adolescent Idiopathic Scoliosis Patients and Healthy Controls
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment and Clinical Assessment
2.2. Bone Qualities and Serum Vit-D Levels of the Clinical Cohort
2.3. SNP Selection and Genotyping of the Clinical Cohort
2.4. Wnt16 Global Knockout and Vit-D Diets in Mice
2.5. MicroCT Measurement and Serum Vit-D Level in Mice
2.6. Messenger Ribonucleic Acid Expression in Mice
2.7. Statistical Analysis
3. Results
3.1. Association between the Individual SNPs and Phenotypes in the Clinical Cohort
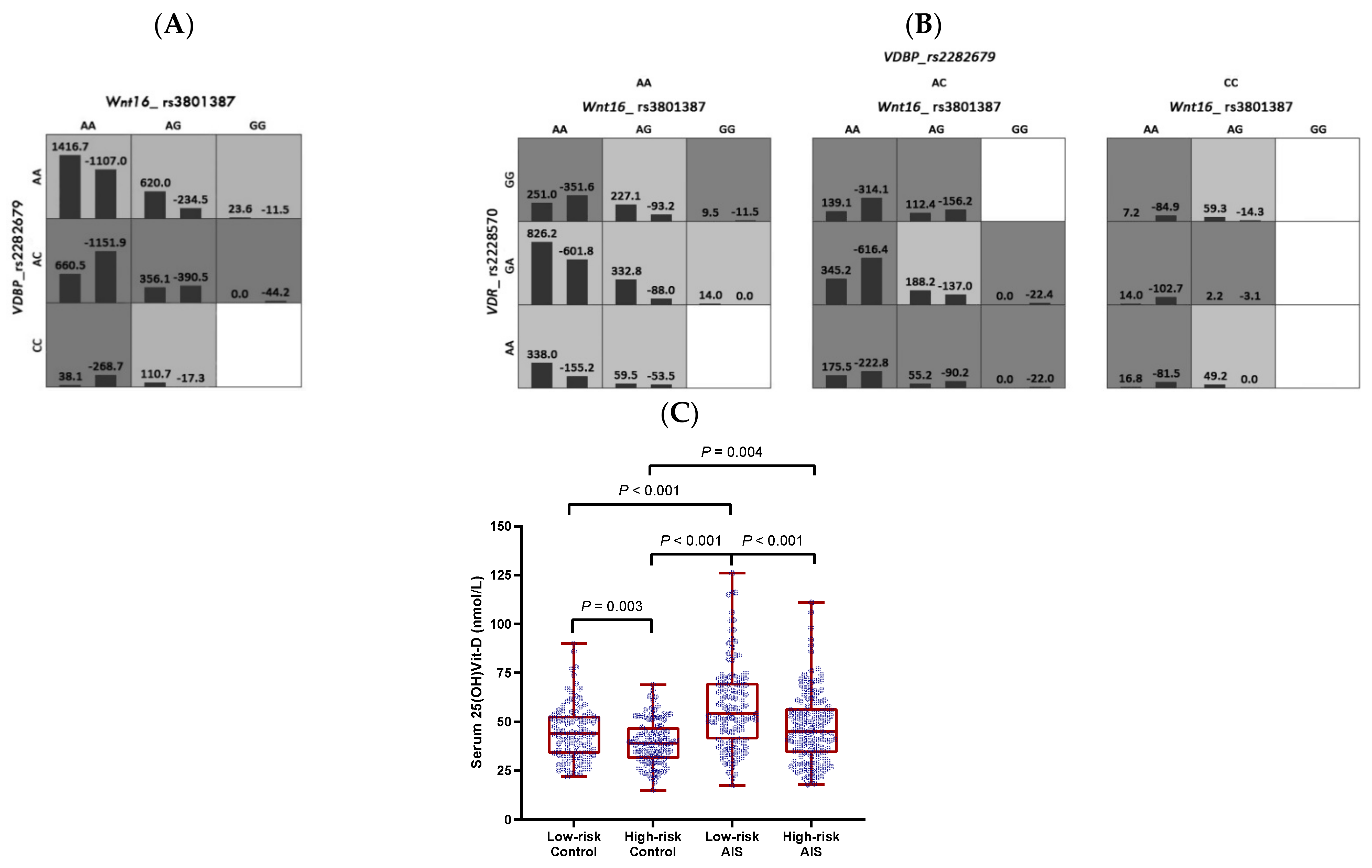
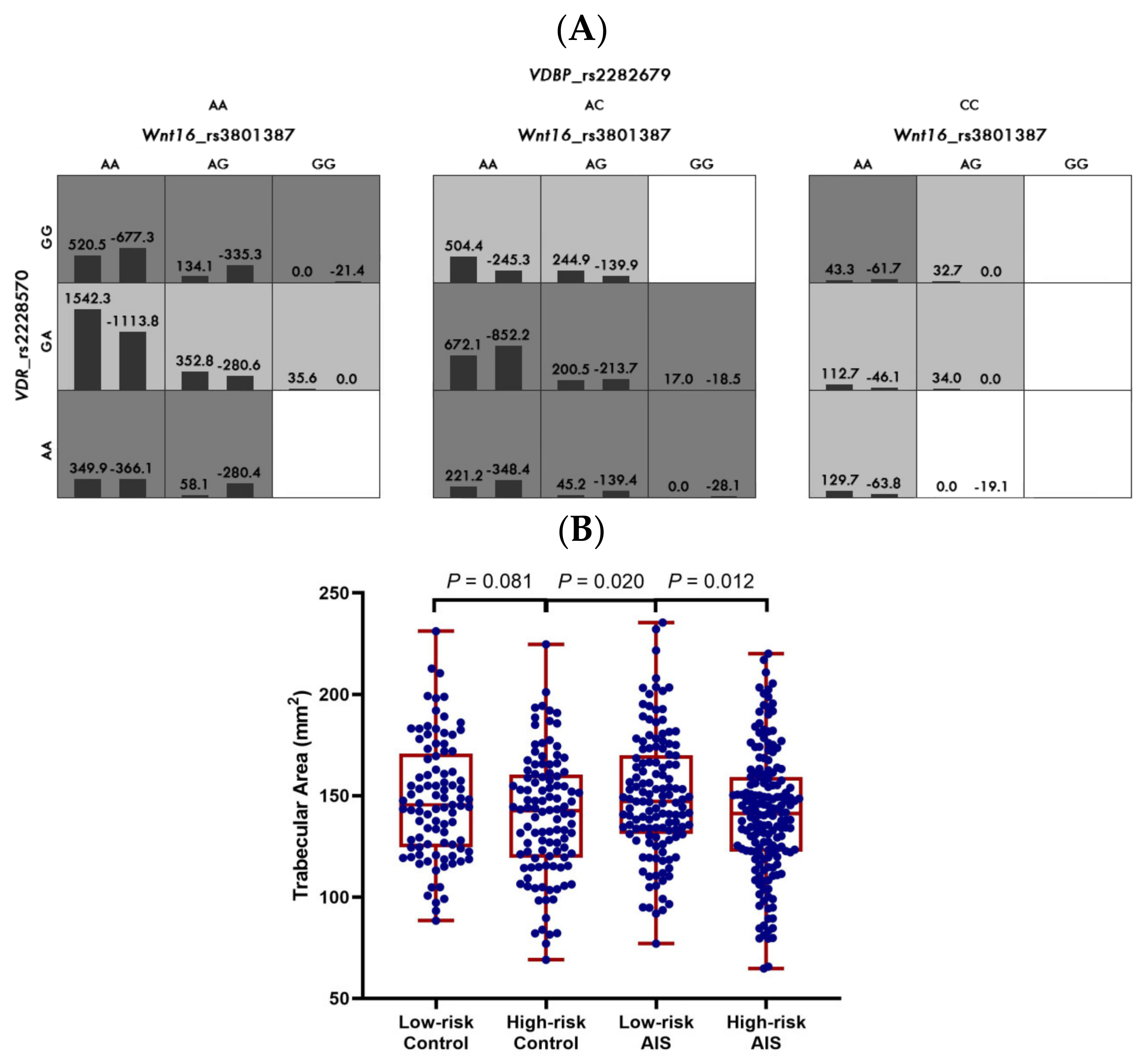
3.2. Gene–Gene Interactions between WNT16, VDR, and VDBP in the Clinical Cohort
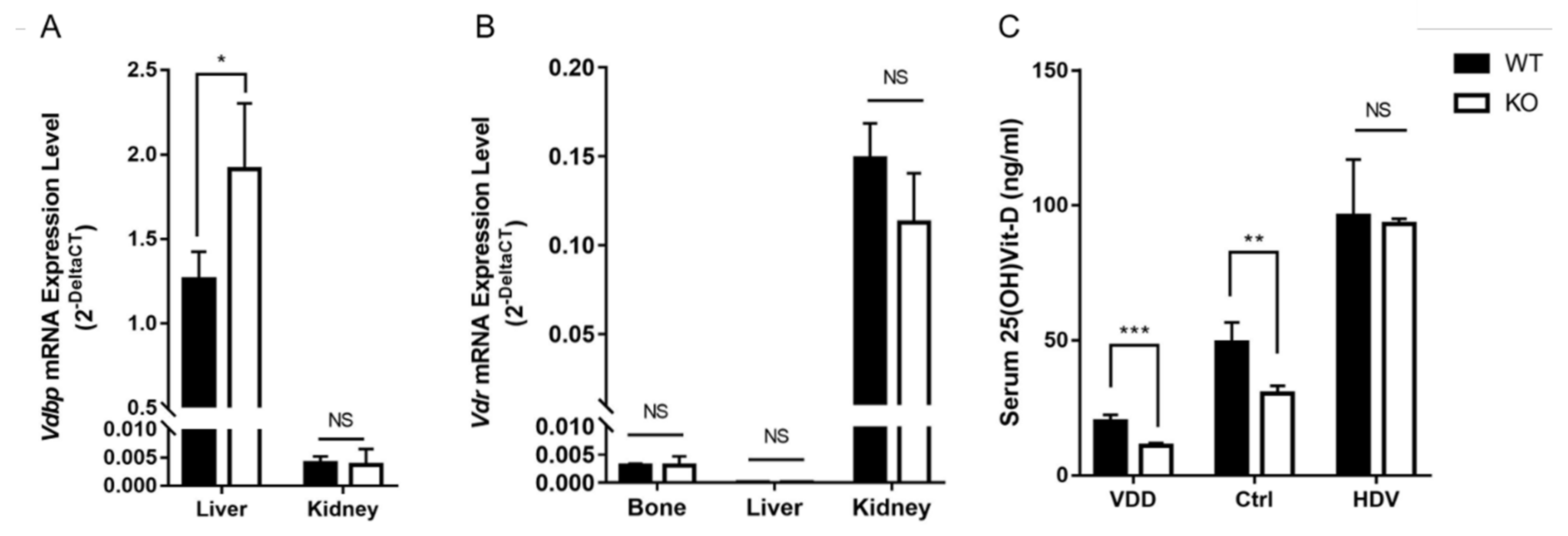
3.3. Serum 25(OH)Vit-D and Vdbp Expression Level in Wnt16 Global Knockout Mice
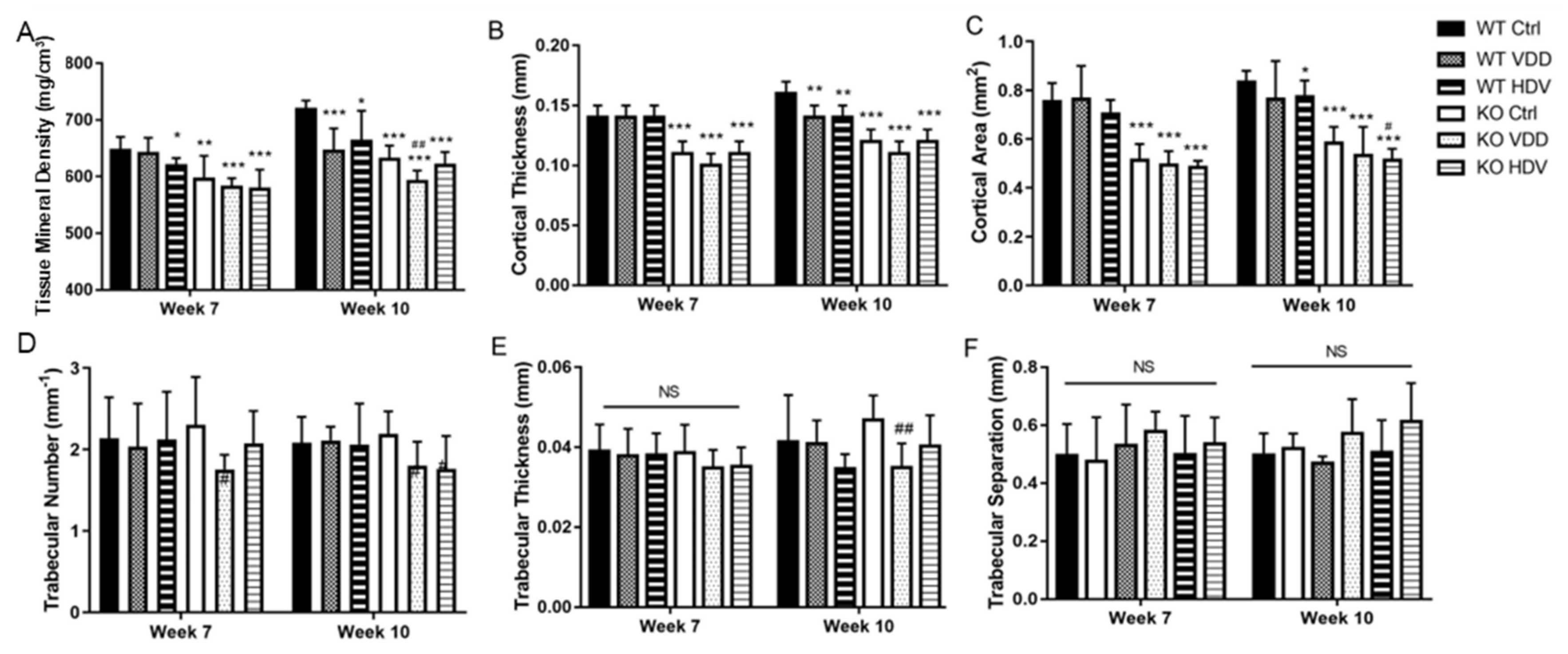
3.4. Bone Qualities in Wnt16 Global Knockout Mice on Different Vit-D Chows
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.C.Y.; Castelein, R.M.; Chu, W.C.W.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primers 2015, 1, 15030. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef]
- Cheng, J.C.Y.; Guo, X.; Sher, A.H. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine 1999, 24, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.Y.; Qin, L.; Cheung, C.S.K.; Sher, A.H.L.; Lee, K.M.; Ng, S.W.E.; Guo, X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J. Bone Miner. Res. 2000, 15, 1587–1595. [Google Scholar] [CrossRef]
- Cheung, C.S.K.; Lee, W.T.K.; Tse, Y.K.; Lee, K.M.; Guo, X.; Qin, L.; Cheng, J.C.Y. Generalized osteopenia in adolescent idiopathic scoliosis—Association with abnormal pubertal growth, bone turnover, and calcium intake? Spine 2006, 31, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hung, V.W.Y.; Yu, F.W.P.; Hung, A.L.H.; Ng, B.K.W.; Cheng, J.C.Y.; Lam, T.P.; Yip, B.H.K. Persistent low-normal bone mineral density in adolescent idiopathic scoliosis with different curve severity: A longitudinal study from presentation to beyond skeletal maturity and peak bone mass. Bone 2019, 133, 115217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.Y.; Hung, V.W.Y.; Lee, W.T.K.; Yeung, H.Y.; Lam, T.P.; Ng, B.K.W.; Guo, X.; Qin, L. Persistent osteopenia in adolescent idiopathic scoliosis—Longitudinal monitoring of bone mineral density until skeletal maturity. Stud. Health Technol. Inform. 2006, 123, 47–51. [Google Scholar]
- Yu, W.S.; Chan, K.Y.; Yu, F.W.P.; Yeung, H.Y.; Ng, B.K.W.; Lee, K.M.; Lam, T.P.; Cheng, J.C.Y. Abnormal bone quality versus low bone mineral density in adolescent idiopathic scoliosis: A case-control study with in vivo high-resolution peripheral quantitative computed tomography. Spine J. 2013, 13, 1493–1499. [Google Scholar] [CrossRef]
- Yu, W.S.; Chan, K.Y.; Yu, F.W.P.; Ng, B.K.W.; Lee, K.M.; Qin, L.; Lam, T.P.; Cheng, J.C.Y. Bone Structural and Mechanical Indices in Adolescent Idiopathic Scoliosis Evaluated by High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT). Bone 2014, 61, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Hung, V.W.Y.; Qin, L.; Cheung, C.S.K.; Lam, T.P.; Ng, B.K.W.; Tse, Y.K.; Guo, X.; Lee, K.M.; Cheng, J.C.Y. Osteopenia: A new prognostic factor of curve progression in adolescent idiopathic scoliosis. J. Bone Jt. Surg. Am. 2005, 87, 2709–2716. [Google Scholar]
- Yip, B.H.K.; Yu, F.W.P.; Wang, Z.; Hung, V.W.Y.; Lam, T.P.; Ng, B.K.W.; Zhu, F.; Cheng, J.C.Y. Prognostic Value of Bone Mineral Density on Curve Progression: A Longitudinal Cohort Study of 513 Girls with Adolescent Idiopathic Scoliosis. Sci. Rep. 2016, 6, 39220. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Yu, Y.E.; Zhang, J.; Cheuk, K.Y.; Ng, B.K.W.; Qiu, Y.; Guo, E.X.; Cheng, J.C.Y.; Lee, W.Y.W. Unique local bone tissue characteristics in iliac crest bone biopsy from adolescent idiopathic scoliosis with severe spinal deformity. Sci. Rep. 2017, 7, 40265. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, J.; Wang, Y.; Cheuk, K.Y.; Hung, A.L.H.; Lam, T.P.; Qiu, Y.; Feng, J.Q.; Lee, W.Y.W.; Cheng, J.C.Y. Abnormal lacuno-canalicular network and negative correlation between serum osteocalcin and Cobb angle indicate abnormal osteocyte function in adolescent idiopathic scoliosis. FASEB J. 2019, 33, 13882–13892. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G. Vitamin D insufficiency: Definition, diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 669–684. [Google Scholar] [CrossRef]
- Cheung, T.F.; Cheuk, K.Y.; Yu, F.W.; Hung, V.W.; Ho, C.S.; Zhu, T.Y.; Ng, B.K.W.; Lee, K.M.; Qin, L.; Ho, S.S.Y.; et al. Prevalence of vitamin D insufficiency among adolescents and its correlation with bone parameters using high-resolution peripheral quantitative computed tomography. Osteoporos. Int. 2016, 27, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, W.Y.W.; Hung, A.L.H.; Tang, M.F.; Li, X.; Kong, A.P.S.; Leung, T.F.; Yung, P.S.H.; To, K.K.W.; Cheng, J.C.Y.; et al. Association of serum 25(OH)Vit-D levels with risk of pediatric fractures: A systematic review and meta-analysis. Osteoporos. Int. 2021, 32, 1287–1300. [Google Scholar] [CrossRef]
- Winzenberg, T.; Powell, S.; Shaw, K.A.; Jones, G. Effects of vitamin D supplementation on bone density in healthy children: Systematic review and meta-analysis. BMJ 2011, 342, c7254. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.T.; Fuleihan, G.E.; Cai, G.Q.; Lamberg-Allardt, C.; Viljakainen, H.T.; Rahme, M.; Grønborg, I.M.; Andersen, R.; Khadilkar, A.; Zulf, M.M.; et al. Vitamin D supplementation for improving bone density in vitamin D-deficient children and adolescents: Systematic review and individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2023, 118, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Kemp, J.P.; Youlten, S.E.; Laurent, L.; Logan, J.G.; Chai, R.C.; Vulpescu, N.A.; Forgetta, V.; Kleinman, A.; Mohanty, S.T.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019, 51, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Kiel, D.P. Genome-Wide Association Studies of Skeletal Phenotypes: What We Have Learned and Where We Are Headed. J. Clin. Endocrinol. Metab. 2012, 97, E1958–E1977. [Google Scholar] [CrossRef] [PubMed]
- Estrada, K.; Styrkarsdottir, U.; Evangelou, E.; Hsu, Y.H.; Duncan, E.L.; Ntzani, E.E.; Oei, L.; Albagha, O.M.E.; Amin, N.; Kemp, J.P.; et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 2012, 44, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, C.; Kemp, J.P.; Estrada, K.; Eriksson, J.; Liu, J.; Reppe, S.; Evans, D.M.; Heppe, D.H.M.; Vandenput, L.; Herrera, L.; et al. Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the WNT16 Locus. PLoS Genet. 2012, 8, e1002718. [Google Scholar] [CrossRef] [PubMed]
- Cobb, J.R. The problem of the primary curve. J. Bone Jt. Surg. Am. 1960, 42, 1413–1425. [Google Scholar] [CrossRef]
- Cheung, C.S.K.; Lee, W.T.K.; Tse, Y.K.; Tang, S.P.; Lee, K.M.; Guo, X.; Qin, L.; Cheng, J.C.Y. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: A study of 598 patients. Spine 2003, 28, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Normal growth and techniques of growth assessment. Clin. Endocrinol. Metab. 1986, 15, 411–451. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Sher, H.L.; Guo, X.; Hung, V.W.; Cheung, A.Y. The effect of vertebral rotation of the lumbar spine on dual energy X-ray absorptiometry measurements: Observational study. Hong Kong Med. J. 2001, 7, 241–245. [Google Scholar] [PubMed]
- Girardi, F.P.; Parvataneni, H.K.; Sandhu, H.S.; Cammisa, F.P.; Grewal, H.; Schneider, R.; Lane, J.M. Correlation between vertebral body rotation and two-dimensional vertebral bone density measurement. Osteoporos. Int. 2001, 12, 738–740. [Google Scholar] [CrossRef]
- Kirmani, S.; Christen, D.; van Lenthe, G.H.; Fischer, P.R.; Bouxsein, M.L.; McCready, L.K.; Melton, L.J., 3rd; Riggs, B.L.; Amin, S.; Müller, R.; et al. Bone Structure at the Distal Radius During Adolescent Growth. J. Bone Miner. Res. 2009, 24, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.; Saw, S.; Sahabdeen, N.B.; Tey, K.T.; Ho, C.S.; Sethi, S.K. Current 25-hydroxyvitamin D assays: Do they pass the test? Clin. Chim. Acta 2012, 413, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M.; de Jongh, R.T. Diet, sun, and lifestyle as determinants of vitamin D status. Ann. N. Y. Acad. Sci. 2014, 1317, 92–98. [Google Scholar] [CrossRef]
- Del Valle, H.B.; Yaktine, A.L.; Taylor, C.L.; Ross, A.C. (Eds.) Dietary Reference Intakes for Calcium and Vitamin D; The National Academies Press: Washington, DC, USA, 2011; pp. 1–1115. [Google Scholar]
- Zheng, H.F.; Tobias, J.H.; Duncan, E.; Evans, D.M.; Eriksson, J.; Paternoster, L.; Yerges-Armstrong, L.M.; Lehtimäki, T.; Bergström, U.; Kähönen, M.; et al. WNT16 influences bone mineral density, cortical bone thickness, bone strength, and osteoporotic fracture risk. PLoS Genet. 2012, 8, e1002745. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhou, H.; Gao, B.; Li, Y.; Liao, Z.; Zhou, T.; Lian, C.; Wu, Z.; Su, D.; Wang, T.; et al. Estrogen promotes the onset and development of idiopathic scoliosis via disproportionate endochondral ossification of the anterior and posterior column in a bipedal rat model. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Wang, L.; Wong, M.W.N.; Wen, C.; Wang, G.; Zhang, G.; Chan, K.M.; Cheung, W.H.; Leung, K.S. Osteogenesis Induced by Extracorporeal Shockwave in Treatment of Delayed Osteotendinous Junction Healing. J. Orthop. Res. 2010, 28, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.H.K.; Suen, P.K.; Fu, L.H.; Cheung, W.H.; Leung, K.S.; Wong, M.W.N.; Qin, L. Extracorporeal shockwave therapy for treatment of delayed tendon-bone insertion healing in a rabbit model: A dose-response study. Am. J. Sports Med. 2012, 40, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Cortes, S.; Perez-Encinas, C.; Escriva, M.D.; Benet, I.; Burgos, J.; Hevia, E.; Pizá, G.; Domenech, P. Anthropometry and body composition profile of girls with nonsurgically treated adolescent idiopathic scoliosis. Spine 2011, 36, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Luttmann-Gibson, H.; Mora, S.; Danik, J.; Bubes, V.; Copeland, T.; LeBoff, M.S.; Cook, N.R.; Lee, I.M.; Buring, J.E.; et al. Association of Body Weight with Response to Vitamin D Supplementation and Metabolism. JAMA Netw. Open 2023, 6, e2250681. [Google Scholar] [CrossRef]
- Ghanbari, F.; Otomo, N.; Gamache, I.; Iwami, T.; Koike, Y.; Khanshour, A.M.; Ikegawa, S.; Wise, C.A.; Terao, C.; Manousaki, D. Interrogating Causal Effects of Body Composition and Puberty-Related Risk Factors on Adolescent Idiopathic Scoliosis: A Two-Sample Mendelian Randomization Study. JBMR Plus 2023, 7, e10830. [Google Scholar] [CrossRef]
- Eun, I.S.; Park, W.W.; Suh, K.T.; Kim, J.I.; Lee, J.S. Association between osteoprotegerin gene polymorphism and bone mineral density in patients with adolescent idiopathic scoliosis. Eur. Spine J. 2009, 18, 1936–1940. [Google Scholar] [CrossRef]
- Lee, J.S.; Suh, K.T.; Eun, I.S. Polymorphism in interleukin-6 gene is associated with bone mineral density in patients with adolescent idiopathic scoliosis. J. Bone Jt. Surg. Br. 2010, 92, 1118–1122. [Google Scholar] [CrossRef][Green Version]
- Dastani, Z.; Li, R.; Richards, B. Genetic Regulation of Vitamin D Levels. Calcif. Tissue Int. 2013, 92, 106–117. [Google Scholar] [CrossRef]
- Ahn, J.; Yu, K.; Stolzenberg-Solomon, R.; Simon, K.C.; McCullough, M.L.; Gallicchio, L.; Ascherio, A.; Helzlsouer, K.; Jacobs, K.B.; Li, Q.; et al. Genome-wide association study of circulating vitamin D levels. Hum. Mol. Genet. 2010, 19, 2739–2745. [Google Scholar] [CrossRef]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Zhang, Z.; He, J.W.; Fu, W.Z.; Zhang, C.Q.; Zhang, Z.L. An analysis of the association between the vitamin D pathway and serum 25-hydroxyvitamin D levels in a healthy Chinese population. J. Bone Miner. Res. 2013, 28, 1784–1792. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144, 132–137. [Google Scholar] [CrossRef]
- Garcia-Ibarbia, C.; Perez-Nunez, M.I.; Olmos, J.M.; Valero, C.; Perez-Aguilar, M.D.; Hernandez, J.L.; Zarrabeitia, M.T.; González-Macías, J.; Riancho, J.A. Missense polymorphisms of the WNT16 gene are associated with bone mass, hip geometry and fractures. Osteoporos. Int. 2013, 24, 2449–2454. [Google Scholar] [CrossRef]
- Koller, D.L.; Zheng, H.F.; Karasik, D.; Yerges-Armstrong, L.; Liu, C.T.; McGuigan, F.; Kemp, J.P.; Giroux, S.; Lai, D.; Edenberg, H.J.; et al. Meta-analysis of genome-wide studies identifies WNT16 and ESR1 SNPs associated with bone mineral density in premenopausal women. J. Bone Miner. Res. 2013, 28, 547–558. [Google Scholar] [CrossRef]
- Price, C.; Herman, B.C.; Lufkin, T.; Goldman, H.M.; Jepsen, K.J. Genetic variation in bone growth patterns defines adult mouse bone fragility. J. Bone Miner. Res. 2005, 20, 1983–1991. [Google Scholar] [CrossRef]
- Bang, W.S.; Lee, D.H.; Kim, K.T.; Cho, D.C.; Sung, J.K.; Han, I.B.; Kim, D.H.; Kwon, B.K.; Kim, C.H.; Park, K.S.; et al. Relationships between vitamin D and paraspinal muscle: Human data and experimental rat model analysis. Spine J. 2018, 18, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Shieh, A.; Gottlieb, C.; Yacoubian, V.; Wang, J.; Hewison, M.; Adams, J.S. Vitamin D Binding Protein and the Biological Activity of Vitamin D. Front. Endocrinol. 2019, 10, 718. [Google Scholar] [CrossRef]
- Kumaratne, M.; Early, G.; Cisneros, J. Vitamin D Deficiency and Association with Body Mass Index and Lipid Levels in Hispanic American Adolescents. Glob. Pediatr. Health 2017, 4, 2333794X17744141. [Google Scholar] [CrossRef] [PubMed]
- Balioglu, M.B.; Aydin, C.; Kargin, D.; Albayrak, A.; Atici, Y.; Tas, S.K.; Kaygusuz, M.A. Vitamin-D measurement in patients with adolescent idiopathic scoliosis. J. Pediatr. Orthop. B 2017, 26, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Danielewicz, A.; Wójciak, M.; Sowa, I.; Kusz, M.; Wessely-Szponder, J.; Dresler, S.; Latalski, M. Metabolic Imbalances and Bone Remodeling Agents in Adolescent Idiopathic Scoliosis: A Study in Postmenarcheal Girls. Int. J. Mol. Sci. 2023, 24, 13286. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.T. Association between body mass index and posterior spine fusion among patients with adolescent idiopathic scoliosis. PLoS ONE 2023, 18, e0286001. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, M.; Saito, W.; Imura, T.; Nakazawa, T.; Shirasawa, E.; Kawakubo, A.; Uchida, K.; Akazawa, T.; Inage, K.; Ohtori, S.; et al. Body Composition in Japanese Girls with Adolescent Idiopathic Scoliosis. Spine Surg. Relat. Res. 2021, 5, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.P.; Yang, G.; Pang, H.; Yip, B.H.K.; Lee, W.Y.W.; Hung, A.L.H.; Tang, N.L.S.; To, K.K.W.; Qiu, Y.; Cheng, J.C.Y. A six years longitudinal cohort study on the changes in bone density and bone quality up to peak bone mass in adolescent idiopathic scoliosis (AIS) with and without 2 years of Calcium and Vit-D supplementation. Stud. Health Technol. Inform. 2021, 280, 31–34. [Google Scholar]
- Brustad, N.; Garland, J.; Thorsen, J.; Sevelsted, A.; Krakauer, M.; Vinding, R.K.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Effect of High-Dose vs Standard-Dose Vitamin D Supplementation in Pregnancy on Bone Mineralization in Offspring Until Age 6 Years: A Prespecified Secondary Analysis of a Double-Blinded, Randomized Clinical Trial. JAMA Pediatr. 2020, 174, 419–427. [Google Scholar] [CrossRef]
- Rosendahl, J.; Valkama, S.; Holmlund-Suila, E.; Enlund-Cerullo, M.; Hauta-Alus, H.; Helve, O.; Hytinantti, T.; Levälahti, E.; Kajantie, E.; Viljakainen, H.; et al. Effect of Higher vs Standard Dosage of Vitamin D3 Supplementation on Bone Strength and Infection in Healthy Infants: A Randomized Clinical Trial. JAMA Pediatr. 2018, 172, 646–654. [Google Scholar] [CrossRef]
- Jones, G. Extrarenal vitamin D activation and interactions between vitamin D(2), vitamin D(3), and vitamin D analogs. Annu. Rev. Nutr. 2013, 33, 23–44. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Newer Concepts of Its Metabolism and Function at the Basic and Clinical Level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.; Hayes, A.; Hanson, E.D.; Pivonka, P.; Sims, N.A.; Gooi, J.H. High dose dietary vitamin D3 increases bone mass and strength in mice. Bone Rep. 2017, 6, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef] [PubMed]
| AIS (n = 318) | Controls (n = 201) | |
|---|---|---|
| Age and stage of puberty | ||
| Age (years) | 14.02 (13.03–15.24) ** | 13.47 (12.88–14.52) |
| Maximum Cobb angle (°) | 27 (22–35) | NA |
| Breast stage | 3 (3–4) | 3 (3–4) |
| Pubic hair stage | 3 (2–4) * | 3 (2–3) |
| Anthropometry | ||
| Body weight (kg) | 43.89 (39.40–48.03) ** | 47.20 (41.40–53.00) |
| Body height (cm) | 155.85 (130.00–175.50) | 156.20 (136.00–170.00) |
| Sitting height (cm) | 83.00 (80.50–85.00) * | 83.50 (81.25–86.15) |
| Arm span (cm) | 156.00 (151.00–161.40) | 155.00 (149.70–159.50) |
| Vitamin D status | ||
| Serum 25(OH)Vit-D (nmol/L) | 50.10 (17.40–126.00) ** | 40.50 (15.00–90.00) |
| Vit-D sufficient | 137 (50.2) ** | 56 (27.9) |
| Vit-D insufficient | 120 (44.0) ** | 130 (64.7) |
| Vit-D deficient | 16 (5.9) | 15 (7.5) |
| Bone parameters | ||
| Left FN aBMD (g/cm2) | 0.68 (0.63–0.74) ** | 0.78 (0.70–0.89) |
| Right FN aBMD (g/cm2) | 0.68 (0.63–0.75) ** | 0.78 (0.71–0.89) |
| Z-score of Left FN aBMD | −0.55 (−0.98–−0.12) ** | 0.10 (−0.67–0.97) |
| Z-score of Right FN aBMD | −0.54 (−0.92–−0.16) ** | 0.03 (−0.64–1.03) |
| Total vBMD (mg/mm3) | 269.40 (228.20–319.10) * | 287.30 (232.50–343.85) |
| Total bone area (mm2) | 183.20 (109.30–275.10) | 183.00 (110.20–264.50) |
| Cortical vBMD (mg/mm3) | 749.80 (665.50–811.80) | 759.00 (668.75–821.70) |
| Cortical thickness (mm) | 0.59 (0.37–0.81) | 0.66 (0.38–0.86) |
| Cortical area (mm2) | 33.70 (20.10–44.70) | 35.60 (20.75–47.25) |
| Cortical bone perimeter (mm) | 55.10 (42.40–69.80) | 55.20 (41.90–68.10) |
| Trabecular vBMD (mg/mm3) | 138.50 (76.50–219.70) ** | 153.40 (72.20–242.80) |
| BV/TV | 0.12(0.10–0.13) ** | 0.13(0.11–0.14) |
| Trabecular number (mm−1) | 1.64 (0.69–2.21) ** | 1.72 (0.91–2.45) |
| Trabecular thickness (mm) | 0.07 (0.07–0.08) * | 0.07 (0.07–0.08) |
| Trabecular area (mm2) | 143.80 (125.00–163.55) | 143.9 (121.30–162.15) |
| Trabecular separation (mm) | 0.54 (0.49–0.61) ** | 0.50 (0.45–0.58) |
| Number of Loci | SNP Combination | CVC | TA (%) | p 1 | |
|---|---|---|---|---|---|
| Serum 25(OH)Vit-D | |||||
| 1 | VDBP_rs2282679 | 10 | 60.36 | 0.001 | |
| 2 | WNT16_rs3801387, VDBP_rs2282679 | 10 | 59.64 | 0.003 | |
| 3 | WNT16_rs3801387, VDBP_rs2282679, VDR_rs2228570 | 10 | 60.08 | 0.006 | |
| Total vBMD | |||||
| 1 | VDR_rs2228570 | 6 | 51.58 | 0.355 | |
| 2 | VDBP_rs2282679, VDR_rs2228570 | 10 | 58.68 | 0.01 | |
| 3 | WNT16_rs3801387, VDBP_rs2282679, VDR_rs2228570 | 10 | 51.39 | 0.381 | |
| Total bone area | |||||
| 1 | VDR_rs2228570 | 10 | 53.23 | 0.189 | |
| 2 | VDBP_rs2282679, VDR_rs2228570 | 10 | 56.7 | 0.041 | |
| 3 | WNT16_rs3801387, VDBP_rs2282679, VDR_rs2228570 | 10 | 55.91 | 0.074 | |
| Cortical bone perimeter | |||||
| 1 | VDR_rs2228570 | 10 | 54.58 | 0.103 | |
| 2 | VDBP_rs2282679, VDR_rs2228570 | 10 | 57.24 | 0.029 | |
| 3 | WNT16_rs3801387, VDBP_rs2282679, VDR_rs2228570 | 10 | 54.88 | 0.117 | |
| Trabecular area | |||||
| 1 | VDR_rs2228570 | 8 | 52.68 | 0.239 | |
| 2 | VDBP_rs2282679, VDR_rs2228570 | 10 | 59.62 | 0.004 | |
| 3 | WNT16_rs3801387, VDBP_rs2282679, VDR_rs2228570 | 10 | 56.73 | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Chen, H.; Cheng, K.-L.; Tang, M.-F.; Wang, Y.; Hung, L.-H.; Cheng, C.-Y.; Mak, K.-L.; Lee, Y.-W. Potential Interaction between WNT16 and Vitamin D on Bone Qualities in Adolescent Idiopathic Scoliosis Patients and Healthy Controls. Biomedicines 2024, 12, 250. https://doi.org/10.3390/biomedicines12010250
Yang G, Chen H, Cheng K-L, Tang M-F, Wang Y, Hung L-H, Cheng C-Y, Mak K-L, Lee Y-W. Potential Interaction between WNT16 and Vitamin D on Bone Qualities in Adolescent Idiopathic Scoliosis Patients and Healthy Controls. Biomedicines. 2024; 12(1):250. https://doi.org/10.3390/biomedicines12010250
Chicago/Turabian StyleYang, Guangpu (Kenneth), Huanxiong Chen, Ka-Lo Cheng, Man-Fung Tang, Yujia Wang, Lik-Hang (Alec) Hung, Chun-Yiu (Jack) Cheng, King-Lun (Kingston) Mak, and Yuk-Wai (Wayne) Lee. 2024. "Potential Interaction between WNT16 and Vitamin D on Bone Qualities in Adolescent Idiopathic Scoliosis Patients and Healthy Controls" Biomedicines 12, no. 1: 250. https://doi.org/10.3390/biomedicines12010250
APA StyleYang, G., Chen, H., Cheng, K.-L., Tang, M.-F., Wang, Y., Hung, L.-H., Cheng, C.-Y., Mak, K.-L., & Lee, Y.-W. (2024). Potential Interaction between WNT16 and Vitamin D on Bone Qualities in Adolescent Idiopathic Scoliosis Patients and Healthy Controls. Biomedicines, 12(1), 250. https://doi.org/10.3390/biomedicines12010250






