The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy
Abstract
1. Introduction
2. Methods
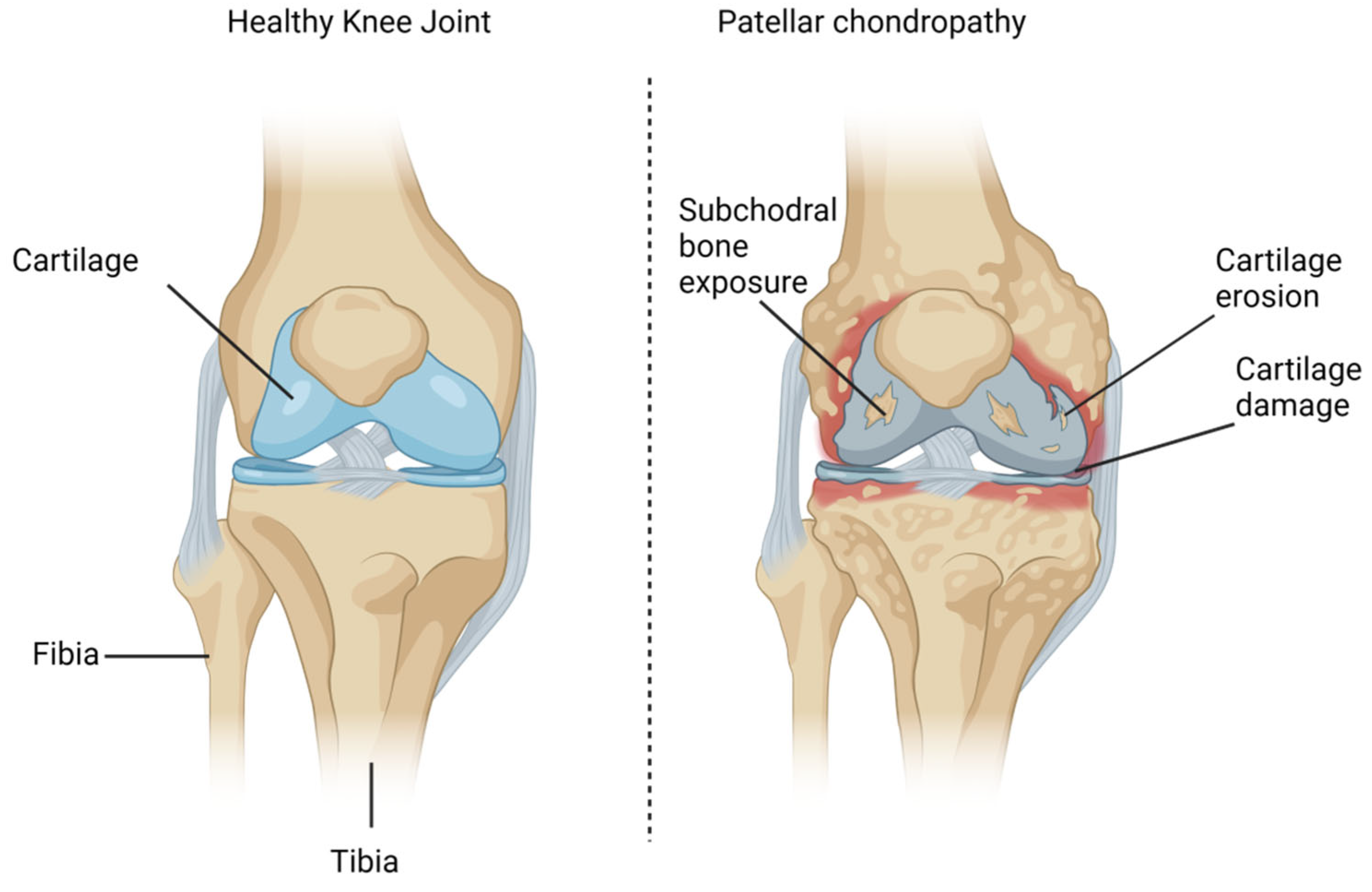
3. Pathophysiology
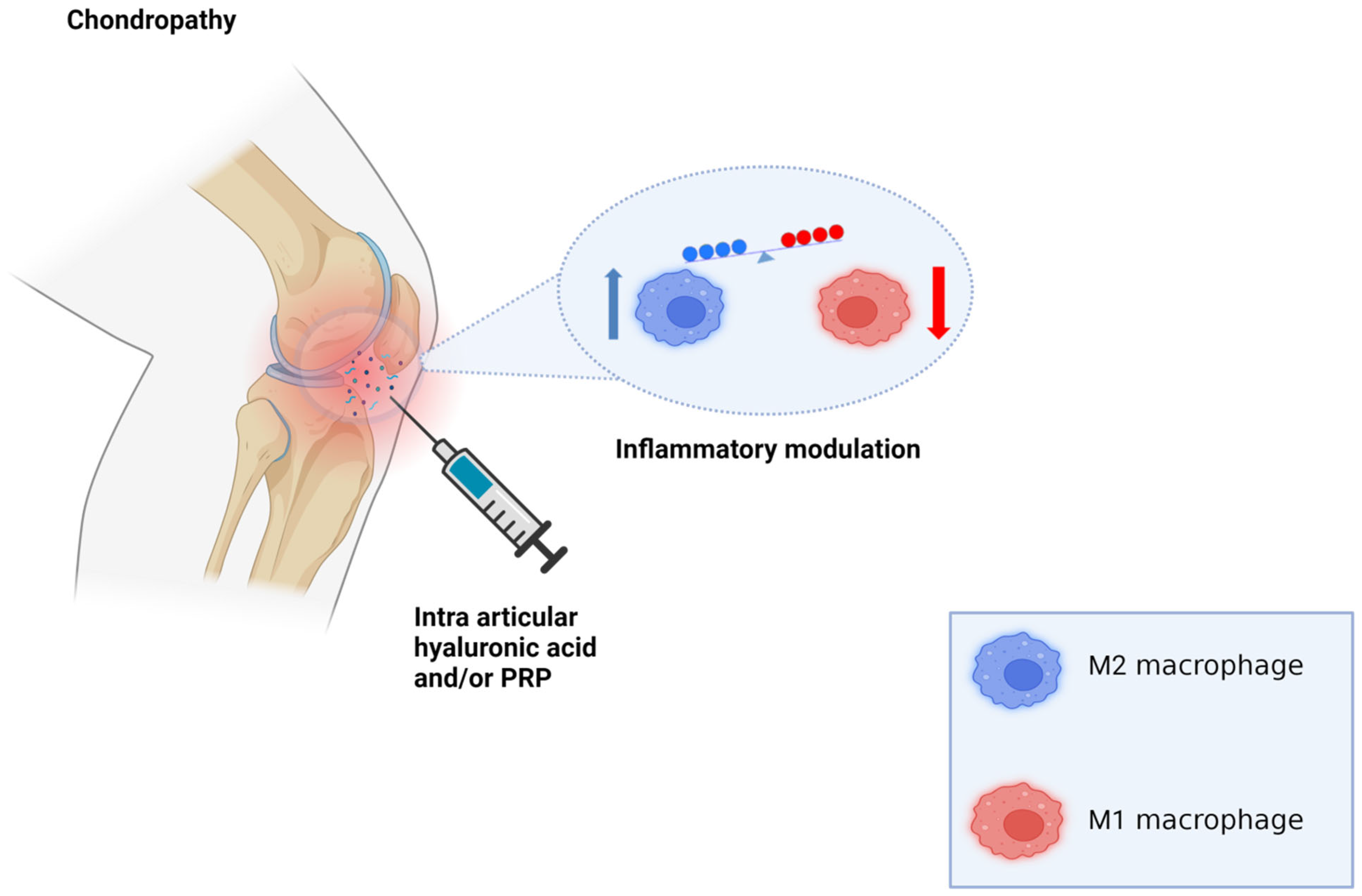
4. Platelet-Rich Plasma
5. Hyaluronic Acid
6. Discussion
7. Author’s Note
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, W.; Li, H.; Hu, K.; Li, L.; Bei, M. Chondromalacia Patellae: Current Options and Emerging Cell Therapies. Stem Cell Res. Ther. 2021, 12, 412. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Sprague, I.S. Outcomes of Prolotherapy in Chondromalacia Patella Patients: Improvements in Pain Level and Function. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Resorlu, H.; Zateri, C.; Nusran, G.; Goksel, F.; Aylanc, N. The Relation between Chondromalacia Patella and Meniscal Tear and the Sulcus Angle/Trochlear Depth Ratio as a Powerful Predictor. J. Back Musculoskelet. Rehabil. 2017, 30, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Ellermann, A.; Rembitzki, I.V.; Scheffler, S.; Herbort, M.; Brüggemann, G.P.; Best, R.; Zantop, T.; Liebau, C. Evaluating the Potential Synergistic Benefit of a Realignment Brace on Patients Receiving Exercise Therapy for Patellofemoral Pain Syndrome: A Randomized Clinical Trial. Arch. Orthop. Trauma Surg. 2016, 136, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R. Knee Joint Osteoarthritis Diagnosis Based on Selected Acoustic Signal Discriminants Using Machine Learning. Appl. Comput. Sci. 2022, 18, 71–85. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN—Part II: Patellofemoral Joint. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Vandenborne, K.; Snyder-Mackler, L. Early Quadriceps Strength Loss After Total Knee Arthroplasty. J. Bone Jt. Surg. Am. 2005, 87, 1047–1053. [Google Scholar] [CrossRef]
- Paravlic, A.H.; Kovač, S.; Pisot, R.; Marusic, U. Neurostructural Correlates of Strength Decrease Following Total Knee Arthroplasty: A Systematic Review of the Literature with Meta-Analysis. Bosn. J. Basic Med. Sci. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Moreno-Garcia, A.; Rodriguez-Merchan, E.C. Orthobiologics: Current Role in Orthopedic Surgery and Traumatology. Arch. Bone Jt. Surg. 2022, 10, 536–542. [Google Scholar] [CrossRef]
- Dhillon, M.S.; Behera, P.; Patel, S.; Shetty, V. Orthobiologics and Platelet Rich Plasma. Indian J. Orthop. 2014, 48, 1–9. [Google Scholar] [CrossRef]
- Purita, J.; Lana, J.F.S.D.; Kolber, M.; Rodrigues, B.L.; Mosaner, T.; Santos, G.S.; Caliari-Oliveira, C.; Huber, S.C. Bone Marrow-Derived Products: A Classification Proposal—Bone Marrow Aspirate, Bone Marrow Aspirate Concentrate or Hybrid? World J. Stem Cells 2020, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Purita, J.; Everts, P.A.; De Mendonça Neto, P.A.T.; de Moraes Ferreira Jorge, D.; Mosaner, T.; Huber, S.C.; Azzini, G.O.M.; da Fonseca, L.F.; Jeyaraman, M.; et al. Platelet-Rich Plasma Power-Mix Gel (Ppm)-An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels 2023, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.R.; Costa Marques, M.R.; Costa, V.C.; Santos, G.S.; Martins, R.A.; da Santos, M.S.; Santana, M.H.A.; Nallakumarasamy, A.; Jeyaraman, M.; Lana, J.V.B.; et al. Intra-Articular Hyaluronic Acid in Osteoarthritis and Tendinopathies: Molecular and Clinical Approaches. Biomedicines 2023, 11, 1061. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E.; et al. Stromal Vascular Fraction for Knee Osteoarthritis—An Update. J. Stem Cells Regen. Med. 2022, 18, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, H.P.; Maheshwer, B.; Wong, S.E.; Chahla, J.; Cole, B.J.; Yanke, A.B. An Update on the Use of Orthobiologics: Use of Biologics for Osteoarthritis. Oper. Tech. Sports Med. 2020, 28, 150759. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef]
- Ghosh, P.; Guidolin, D. Potential Mechanism of Action of Intra-Articular Hyaluronan Therapy in Osteoarthritis: Are the Effects Molecular Weight Dependent? Semin. Arthritis Rheum. 2002, 32, 10–37. [Google Scholar] [CrossRef]
- Örsçelik, A.; Akpancar, S.; Seven, M.M.; Erdem, Y.; Koca, K. The Efficacy of Platelet Rich Plasma and Prolotherapy in Chondromalacia Patella Treatment. Spor Hekim. Derg. 2020, 55, 028–037. [Google Scholar] [CrossRef]
- Chang, K.-V.; Hung, C.-Y.; Aliwarga, F.; Wang, T.-G.; Han, D.-S.; Chen, W.-S. Comparative Effectiveness of Platelet-Rich Plasma Injections for Treating Knee Joint Cartilage Degenerative Pathology: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2014, 95, 562–575. [Google Scholar] [CrossRef]
- Subasi, V. Effectiveness of Platelet-Rich Plasma Treatment in Chondromalacia Patellae. J. Acad. Res. Med. 2017, 7, 36–38. [Google Scholar] [CrossRef]
- Laver, L.; Marom, N.; Dnyanesh, L.; Mei-Dan, O.; Espregueira-Mendes, J.; Gobbi, A. PRP for Degenerative Cartilage Disease: A Systematic Review of Clinical Studies. Cartilage 2017, 8, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Jorquera, C.; de Dicastillo, L.L.; Fiz, N.; Knörr, J.; Beitia, M.; Aizpurua, B.; Azofra, J.; Delgado, D. Real-World Evidence to Assess the Effectiveness of Platelet-Rich Plasma in the Treatment of Knee Degenerative Pathology: A Prospective Observational Study. Ther. Adv. Musculoskelet. 2022, 14, 1759720X221100304. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.; Safi, A.; Komzák, M.; Jajtner, P.; Puskeiler, M.; Hartová, P. Platelet-Rich Plasma in Patients with Tibiofemoral Cartilage Degeneration. Arch. Orthop. Trauma Surg. 2013, 133, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, I.I. Effectiveness of Intra-Articular Injection of Platelet-Rich Plasma in Isolated Patellofemoral Arthritis. Egypt. Orthop. J. 2022, 57, 152. [Google Scholar] [CrossRef]
- Hart, J.M.; Kuenze, C.; Bodkin, S.; Hart, J.; Denny, C.; Diduch, D.R. Prospective, Randomized, Double Blind Evaluation of the Efficacy of a Single Dose Hyaluronic Acid for the Treatment of Patellofemoral Chondromalacia. Orthop. J. Sports Med. 2018, 6, 2325967118S00118. [Google Scholar] [CrossRef]
- Zhang, S.; Jia, M.; Luo, Y.; Wang, X.; Shi, Z.; Xiao, J. Hyaluronate acid for treatment of chondromalacia patellae: A 52-week follow-up study. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, S.R.; da Mota e Albuquerque, R.F.; Helito, C.P.; Camanho, G.L. The Role of Viscosupplementation in Patellar Chondropathy. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X211015005. [Google Scholar] [CrossRef] [PubMed]
- Astur, D.C.; Angelini, F.B.; Santos, M.A.; Arliani, G.G.; Belangero, P.S.; Cohen, M. Use of Exogenous Hyaluronic Acid for the Treatment of Patellar Chondropathy—A Six-Month Randomized Controlled Trial. Rev. Bras. Ortop. 2019, 54, 549–555. [Google Scholar] [CrossRef]
- Cole, B.J.; Karas, V.; Hussey, K.; Merkow, D.B.; Pilz, K.; Fortier, L.A. Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-Articular Biology for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 339–346. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The Regenerative Mechanisms of Platelet-Rich Plasma: A Review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef]
- Krakowski, P.; Karpiński, R.; Maciejewski, R.; Jonak, J.; Jurkiewicz, A. Short-Term Effects of Arthroscopic Microfracturation of Knee Chondral Defects in Osteoarthritis. Appl. Sci. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; dos Santos, R.G.; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis—A Review. J. Clin. Orthop. Trauma 2020, 11, S849–S855. [Google Scholar] [CrossRef] [PubMed]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, Metabolic and Genetic Considerations to Optimise Regenerative Medicine Outcome for Knee Osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.K.; Donnellan, J.; Ryan, D.; Torreggiani, W.C. Correlation between Subcutaneous Knee Fat Thickness and Chondromalacia Patellae on Magnetic Resonance Imaging of the Knee. Can. Assoc. Radiol. J. 2013, 64, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bay-Jensen, A.C.; Lories, R.J.; Abramson, S.; Spector, T.; Pastoureau, P.; Christiansen, C.; Attur, M.; Henriksen, K.; Goldring, S.R.; et al. The Coupling of Bone and Cartilage Turnover in Osteoarthritis: Opportunities for Bone Antiresorptives and Anabolics as Potential Treatments? Ann. Rheum. Dis. 2014, 73, 336–348. [Google Scholar] [CrossRef]
- Kadri, A.; Ea, H.K.; Bazille, C.; Hannouche, D.; Lioté, F.; Cohen-Solal, M.E. Osteoprotegerin Inhibits Cartilage Degradation through an Effect on Trabecular Bone in Murine Experimental Osteoarthritis. Arthritis Rheum. 2008, 58, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Deberg, M.A.; Piccardi, N.; Msika, P.; Reginster, J.Y.L.; Henrotin, Y.E. Subchondral Bone Osteoblasts Induce Phenotypic Changes in Human Osteoarthritic Chondrocytes. Osteoarthr. Cartil. 2005, 13, 988–997. [Google Scholar] [CrossRef]
- Lajeunesse, D. The Role of Bone in the Treatment of Osteoarthritis. Osteoarthr. Cartil. 2004, 12, 34–38. [Google Scholar] [CrossRef]
- Miller, R.E.; Miller, R.J.; Malfait, A.-M. Osteoarthritis joint pain: The cytokine connection. Cytokine 2014, 70, 185–193. [Google Scholar] [CrossRef]
- Pan, J.; Wang, B.; Li, W.; Zhou, X.; Scherr, T.; Yang, Y.; Price, C.; Wang, L. Elevated Cross-Talk between Subchondral Bone and Cartilage in Osteoarthritic Joints. Bone 2012, 51, 212–217. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B. Musculoskeletal Regeneration. Musculoskelet. Regen. 2017, 3, e15. [Google Scholar] [CrossRef][Green Version]
- Marx, R.E. Platelet-Rich Plasma: Evidence to Support Its Use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Rui, S.; Yuan, Y.; Du, C.; Song, P.; Chen, Y.; Wang, H.; Fan, Y.; Armstrong, D.G.; Deng, W.; Li, L. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transpl. 2021, 30, 9636897211017833. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.Q.; Pinto, N.R.; Bielecki, T. Classification of Platelet Concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for Topical and Infiltrative Use in Orthopedic and Sports Medicine: Current Consensus, Clinical Implications and Perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Purita, J.; Paulus, C.; Huber, S.C.; Rodrigues, B.L.; Rodrigues, A.A.; Santana, M.H.; Madureira, J.L.; Malheiros Luzo, Â.C.; Belangero, W.D.; et al. Contributions for Classification of Platelet Rich Plasma—Proposal of a New Classification: MARSPILL. Regen. Med. 2017, 12, 565–574. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-Rich Plasma: A Milieu of Bioactive Factors. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet Rich Plasma: A Short Overview of Certain Bioactive Components. Open Med. 2016, 1, 242–247. [Google Scholar] [CrossRef]
- Parrish, W.R.; Roides, B.; Hwang, J.; Mafilios, M.; Story, B.; Bhattacharyya, S. Normal Platelet Function in Platelet Concentrates Requires Non-Platelet Cells: A Comparative in Vitro Evaluation of Leucocyte-Rich (Type 1a) and Leucocyte-Poor (Type 3b) Platelet Concentrates. BMJ Open Sport. Exerc. Med. 2016, 2, e000071. [Google Scholar] [CrossRef]
- Ganguly, P.; Fiz, N.; Beitia, M.; Owston, H.E.; Delgado, D.; Jones, E.; Sánchez, M. Effect of Combined Intraosseous and Intraarticular Infiltrations of Autologous Platelet-Rich Plasma on Subchondral Bone Marrow Mesenchymal Stromal Cells from Patients with Hip Osteoarthritis. J. Clin. Med. 2022, 11, 3891. [Google Scholar] [CrossRef]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-Rich Plasma: From Basic Science to Clinical Applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Meheux, C.J.; McCulloch, P.C.; Lintner, D.M.; Varner, K.E.; Harris, J.D. Efficacy of Intra-Articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy 2016, 32, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Cheng, C.; Sun, X.; Yan, Y.; Zhang, Q.; Wang, W.; Guo, W. Efficacy and Safety of Intra-Articular Platelet-Rich Plasma in Osteoarthritis Knee: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2021, 2021, 2191926. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-B.; Kim, J.-H.; Ha, C.-W.; Lee, D.-H. Clinical Efficacy of Platelet-Rich Plasma Injection and Its Association With Growth Factors in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized Double-Blind Controlled Clinical Trial As Compared With Hyaluronic Acid. Am. J. Sports Med. 2021, 49, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.-Y.; Zhao, K.; Ruan, J.; Xue, J. Effectiveness of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Clinical Trials. Orthop. J. Sports Med. 2021, 9, 2325967120973284. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Hsu, C.-W.; Lin, G.-C.; Lin, H.-S.; Chou, Y.-J.; Liou, I.-H.; Sun, S.-F. Comparing Efficacy of a Single Intraarticular Injection of Platelet-Rich Plasma (PRP) Combined with Different Hyaluronans for Knee Osteoarthritis: A Randomized-Controlled Clinical Trial. BMC Musculoskelet. Disord. 2022, 23, 954. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative Evaluation of MSCs from Bone Marrow and Adipose Tissue Seeded in PRP-Derived Scaffold for Cartilage Regeneration. Biomaterials 2012, 33, 7008–7018. [Google Scholar] [CrossRef]
- Van Buul, G.M.; Koevoet, W.L.M.; Kops, N.; Bos, P.K.; Verhaar, J.A.N.; Weinans, H.; Bernsen, M.R.; Van Osch, G.J.V.M. Platelet-Rich Plasma Releasate Inhibits Inflammatory Processes in Osteoarthritic Chondrocytes. Am. J. Sports Med. 2011, 39, 2362–2370. [Google Scholar] [CrossRef]
- Giannopoulou, M.; Dai, C.; Tan, X.; Wen, X.; Michalopoulos, G.K.; Liu, Y. Hepatocyte Growth Factor Exerts Its Anti-Inflammatory Action by Disrupting Nuclear Factor-κB Signaling. Am. J. Pathol. 2008, 173, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Marathe, A.; Patel, S.J.; Song, B.; Sliepka, J.M.; Shybut, T.S.; Lee, B.H.; Jayaram, P. Double-Spin Leukocyte-Rich Platelet-Rich Plasma Is Predominantly Lymphocyte Rich With Notable Concentrations of Other White Blood Cell Subtypes. Arthrosc. Sports Med. Rehabil. 2022, 4, e335–e341. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.I.; Whitney, K.; Evans, T.; LaPrade, R.F. Platelet-Rich Plasma and Cartilage Repair. Curr. Rev. Musculoskelet. Med. 2018, 11, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Moussa, M.; Lajeunesse, D.; Hilal, G.; El Atat, O.; Haykal, G.; Serhal, R.; Chalhoub, A.; Khalil, C.; Alaaeddine, N. Platelet Rich Plasma (PRP) Induces Chondroprotection via Increasing Autophagy, Anti-Inflammatory Markers, and Decreasing Apoptosis in Human Osteoarthritic Cartilage. Exp. Cell Res. 2017, 352, 146–156. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy Maintains Stemness by Preventing Senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Khosraviani, S.; Noel, S.; Mohan, D.; Donner, T.; Hamad, A.R.A. Interleukin-10 Paradox: A Potent Immunoregulatory Cytokine That Has Been Difficult to Harness for Immunotherapy. Cytokine 2015, 74, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27. [Google Scholar] [CrossRef]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in Fibrosis: Novel Roles and Mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Cavallo, C.; Filardo, G.; Mariani, E.; Kon, E.; Marcacci, M.; Pereira Ruiz, M.T.; Facchini, A.; Grigolo, B. Comparison of Platelet-Rich Plasma Formulations for Cartilage Healing: An in Vitro Study. J. Bone Jt. Surg.–Ser. A 2014, 96, 423–429. [Google Scholar] [CrossRef]
- Sánchez, M.; Anitua, E.; Azofra, J.; Aguirre, J.J.; Andia, I. Intra-Articular Injection of an Autologous Preparation Rich in Growth Factors for the Treatment of Knee OA: A Retrospective Cohort Study. Clin. Exp. Rheumatol. 2008, 26, 910–913. [Google Scholar] [PubMed]
- Opneja, A.; Kapoor, S.; Stavrou, E.X. Contribution of Platelets, the Coagulation and Fibrinolytic Systems to Cutaneous Wound Healing. Thromb. Res. 2019, 179, 56–63. [Google Scholar] [CrossRef]
- Nurden, A.T.; Nurden, P.; Sanchez, M.; Andia, I.; Anitua, E. Platelets and Wound Healing. Front. Biosci. 2008, 13, 3532–3548. [Google Scholar] [CrossRef]
- Von Hundelshausen, P.; Koenen, R.R.; Sack, M.; Mause, S.F.; Adriaens, W.; Proudfoot, A.E.I.; Hackeng, T.M.; Weber, C. Heterophilic Interactions of Platelet Factor 4 and RANTES Promote Monocyte Arrest on Endothelium. Blood 2005, 105, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.Q.; Kao, K.J. Effect of CXC Chemokine Platelet Factor 4 on Differentiation and Function of Monocyte-Derived Dendritic Cells. Int. Immunol. 2003, 15, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.D.; Petersen, F. The CXC-Chemokine Platelet Factor 4 Promotes Monocyte Survival and Induces Monocyte Differentiation into Macrophages. Blood 2000, 95, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Kzhyshkowska, J.; Köthe, K.; Muller-Molinet, I.; Kannookadan, S.; Utikal, J.; Goerdt, S. Mφ1 and Mφ2 Can Be Re-Polarized by Th2 or Th1 Cytokines, Respectively, and Respond to Exogenous Danger Signals. Immunobiology 2006, 211, 473–486. [Google Scholar] [CrossRef]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef]
- Lana, J.F.; Huber, S.C.; Purita, J.; Tambeli, C.H.; Santos, G.S.; Paulus, C.; Annichino-Bizzacchi, J.M. Leukocyte-Rich PRP versus Leukocyte-Poor PRP—The Role of Monocyte/Macrophage Function in the Healing Cascade. J. Clin. Orthop. Trauma 2019, 10, S7–S12. [Google Scholar] [CrossRef]
- Meszaros, A.J.; Reichner, J.S.; Albina, J.E. Macrophage-Induced Neutrophil Apoptosis. J. Immunol. 2000, 165, 435–441. [Google Scholar] [CrossRef]
- Ramaswamy Reddy, S.H.; Reddy, R.; Babu, N.C.; Ashok, G.N. Stem-Cell Therapy and Platelet-Rich Plasma in Regenerative Medicines: A Review on Pros and Cons of the Technologies. J. Oral Maxillofac. Pathol. 2018, 22, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Latalski, M.; Walczyk, A.; Fatyga, M.; Rutz, E.; Szponder, T.; Bielecki, T.; Danielewicz, A. Allergic Reaction to Platelet-Rich Plasma (PRP). Medicine 2019, 98, e14702. [Google Scholar] [CrossRef] [PubMed]
- Cömert Kiliç, S.; Güngörmüş, M. Is Arthrocentesis plus Platelet-Rich Plasma Superior to Arthrocentesis plus Hyaluronic Acid for the Treatment of Temporomandibular Joint Osteoarthritis: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yan, W.; Leng, X.; Wang, J.; Hu, X.; Cheng, J.; Ao, Y. Efficacy of Platelet-Rich Plasma Versus Placebo in the Treatment of Tendinopathy: A Meta-Analysis of Randomized Controlled Trials. Clin. J. Sport Med. 2023, 33, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lisignoli, G.; Cristino, S.; Piacentini, A.; Cavallo, C.; Caplan, A.I.; Facchini, A. Hyaluronan-Based Polymer Scaffold Modulates the Expression of Inflammatory and Degradative Factors in Mesenchymal Stem Cells: Involvement of Cd44 and Cd54. J. Cell. Physiol. 2006, 207, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Rugolo, C.A.; Calatroni, A. Differential Effect of Molecular Mass Hyaluronan on Lipopolysaccharide-Induced Damage in Chondrocytes. Innate Immun. 2010, 16, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; de la Motte, C.A. Hyaluronan Cross-Linking: A Protective Mechanism in Inflammation? Trends Immunol. 2005, 26, 637–643. [Google Scholar] [CrossRef]
- Tanimoto, K.; Ohno, S.; Fujimoto, K.; Honda, K.; Ijuin, C.; Tanaka, N.; Doi, T.; Nakahara, M.; Tanne, K. Proinflammatory Cytokines Regulate the Gene Expression of Hyaluronic Acid Synthetase in Cultured Rabbit Synovial Membrane Cells. Connect. Tissue Res. 2001, 42, 187–195. [Google Scholar] [CrossRef]
- Maheu, E.; Rannou, F.; Reginster, J.Y. Efficacy and Safety of Hyaluronic Acid in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 2016, 45, S28–S33. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The Effects of the Molecular Weights of Hyaluronic Acid on the Immune Responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef]
- Zhang, B.; Du, Y.; He, Y.; Liu, Y.; Zhang, G.; Yang, C.; Gao, F. INT-HA Induces M2-like Macrophage Differentiation of Human Monocytes via TLR4-miR-935 Pathway. Cancer Immunol. Immunother. 2019, 68, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and Safety of Repeated Courses of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review. Semin Arthritis Rheum. 2018, 48, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mordin, M.; Parrish, W.; Masaquel, C.; Bisson, B.; Copley-Merriman, C. Intra-Articular Hyaluronic Acid for Osteoarthritis of the Knee in the United States: A Systematic Review of Economic Evaluations. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2021, 14, 11795441211047284. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Branco, J.; Luisa Brandi, M.; Guillemin, F.; Hochberg, M.C.; Kanis, J.A.; Kvien, T.K.; Martel-Pelletier, J.; et al. An Algorithm Recommendation for the Management of Knee Osteoarthritis in Europe and Internationally: A Report from a Task Force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Zavan, B.; Vindigni, V.; Schiavinato, A.; Pozzuoli, A.; Iacobellis, C.; Abatangelo, G. In Vitro Response of Osteoarthritic Chondrocytes and Fibroblast-like Synoviocytes to a 500-730 kDa Hyaluronan Amide Derivative. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2073–2081. [Google Scholar] [CrossRef]
- Kruel, A.V.S.; Ribeiro, L.L.; Gusmão, P.D.; Huber, S.C.; Lana, J.F.S.D. Orthobiologics in the Treatment of Hip Disorders. World J. Stem Cells 2021, 13, 304–316. [Google Scholar] [CrossRef]
- Julovi, S.M.; Yasuda, T.; Shimizu, M.; Hiramitsu, T.; Nakamura, T. Inhibition of Interleukin-1β-Stimulated Production of Matrix Metalloproteinases by Hyaluronan via CD44 in Human Articular Cartilage. Arthritis Rheum. 2004, 50, 516–525. [Google Scholar] [CrossRef]
- Kalaci, A.; Yilmaz, R.H.; Aslan, B.; Söğüt, S.; Yanat, A.N.; Uz, E. Effects of Hyaluronan on Nitric Oxide Levels and Superoxide Dismutase Activities in Synovial Fluid in Knee Osteoarthritis. Clin. Rheumatol. 2007, 26, 1306–1311. [Google Scholar] [CrossRef]
- Karna, E.; Miltyk, W.; Surażyński, A.; Pałka, J.A. Protective Effect of Hyaluronic Acid on Interleukin-1-Induced Deregulation of Βeta 1 -Integrin and Insulin-like Growth Factor-I Receptor Signaling and Collagen Biosynthesis in Cultured Human Chondrocytes. Mol. Cell. Biochem. 2008, 308, 57–64. [Google Scholar] [CrossRef]
- Abate, M.; Pelotti, P.; De Amicis, D.; Di Iorio, A.; Galletti, S.; Salini, V. Viscosupplementation with Hyaluronic Acid in Hip Osteoarthritis (A Review). Upsala J. Med. Sci. 2008, 113, 261–278. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and Signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Gomis, A.; Miralles, A.; Schmidt, R.F.; Belmonte, C. Intra-Articular Injections of Hyaluronan Solutions of Different Elastoviscosity Reduce Nociceptive Nerve Activity in a Model of Osteoarthritic Knee Joint of the Guinea Pig. Osteoarthr. Cartil. 2009, 17, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, K.; Cook, J.L.; Kreeger, J.M. Mechanisms of Action and Potential Uses of Hyaluronan in Dogs with Osteoarthritis. J. Am. Vet. Med. Assoc. 2002, 221, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gallego, L.; Prieto, J.G.; Coronel, P.; Gamazo, L.E.; Gimeno, M.; Alvarez, A.I. Apoptosis and Nitric Oxide in an Experimental Model of Osteoarthritis in Rabbit after Hyaluronic Acid Treatment. J. Orthop. Res. 2005, 23, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Bernetti, A.; Agostini, F.; Paoloni, M.; Raele, M.V.; Farì, G.; Megna, M.; Mangone, M. Could Hyaluronic Acid Be Considered as a Senomorphic Agent in Knee Osteoarthritis? A Systematic Review. Biomedicines 2023, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Lee, J.Y.; Shim, K.-S.; Lee, S.; Min, K.; Bae, J.-H.; Kim, H.-J.; Park, K.; Song, H.-R. Attenuation of Inflammation and Cartilage Degradation by Sulfasalazine-Containing Hyaluronic Acid on Osteoarthritis Rat Model. Int. J. Biol. Macromol. 2018, 114, 341–348. [Google Scholar] [CrossRef]
- Furuta, J.; Ariyoshi, W.; Okinaga, T.; Takeuchi, J.; Mitsugi, S.; Tominaga, K.; Nishihara, T. High Molecular Weight Hyaluronic Acid Regulates MMP13 Expression in Chondrocytes via DUSP10/MKP5. J. Orthop. Res. 2017, 35, 331–339. [Google Scholar] [CrossRef]
- Altman, R.D.; Moskowitz, R. Intraarticular Sodium Hyaluronate (Hyalgan) in the Treatment of Patients with Osteoarthritis of the Knee: A Randomized Clinical Trial. Hyalgan Study Group. J. Rheumatol. 1998, 25, 2203–2212. [Google Scholar]
- Kolarz, G.; Kotz, R.; Hochmayer, I. Long-Term Benefits and Repeated Treatment Cycles of Intra-Articular Sodium Hyaluronate (Hyalgan) in Patients with Osteoarthritis of the Knee. Semin. Arthritis Rheum. 2003, 32, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Martini, L.; Nicoli Aldini, N.; Abatangelo, G.; Frizziero, A.; Fini, M. Effects of Intra-Articular Hyaluronic Acid Associated to Chitlac (Arty-Duo®) in a Rat Knee Osteoarthritis Model. J. Orthop. Res. 2019, 37, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, E.; Mattiuzzo, E.; Belluzzi, E.; Elia, R.; Benetti, A.; Venerando, R.; Vindigni, V.; Ruggieri, P.; Brun, P. Anti-Inflammatory Performance of Lactose-Modified Chitosan and Hyaluronic Acid Mixtures in an In Vitro Macrophage-Mediated Inflammation Osteoarthritis Model. Cells 2020, 9, E1328. [Google Scholar] [CrossRef] [PubMed]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The Viability and Anti-Inflammatory Effects of Hyaluronic Acid-Chitlac-Tracimolone Acetonide- β-Cyclodextrin Complex on Human Chondrocytes. Cartilage 2021, 13, 920S–924S. [Google Scholar] [CrossRef] [PubMed]
- Hollander, A.P.; Dickinson, S.C.; Sims, T.J.; Brun, P.; Cortivo, R.; Kon, E.; Marcacci, M.; Zanasi, S.; Borrione, A.; De Luca, C.; et al. Maturation of Tissue Engineered Cartilage Implanted in Injured and Osteoarthritic Human Knees. Tissue Eng. 2006, 12, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Weglein, A.; Sampson, S.E.; Vicente, E.F.; Huber, S.C.; Souza, C.V.; Ambach, M.A.; Vincent, H.; Urban-Paffaro, A.; Onodera, C.M.K.; et al. Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee. J. Stem Cells Regen. Med. 2016, 12, 69–78. [Google Scholar] [PubMed]
- Levett, P.A.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Hyaluronic Acid Enhances the Mechanical Properties of Tissue-Engineered Cartilage Constructs. PLoS ONE 2014, 9, e113216. [Google Scholar] [CrossRef] [PubMed]
- Sebbagh, P.; Cannone, A.; Gremion, G.; Gremeaux, V.; Raffoul, W.; Hirt-Burri, N.; Michetti, M.; Abdel-Sayed, P.; Laurent, A.; Wardé, N.; et al. Current Status of PRP Manufacturing Requirements & European Regulatory Frameworks: Practical Tools for the Appropriate Implementation of PRP Therapies in Musculoskeletal Regenerative Medicine. Bioengineering 2023, 10, 292. [Google Scholar] [CrossRef]
- Kuffler, D.P. Variables Affecting the Potential Efficacy of PRP in Providing Chronic Pain Relief. J. Pain Res. 2018, 12, 109–116. [Google Scholar] [CrossRef]
- Gupta, V.; Parihar, A.S.; Pathak, M.; Sharma, V.K. Comparison of Platelet-Rich Plasma Prepared Using Two Methods: Manual Double Spin Method versus a Commercially Available Automated Device. Indian Dermatol. Online J. 2020, 11, 575–579. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. Cutan. Aesthetic Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, E.M.; Ebel, J.A.; Reynolds, S.B.; Arnold, R.M.; Brown, D.E. The Cost-Effectiveness of Platelet-Rich Plasma Compared With Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Niazi, F.; Dysart, S. Cost-Effectiveness of Treating Early to Moderate Stage Knee Osteoarthritis with Intra-Articular Hyaluronic Acid Compared to Conservative Interventions. Adv. Ther. 2020, 37, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Onkarappa, R.S.; Chauhan, D.K.; Saikia, B.; Karim, A.; Kanojia, R.K. Metabolic Syndrome and Its Effects on Cartilage Degeneration vs Regeneration: A Pilot Study Using Osteoarthritis Biomarkers. Indian J. Orthop. 2020, 54, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E. Mini-Review: The Contribution of Adipokines to Joint Inflammation in Inflammatory Rheumatic Diseases. Front. Endocrinol. 2020, 11, 606560. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Pavanello, C.; Calabresi, L.; Ruscica, M. Nutraceutical Approaches to Metabolic Syndrome. Ann. Med. 2017, 49, 678–697. [Google Scholar] [CrossRef]
- DAYI, T.; OZGOREN, M. Effects of the Mediterranean Diet on the Components of Metabolic Syndrome. J. Prev. Med. Hyg. 2022, 63, E56–E64. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Ramirez-Jimenez, M.; Ortega, J.F.; Mora-Rodriguez, R. Effectiveness of Aerobic Exercise Programs for Health Promotion in Metabolic Syndrome. Med. Sci. Sports Exerc. 2019, 51, 1876–1883. [Google Scholar] [CrossRef]
- Zeng, C.-Y.; Zhang, Z.-R.; Tang, Z.-M.; Hua, F.-Z. Benefits and Mechanisms of Exercise Training for Knee Osteoarthritis. Front. Physiol. 2021, 12, 794062. [Google Scholar] [CrossRef]
- Bliddal, H.; Leeds, A.R.; Christensen, R. Osteoarthritis, Obesity and Weight Loss: Evidence, Hypotheses and Horizons—A Scoping Review. Obes. Rev. 2014, 15, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Kurt, M.; Öner, A.Y.; Uçar, M.; Aladağ Kurt, S. The Relationship between Patellofemoral Arthritis and Fat Tissue Volume, Body Mass Index and Popliteal Artery Intima-Media Thickness through 3T Knee MRI. Turk. J. Med. Sci. 2019, 49, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Kızılgöz, V.; Kantarci, M.; Aydın, S. Association between the Subcutaneous Fat Thickness of the Knee and Chondromalacia Patella: A Magnetic Resonance Imaging-Based Study. J. Int. Med. Res. 2023, 51, 3000605231183581. [Google Scholar] [CrossRef] [PubMed]

| Grade | Radiological Observations |
|---|---|
| Grade 0 (normal) | No radiological findings |
| Grade I | Softening and swelling, edema |
| Grade II | Fragmentation and fissuring in an area of about 1.27 cm2 (half an inch) in diameter |
| Grade III | Acute fragmentation and fissuring in an area greater than 1.27 cm2 (half an inch) in diameter |
| Grade IV | Severe cartilage denudation and erosion down to the subchondral bone compartment |
| Growth Factor | Abbreviation | Biological Function |
|---|---|---|
| Insulin-like growth factor | IGF | Promotes cell growth and differentiation, stimulates collagen synthesis and cell recruitment from bone, endothelium, epithelium and other tissues. |
| Vascular endothelial growth factor | VEGF | Stimulates angiogenesis, chemotaxis of macrophages and neutrophils, migration and mitosis of endothelial cells, and increases permeability of blood vessels. |
| Hepatocyte growth factor | HGF | HGF is secreted by mesenchymal cells and stimulates mitogenesis, cell motility, and matrix invasion. |
| Fibroblast growth factor | FGF | Regulates cellular proliferation, survival, migration, and differentiation. |
| Epidermal growth factor | EGF | Sustains proliferation and differentiation of epithelial cells, promotes secretion of cytokines by mesenchymal and epithelial cells. |
| Transforming growth factor-β | TGF-β | Increases synthesis of collagen type 1, stimulates angionesis and immune cell chemotaxis, and inhibits osteoclast formation and bone resorption. |
| Platelet-derived growth factor | PDGF | Increases expression of collagen, proliferation of bone cells, fibroblast chemotaxis and proliferative activity, and induces macrophage activation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, F.R.; Santos, M.d.S.; Martins, R.A.; Costa, C.B.; Hamdan, P.C.; Da Silva, M.B.; Azzini, G.O.M.; Pires, L.; Menegassi, Z.; Santos, G.S.; et al. The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy. Biomedicines 2024, 12, 6. https://doi.org/10.3390/biomedicines12010006
Costa FR, Santos MdS, Martins RA, Costa CB, Hamdan PC, Da Silva MB, Azzini GOM, Pires L, Menegassi Z, Santos GS, et al. The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy. Biomedicines. 2024; 12(1):6. https://doi.org/10.3390/biomedicines12010006
Chicago/Turabian StyleCosta, Fábio Ramos, Márcia da Silva Santos, Rubens Andrade Martins, Cláudia Bruno Costa, Paulo César Hamdan, Marcos Britto Da Silva, Gabriel Ohana Marques Azzini, Luyddy Pires, Zartur Menegassi, Gabriel Silva Santos, and et al. 2024. "The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy" Biomedicines 12, no. 1: 6. https://doi.org/10.3390/biomedicines12010006
APA StyleCosta, F. R., Santos, M. d. S., Martins, R. A., Costa, C. B., Hamdan, P. C., Da Silva, M. B., Azzini, G. O. M., Pires, L., Menegassi, Z., Santos, G. S., & Lana, J. F. (2024). The Synergistic Effects of Hyaluronic Acid and Platelet-Rich Plasma for Patellar Chondropathy. Biomedicines, 12(1), 6. https://doi.org/10.3390/biomedicines12010006







