Unexpected and Synergistical Effects of All-Trans Retinoic Acid and TGF-β2 on Biological Aspects of 2D and 3D Cultured ARPE19 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. 2D and 3D Cultures of Human Retinal Pigment Epithelium Cells, ARPE19
2.2. Barrier Function of 2D ARPE19 Monolayer by TEER
2.3. Measurement of Real-Time Cellular Metabolic Functions
2.4. Measurement of Reactive Oxygen Species (ROS) Levels
2.5. Measurements of Physical Property, Size, and Solidity of 3D ARPE19 Spheroids
2.6. Other Analytical Methods
3. Results
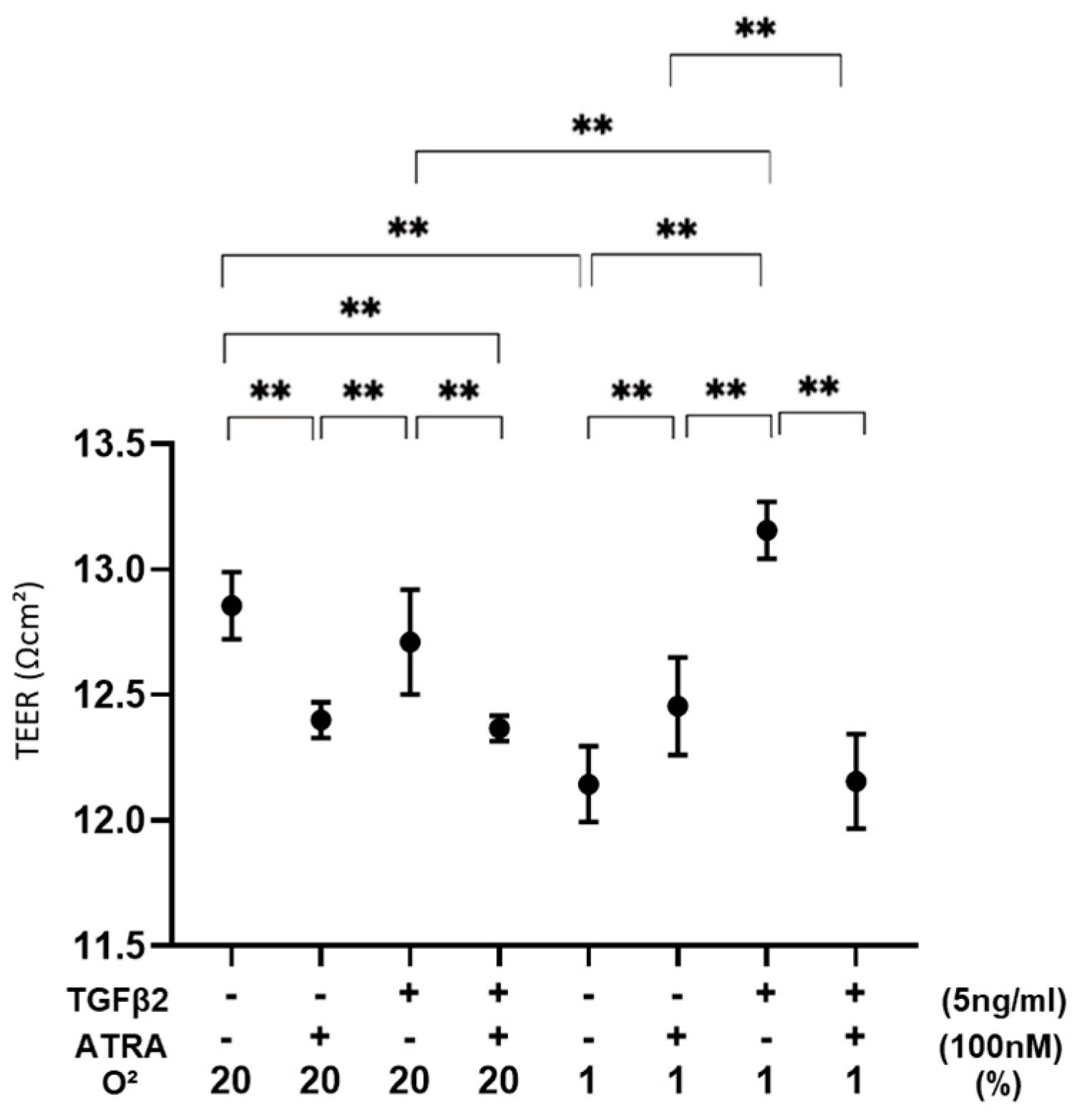
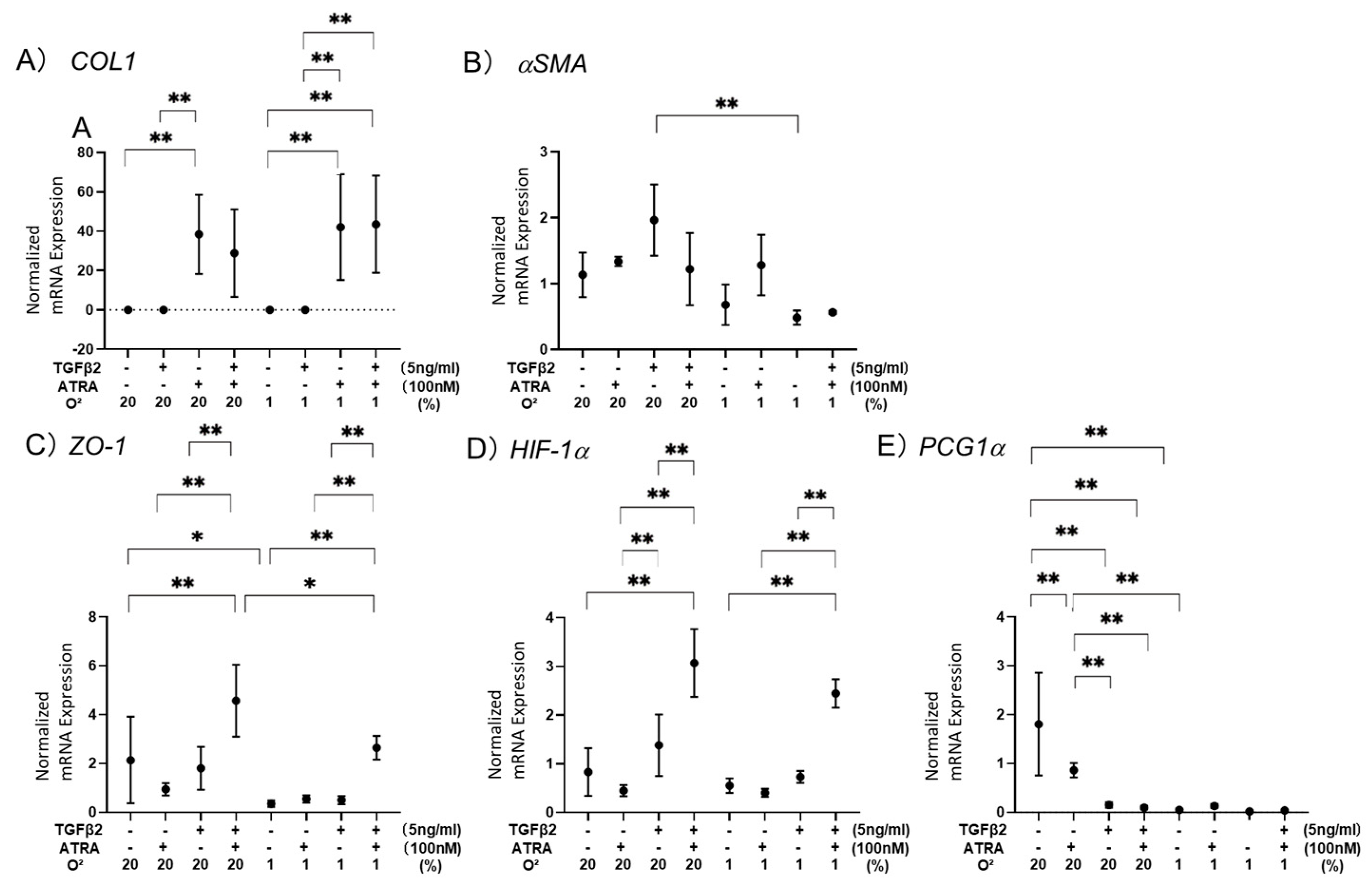
3.1. Effects of ATRA on the Barrier Function of TGF-β2-Teated or UNTREATED ARPE19 Cell Monolayer under Different Oxygen Conditions
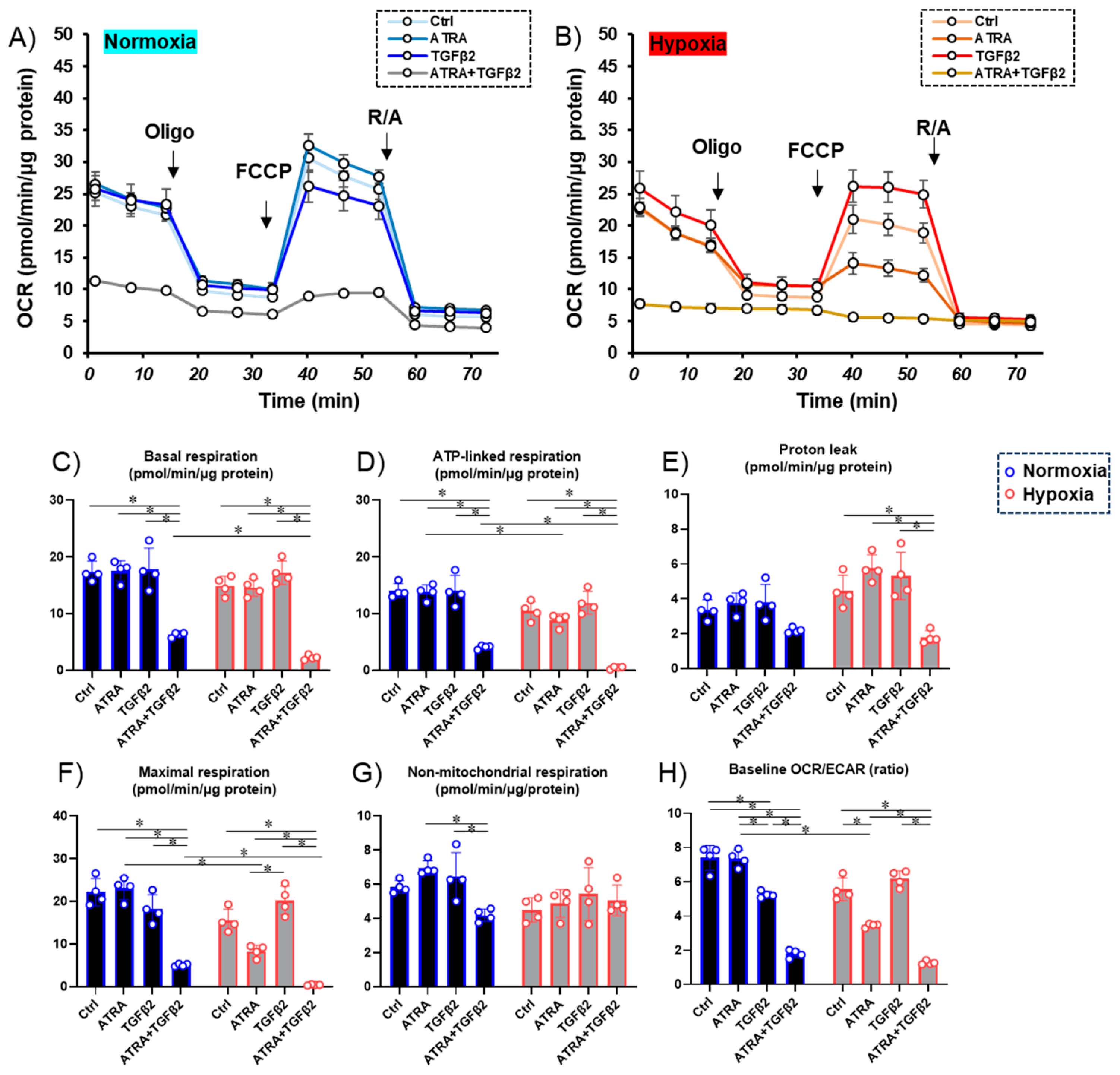
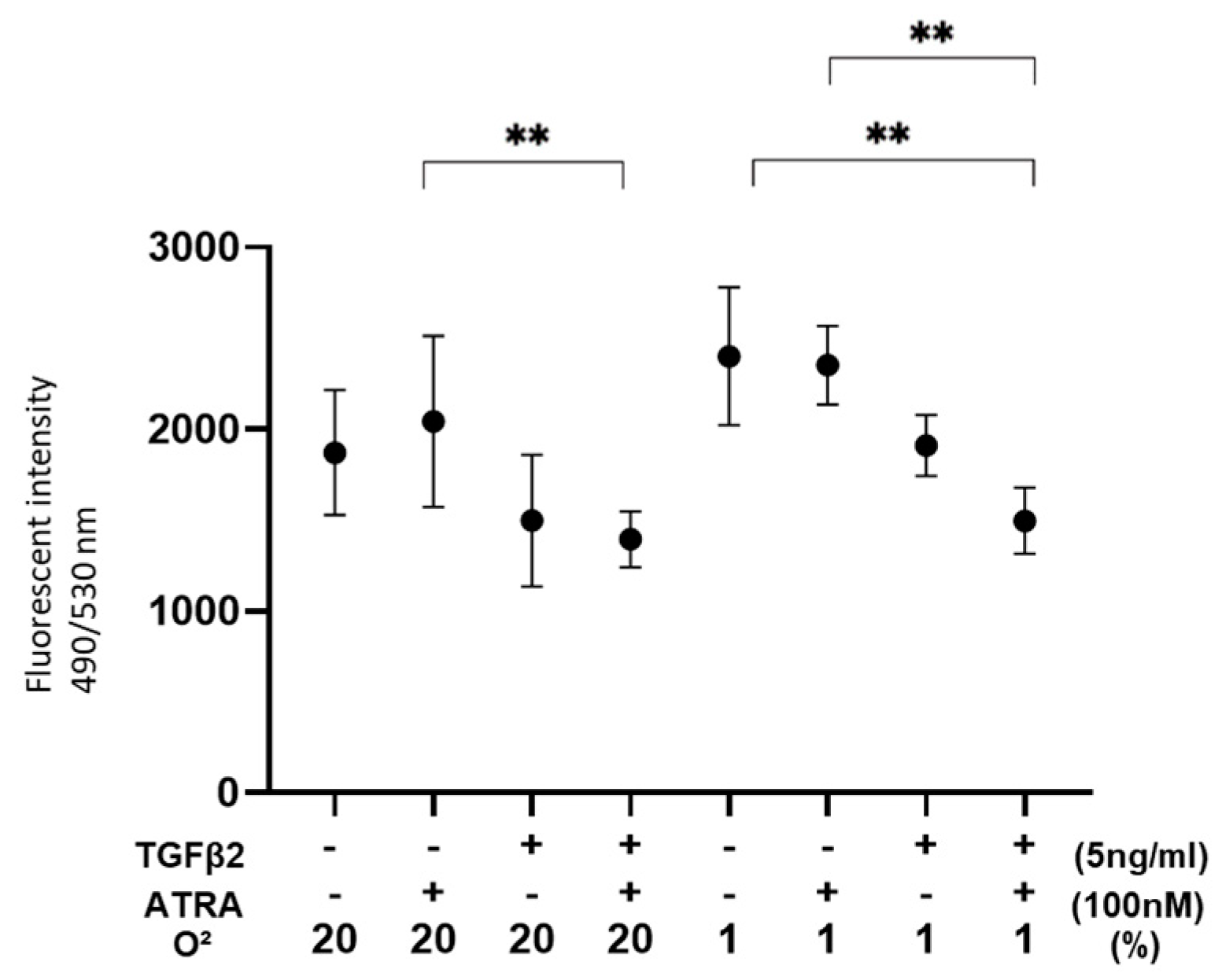
3.2. Effects of ATRA on Cellular Metabolic Functions and ROS Levels of TGF-β2-Teated or Untreated 2D Cultured ARPE-19 Cells under Different Oxygen Conditions
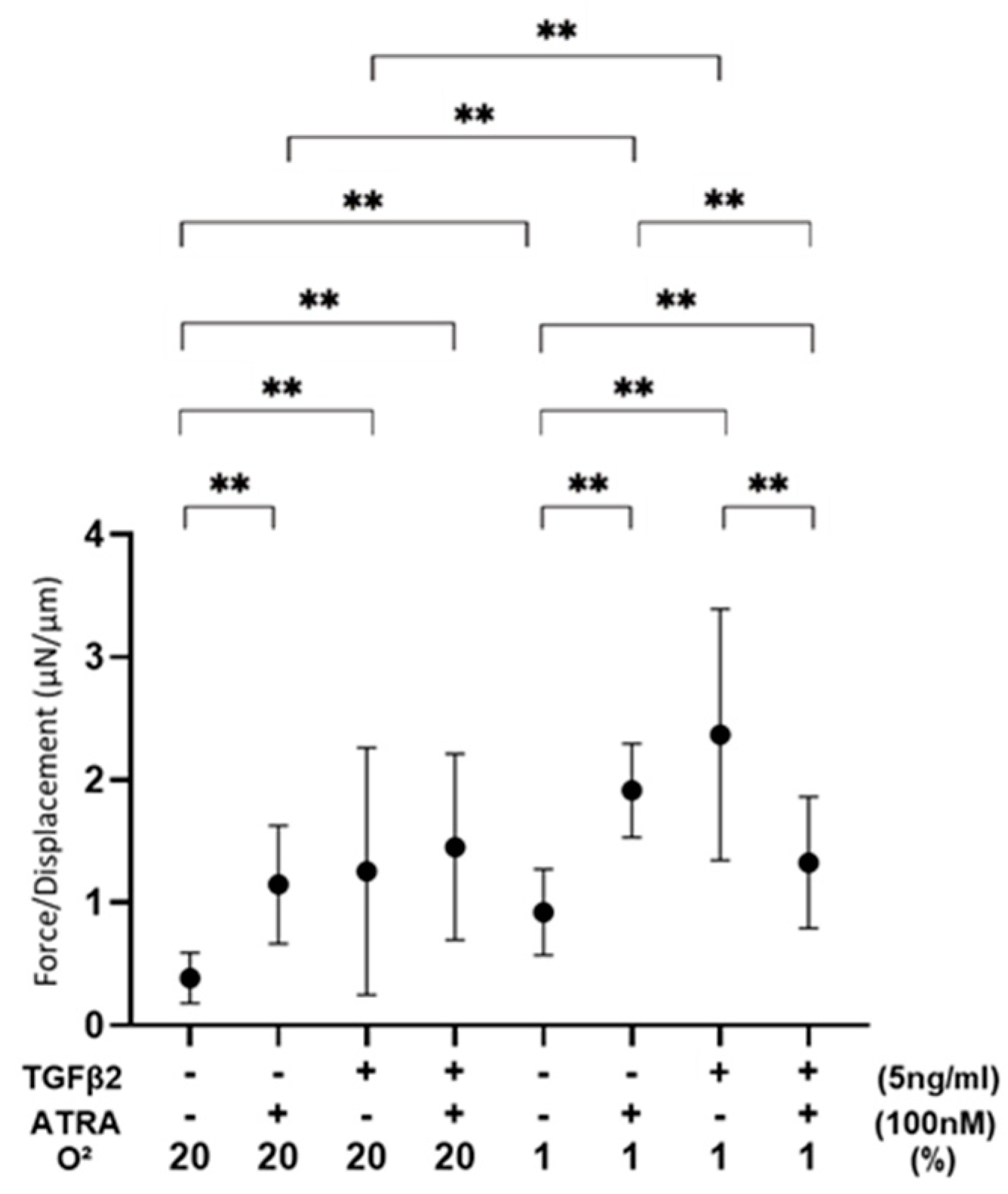
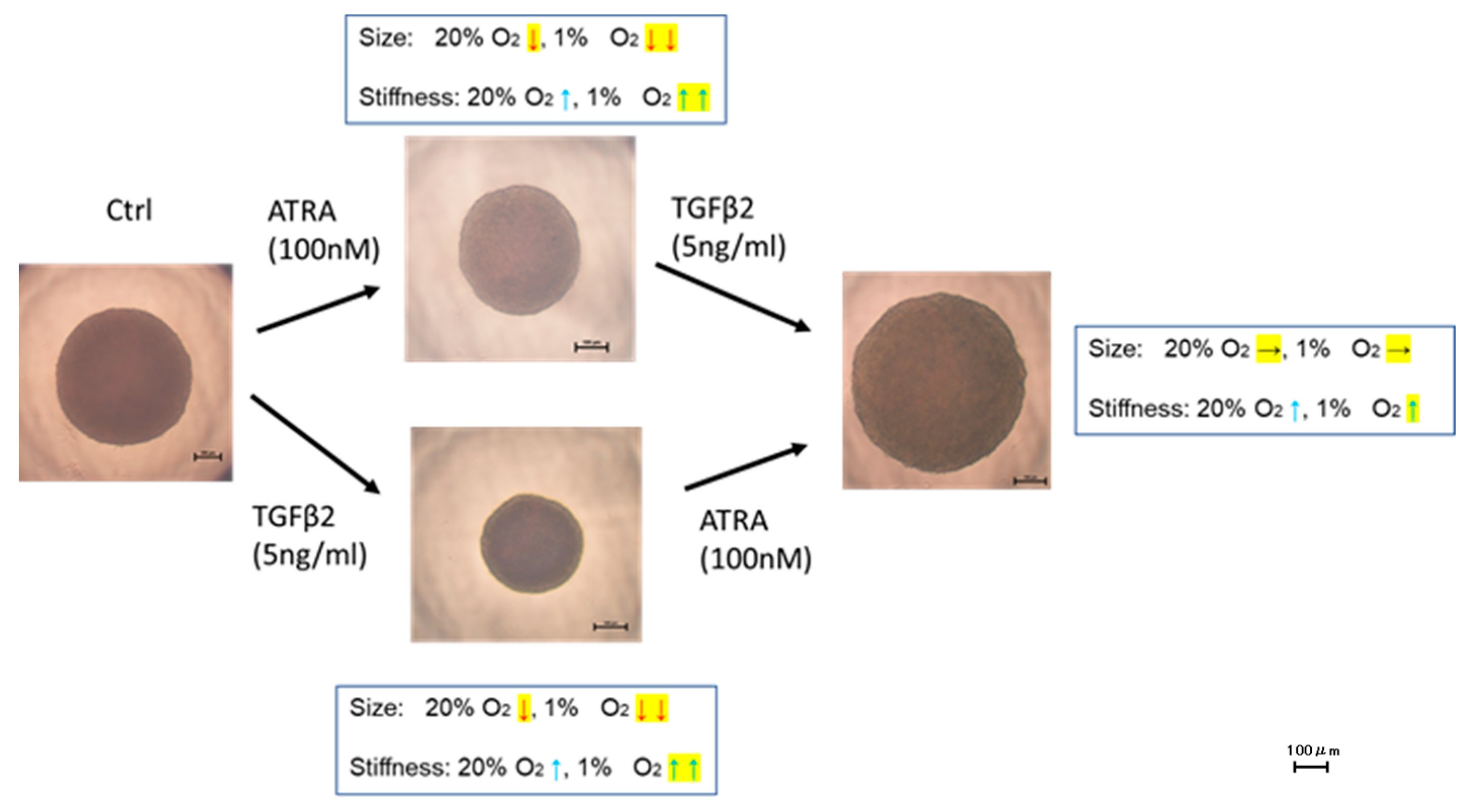
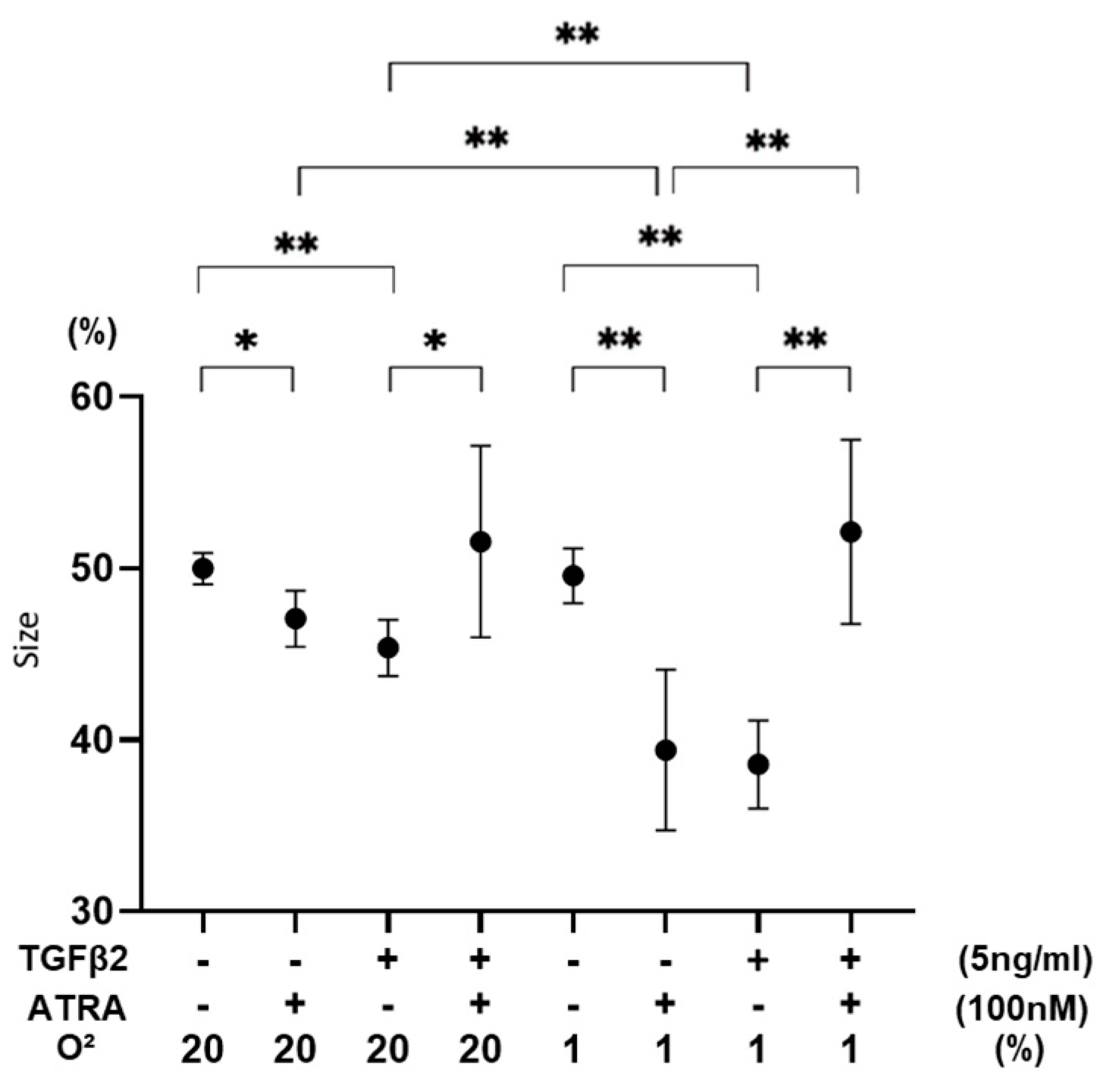
3.3. Effects of ATRA on Physical Properties of TGF-β2-Teated or Untreated 3D ARPE19 Spheroids under Different Oxygen Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adamis, A.P.; Shima, D.T.; Yeo, K.T.; Yeo, T.K.; Brown, L.F.; Berse, B.; Damore, P.A.; Folkman, J. Synthesis and Secretion of Vascular Permeability Factor/Vascular Endothelial Growth Factor by Human Retinal Pigment Epithelial Cells. Biochem. Biophys. Res. Commun. 1993, 193, 631–638. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.; Saint-Geniez, M. EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration. Int. J. Mol. Sci. 2020, 21, 4271. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.B., Jr.; Roberts, A.B.; Sporn, M.B.; Danielpour, D.; Dart, L.L.; Michels, R.G.; de Bustros, S.; Enger, C.; Kato, H.; Lansing, M. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Investig. 1989, 83, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.; Heimann, K.; Bartz-Schmidt, K.-U.; Fontana, A.; Schraermeyer, U.; Thumann, G.; Weller, M. Apoptosis in Proliferative Vitreoretinal Disorders: Possible Involvement of TGF-β-induced RPE cell Apoptosis. Exp. Eye Res. 1997, 65, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Kon, C.H.; Occleston, N.L.; Aylward, G.W.; Khaw, P.T. Expression of vitreous cytokines in proliferative vitreoretinopathy: A prospective study. Investig. Ophthalmol. Vis. Sci. 1999, 40, 705–712. [Google Scholar]
- González-Avila, G.; Lozano, D.; Manjarrez, M.-E.; Ruiz, V.M.; Terán, L.; Vadillo-Ortega, F.; Selman, M. Influence on Collagen Metabolism of Vitreous from Eyes with Proliferative Vitreoretinopathy. Ophthalmology 1995, 102, 1400–1405. [Google Scholar] [CrossRef]
- Arjamaa, O.; Nikinmaa, M. Oxygen-dependent diseases in the retina: Role of hypoxia-inducible factors. Exp. Eye Res. 2006, 83, 473–483. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Bressler, S.B. Introduction: Understanding the Role of Angiogenesis and Antiangiogenic Agents in Age-Related Macular Degeneration. Ophthalmology 2009, 116, S1–S7. [Google Scholar] [CrossRef]
- Axel, D.I.; Frigge, A.; Dittmann, J.; Runge, H.; Spyridopoulos, I.; Riessen, R.; Viebahn, R.; Karsch, K.R. All-trans retinoic acid regulates proliferation, migration, differentiation, and extracellular matrix turnover of human arterial smooth muscle cells. Cardiovasc. Res. 2001, 49, 851–862. [Google Scholar] [CrossRef]
- Xu, Q.; Kopp, J.B. Retinoid and TGF-β Families: Crosstalk in Development, Neoplasia, Immunity, and Tissue Repair. Semin. Nephrol. 2012, 32, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Na, S.-Y.; Kang, B.Y.; Chung, S.W.; Han, S.-J.; Ma, X.; Trinchieri, G.; Im, S.-Y.; Lee, J.W.; Kim, T.S. Retinoids Inhibit Interleukin-12 Production in Macrophages through Physical Associations of Retinoid X Receptor and NFκB. J. Biol. Chem. 1999, 274, 7674–7680. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, Y.; Hu, K.; Dai, C.; Liu, Y. Hepatocyte Growth Factor Receptor Signaling Mediates the Anti-Fibrotic Action of 9-cis-Retinoic Acid in Glomerular Mesangial Cells. Am. J. Pathol. 2005, 167, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-L.; Chang, W.-T.; Hung, K.-C.; Li, E.I.; Chuang, C.-C. Inhibition of transforming growth factor-β-induced liver fibrosis by a retinoic acid derivative via the suppression of Col 1A2 promoter activity. Biochem. Biophys. Res. Commun. 2008, 373, 219–223. [Google Scholar] [CrossRef]
- Liu, Y.; Kimura, K.; Orita, T.; Teranishi, S.; Suzuki, K.; Sonoda, K.-H. Inhibition by All-Trans-Retinoic Acid of Transforming Growth Factor-β–Induced Collagen Gel Contraction Mediated by Human Tenon Fibroblasts. Investig. Opthalmol. Vis. Sci. 2014, 55, 4199–4205. [Google Scholar] [CrossRef]
- Liang, L.; Wang, X.; Zheng, Y.; Liu, Y. All-trans-retinoic acid modulates TGF-β-induced apoptosis, proliferation, migration and extracellular matrix synthesis of conjunctival fibroblasts by inhibiting PI3K/AKT signaling. Mol. Med. Rep. 2019, 20, 2929–2935. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, Z.; Tan, J.; Liu, S.; Hu, S.; Tao, H.; Tang, R. All-trans retinoic acid stimulates the secretion of TGF-β2 via the phospholipase C but not the adenylyl cyclase signaling pathway in retinal pigment epithelium cells. BMC Ophthalmol. 2019, 19, 23. [Google Scholar] [CrossRef]
- Mao, Y.-M.; Tan, Q.-Q.; Zhou, G.-M.; Xiang, X.-L.; Lin, J.; Liao, X. All-trans retinoic acid regulates the expression of MMP-2 and TGF-β2 via RDH5 in retinal pigment epithelium cells. Int. J. Ophthalmol. 2023, 16, 849–854. [Google Scholar] [CrossRef]
- Al-Qassab, Y.; Grassilli, S.; Brugnoli, F.; Vezzali, F.; Capitani, S.; Bertagnolo, V. Protective role of all-trans retinoic acid (ATRA) against hypoxia-induced malignant potential of non-invasive breast tumor derived cells. BMC Cancer 2018, 18, 1194. [Google Scholar] [CrossRef]
- Hikage, F.; Atkins, S.; Kahana, A.; Smith, T.J.; Chun, T.-H. HIF2A–LOX Pathway Promotes Fibrotic Tissue Remodeling in Thyroid-Associated Orbitopathy. Endocrinology 2019, 160, 20–35. [Google Scholar] [CrossRef]
- Ida, Y.; Hikage, F.; Itoh, K.; Ida, H.; Ohguro, H. Prostaglandin F2α agonist-induced suppression of 3T3-L1 cell adipogenesis affects spatial formation of extra-cellular matrix. Sci. Rep. 2020, 10, 7958. [Google Scholar] [CrossRef] [PubMed]
- Ota, C.; Ida, Y.; Ohguro, H.; Hikage, F. ROCK inhibitors beneficially alter the spatial configuration of TGFβ2-treated 3D organoids from a human trabecular meshwork (HTM). Sci. Rep. 2020, 10, 20292. [Google Scholar] [CrossRef] [PubMed]
- Oouchi, Y.; Watanabe, M.; Ida, Y.; Ohguro, H.; Hikage, F. Rosiglitasone and ROCK Inhibitors Modulate Fibrogenetic Changes in TGF-β2 Treated Human Conjunctival Fibroblasts (HconF) in Different Manners. Int. J. Mol. Sci. 2021, 22, 7335. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sato, T.; Watanabe, M.; Higashide, M.; Tsugeno, Y.; Umetsu, A.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Hypoxia Differently Affects TGF-β2-Induced Epithelial Mesenchymal Transitions in the 2D and 3D Culture of the Human Retinal Pigment Epithelium Cells. Int. J. Mol. Sci. 2022, 23, 5473. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chang, Y.-S.; Hsieh, M.-C.; Wu, H.-J.; Wu, M.-H.; Lin, C.-W.; Wu, W.-C.; Kao, Y.-H. All-trans retinoic acid suppresses the adhering ability of ARPE-19 cells via mitogen-activated protein kinase and focal adhesion kinase. J. Pharmacol. Sci. 2016, 132, 262–270. [Google Scholar] [CrossRef]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a novel ROCK inhibitor, on trabecular meshwork and Schlemm’s canal endothelial cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef]
- Itoh, K.; Hikage, F.; Ida, Y.; Ohguro, H. Prostaglandin F2α Agonists Negatively Modulate the Size of 3D Organoids from Primary Human Orbital Fibroblasts. Investig. Opthalmol. Vis. Sci. 2020, 61, 13. [Google Scholar] [CrossRef]
- Nagamoto, T.; Eguchi, G.; Beebe, D.C. Alpha-smooth muscle actin expression in cultured lens epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1122–1129. [Google Scholar]
- Cvekl, A.; Wang, W.-L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009, 89, 280–291. [Google Scholar] [CrossRef]
- Kampmann, E.; Mey, J. Retinoic acid enhances Erk phosphorylation in the chick retina. Neurosci. Lett. 2007, 426, 18–22. [Google Scholar] [CrossRef]
- Warkany, J.; Schraffenberger, E. congenital malformations induced in rats by maternal vitamin a deficiency: Defects of the eye. Arch. Ophthalmol. 1946, 35, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Roth, C.B.; Warkany, J. An analysis of the syndrome of malformations induced by maternal vitamin a deficiency. Effects of restoration of vitamin a at various times during gestation. Am. J. Anat. 1953, 92, 189–217. [Google Scholar] [CrossRef] [PubMed]
- Dickman, E.D.; Thaller, C.; Smith, S.M. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development 1997, 124, 3111–3121. [Google Scholar] [CrossRef] [PubMed]
- Dupé, V.; Matt, N.; Garnier, J.-M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041. [Google Scholar] [CrossRef]
- Ghyselinck, N.B.; Dupé, V.; Dierich, A.; Messaddeq, N.; Garnier, J.M.; Rochette-Egly, C.; Chambon, P.; Mark, M. Role of the retinoic acid receptor beta (RARbeta) during mouse development. Int. J. Dev. Biol. 1997, 41, 425–447. [Google Scholar]
- Mori, M.; Ghyselinck, N.B.; Chambon, P.; Mark, M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1312–1318. [Google Scholar]
- Mizee, M.R.; Wooldrik, D.; Lakeman, K.A.; van het Hof, B.; Drexhage, J.A.; Geerts, D.; Bugiani, M.; Aronica, E.; Mebius, R.E.; Prat, A.; et al. Retinoic Acid Induces Blood–Brain Barrier Development. J. Neurosci. 2013, 33, 1660–1671. [Google Scholar] [CrossRef]
- Pollock, L.M.; Xie, J.; Bell, B.A.; Anand-Apte, B. Retinoic acid signaling is essential for maintenance of the blood-retinal barrier. FASEB J. 2018, 32, 5674–5684. [Google Scholar] [CrossRef]
- Nishikiori, N.; Osanai, M.; Chiba, H.; Kojima, T.; Mitamura, Y.; Ohguro, H.; Sawada, N. Glial Cell–Derived Cytokines Attenuate the Breakdown of Vascular Integrity in Diabetic Retinopathy. Diabetes 2007, 56, 1333–1340. [Google Scholar] [CrossRef]
- Wu, W.-C.; Hu, D.-N.; Mehta, S.; Chang, Y.-C. Effects of Retinoic Acid on Retinal Pigment Epithelium from Excised Membranes from Proliferative Vitreoretinopathy. J. Ocul. Pharmacol. Ther. 2005, 21, 44–54. [Google Scholar] [CrossRef]
- Dong, X.; Chen, N.; Xie, L.; Wang, S. Prevention of experimental proliferative vitreoretinopathy with a biodegradable intravitreal drug delivery system of all-trans retinoic acid. Retina 2006, 26, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Araiz, J.J.; Refojo, M.F.; Arroyo, M.H.; Leong, F.L.; Albert, D.M.; Tolentino, F.I. Antiproliferative effect of retinoic acid in in-travitreous silicone oil in an animal model of proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 1993, 34, 522–530. [Google Scholar]
- Du, Y.-H.; Hirooka, K.; Miyamoto, O.; Bao, Y.-Q.; Zhang, B.; An, J.-B.; Ma, J.-X. Retinoic acid suppresses the adhesion and migration of human retinal pigment epithelial cells. Exp. Eye Res. 2013, 109, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Kao, Y.-H.; Hu, D.-N.; Tsai, L.-Y.; Wu, W.-C. All-trans retinoic acid remodels extracellular matrix and suppresses laminin-enhanced contractility of cultured human retinal pigment epithelial cells. Exp. Eye Res. 2009, 88, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-Trans Retinoic Acid Modulates DNA Damage Response and the Expression of the VEGF-A and MKI67 Genes in ARPE-19 Cells Subjected to Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Establishment of appropriate glaucoma models using dexamethasone or TGFβ2 treated three-dimension (3D) cultured human trabecular meshwork (HTM) cells. Sci. Rep. 2021, 11, 19369. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Ohguro, H.; Ota, C.; Hikage, F. Diverse effects of pan-ROCK and ROCK2 inhibitors on 2 D and 3D cultured human trabecular meshwork (HTM) cells treated with TGFβ2. Sci. Rep. 2021, 11, 15286. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Ohguro, H.; Hikage, F. Screening of the Drug-Induced Effects of Prostaglandin EP2 and FP Agonists on 3D Cultures of Dexamethasone-Treated Human Trabecular Meshwork Cells. Biomedicines 2021, 9, 930. [Google Scholar] [CrossRef]
- Watanabe, M.; Ida, Y.; Furuhashi, M.; Tsugeno, Y.; Hikage, F.; Ohguro, H. Pan-ROCK and ROCK2 Inhibitors Affect Dexamethasone-Treated 2D- and 3D-Cultured Human Trabecular Meshwork (HTM) Cells in Opposite Manners. Molecules 2021, 26, 6382. [Google Scholar] [CrossRef]
- Endo, K.; Sato, T.; Umetsu, A.; Watanabe, M.; Hikage, F.; Ida, Y.; Ohguro, H.; Furuhashi, M. 3D culture induction of adipogenic differentiation in 3T3-L1 preadipocytes exhibits adipocyte-specific molecular expression patterns and metabolic functions. Heliyon 2023, 9, e20713. [Google Scholar] [CrossRef]
- Watanabe, M.; Sato, T.; Tsugeno, Y.; Umetsu, A.; Suzuki, S.; Furuhashi, M.; Ida, Y.; Hikage, F.; Ohguro, H. Human Trabecular Meshwork (HTM) Cells Treated with TGF-β2 or Dexamethasone Respond to Compression Stress in Different Manners. Biomedicines 2022, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yano, T.; Sato, T.; Umetsu, A.; Higashide, M.; Furuhashi, M.; Ohguro, H. mTOR Inhibitors Modulate the Physical Properties of 3D Spheroids Derived from H9c2 Cells. Int. J. Mol. Sci. 2023, 24, 11459. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Watanabe, M.; Takada, K.; Sato, T.; Hikage, F.; Umetsu, A.; Muramatsu, J.; Furuhashi, M.; Ohguro, H. Modulation of Epithelial–Mesenchymal Transition Is a Possible Underlying Mechanism for Inducing Chemoresistance in MIA PaCa-2 Cells against Gemcitabine and Paclitaxel. Biomedicines 2024, 12, 1011. [Google Scholar] [CrossRef]
- Ohguro, H.; Ida, Y.; Hikage, F.; Umetsu, A.; Ichioka, H.; Watanabe, M.; Furuhashi, M. STAT3 Is the Master Regulator for the Forming of 3D Spheroids of 3T3-L1 Preadipocytes. Cells 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Saitoh, M.; Miyazawa, K. Smad3–STAT3 crosstalk in pathophysiological contexts. Acta Biochim. Biophys. Sin. 2018, 50, 82–90. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Alatshan, A.; Kovács, G.E.; Aladdin, A.; Czimmerer, Z.; Tar, K.; Benkő, S. All-Trans Retinoic Acid Enhances both the Signaling for Priming and the Glycolysis for Activation of NLRP3 Inflammasome in Human Macrophage. Cells 2020, 9, 1591. [Google Scholar] [CrossRef]
- Yoyen-Ermis, D.; Tunali, G.; Tavukcuoglu, E.; Horzum, U.; Ozkazanc, D.; Sutlu, T.; Buyukasik, Y.; Esendagli, G. Myeloid maturation potentiates STAT3-mediated atypical IFN-γ signaling and upregulation of PD-1 ligands in AML and MDS. Sci. Rep. 2019, 9, 11697. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A Nuclear Factor Induced by Hypoxia via De Novo Protein Synthesis Binds to the Human Erythropoietin Gene Enhancer at a Site Required for Transcriptional Activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Lin, M.; Hu, Y.; Chen, Y.; Zhou, K.K.; Jin, J.; Zhu, M.; Le, Y.-Z.; Ge, J.; Ma, J.-X. Impacts of Hypoxia-Inducible Factor-1 Knockout in the Retinal Pigment Epithelium on Choroidal Neovascularization. Investig. Opthalmol. Vis. Sci. 2012, 53, 6197–6206. [Google Scholar] [CrossRef]
- Lazzara, F.; Trotta, M.C.; Platania, C.B.M.; D’amico, M.; Petrillo, F.; Galdiero, M.; Gesualdo, C.; Rossi, S.; Drago, F.; Bucolo, C. Stabilization of HIF-1α in Human Retinal Endothelial Cells Modulates Expression of miRNAs and Proangiogenic Growth Factors. Front. Pharmacol. 2020, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, V.; Platania, C.B.M.; Lazzara, F.; Conti, F.; Pizzo, C.; Reibaldi, M.; Russo, A.; Fallico, M.; Ortisi, E.; Pignatelli, F.; et al. TGF-β Serum Levels in Diabetic Retinopathy Patients and the Role of Anti-VEGF Therapy. Int. J. Mol. Sci. 2020, 21, 9558. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Nahomi, R.B.; Nagaraj, R.H. The role of HIF-1α in the TGF-β2-mediated epithelial-to-mesenchymal transition of human lens epithelial cells. J. Cell. Biochem. 2018, 119, 6814–6827. [Google Scholar] [CrossRef]
- Lai, K.; Luo, C.; Zhang, X.; Ye, P.; Zhang, Y.; He, J.; Yao, K. Regulation of angiogenin expression and epithelial-mesenchymal transition by HIF-1α signaling in hypoxic retinal pigment epithelial cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 1594–1607. [Google Scholar] [CrossRef]
- Tormos, K.V.; Chandel, N.S. Inter-connection between mitochondria and HIFs. J. Cell. Mol. Med. 2010, 14, 795–804. [Google Scholar] [CrossRef]
- Shu, D.Y.; Butcher, E.R.; Saint-Geniez, M. Suppression of PGC-1α Drives Metabolic Dysfunction in TGFβ2-Induced EMT of Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4701. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higashide, M.; Watanabe, M.; Sato, T.; Ogawa, T.; Umetsu, A.; Suzuki, S.; Furuhashi, M.; Ohguro, H.; Nishikiori, N. Unexpected and Synergistical Effects of All-Trans Retinoic Acid and TGF-β2 on Biological Aspects of 2D and 3D Cultured ARPE19 Cells. Biomedicines 2024, 12, 2228. https://doi.org/10.3390/biomedicines12102228
Higashide M, Watanabe M, Sato T, Ogawa T, Umetsu A, Suzuki S, Furuhashi M, Ohguro H, Nishikiori N. Unexpected and Synergistical Effects of All-Trans Retinoic Acid and TGF-β2 on Biological Aspects of 2D and 3D Cultured ARPE19 Cells. Biomedicines. 2024; 12(10):2228. https://doi.org/10.3390/biomedicines12102228
Chicago/Turabian StyleHigashide, Megumi, Megumi Watanabe, Tatsuya Sato, Toshifumi Ogawa, Araya Umetsu, Soma Suzuki, Masato Furuhashi, Hiroshi Ohguro, and Nami Nishikiori. 2024. "Unexpected and Synergistical Effects of All-Trans Retinoic Acid and TGF-β2 on Biological Aspects of 2D and 3D Cultured ARPE19 Cells" Biomedicines 12, no. 10: 2228. https://doi.org/10.3390/biomedicines12102228
APA StyleHigashide, M., Watanabe, M., Sato, T., Ogawa, T., Umetsu, A., Suzuki, S., Furuhashi, M., Ohguro, H., & Nishikiori, N. (2024). Unexpected and Synergistical Effects of All-Trans Retinoic Acid and TGF-β2 on Biological Aspects of 2D and 3D Cultured ARPE19 Cells. Biomedicines, 12(10), 2228. https://doi.org/10.3390/biomedicines12102228








