Influence of Polymerization Protocol on Adhesion and Proliferation of Streptococcus mutans on Three Dental Composite Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Realization of Composite Disks
2.2. Saliva Collection
2.3. Bacterial Strain
- (I)
- Planktonic CFU count of the bacterial cells (CFU/mL);
- (II)
- Sessile CFU count of the cultivable cells on composite disks (CFU/mL);
- (III)
- Biomass evaluation of the biofilm produced on composite disks using Hucker’s crystal violet staining method (OD570nm).
2.4. Planktonic CFU Count
2.5. Sessile CFU Count
2.6. Biomass Quantification by Optical Density (OD570nm)
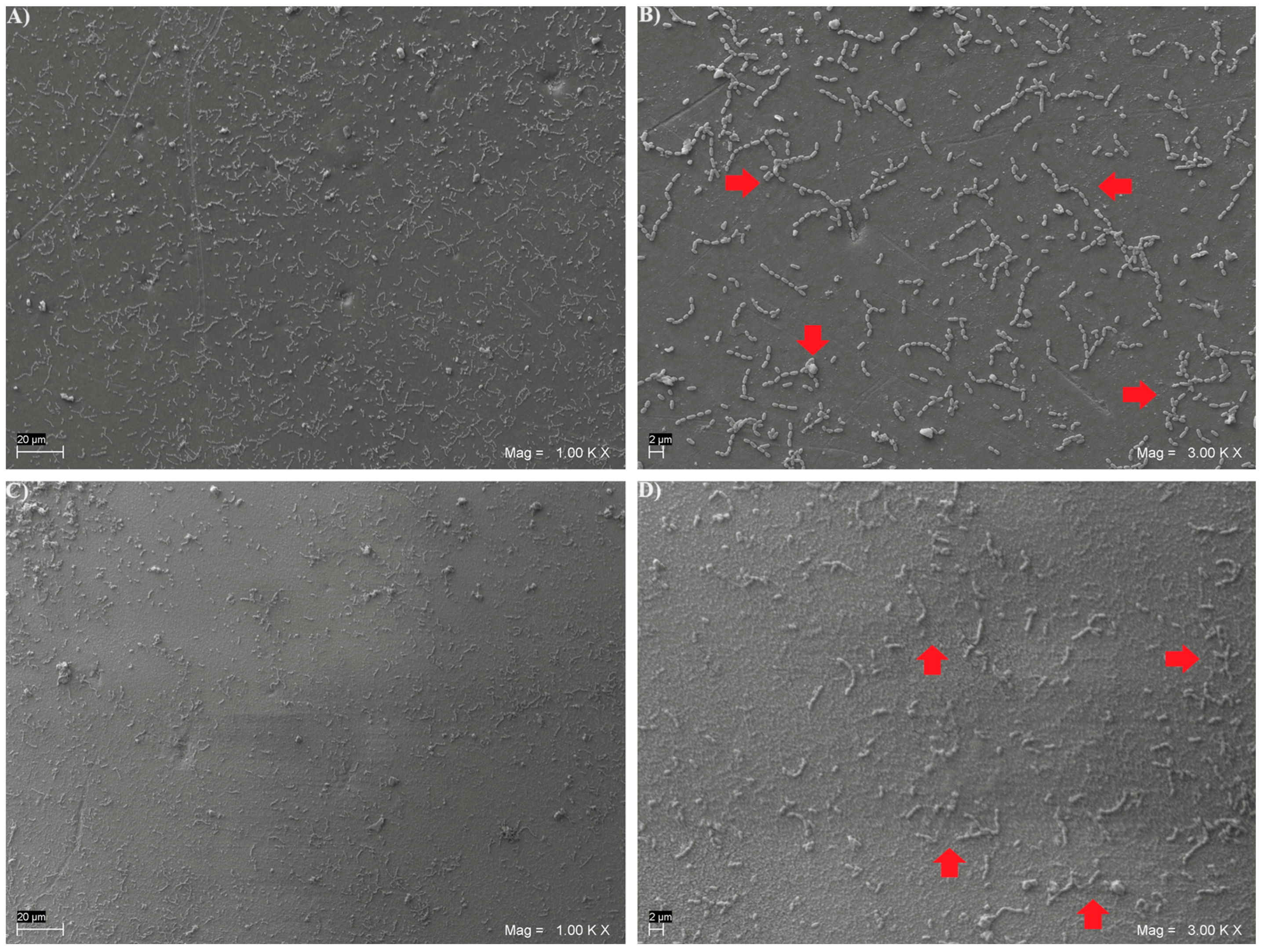
2.7. Scanning Electron Microscope (SEM) Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
- The intrinsic ability of Streptococcus mutans to proliferate seems impaired in the presence of composites subjected to additional heat-curing protocols, with a general trend towards a reduction in both sessile and planktonic CFUs;
- Where there is an inability to reduce sessile CFUs, heat-curing still seems to inhibit the production of biofilm glycoproteins.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beighton, D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 2005, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, S.; Zhuang, P.; Liu, J.; Wang, Y.; Lin, H. Characterization of acid-tolerance-associated small RNAs in clinical isolates of Streptococcus mutans: Potential biomarkers for caries prevention. Mol. Med. Rep. 2017, 16, 9242–9250. [Google Scholar] [CrossRef][Green Version]
- Hahnel, S.; Henrich, A.; Rosentritt, M.; Handel, G.; Burgers, R. Influence of artificial ageing on surface properties and Streptococcus mutans adhesion to dental composite materials. J. Mater. Sci. Mater. Med. 2010, 21, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M. Transmission electron microscopy of early plaque formation on dental materials in vivo. Eur. J. Oral Sci. 1999, 107, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.; Cavalcante, L.M.; Silikas, N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J. Dent. Biomech. 2010, 2010, 131630. [Google Scholar] [CrossRef] [PubMed]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef]
- Hasan, S.; Danishuddin, M.; Adil, M.; Singh, K.; Verma, P.K.; Khan, A.U. Efficacy of E. officinalis on the cariogenic properties of Streptococcus mutans: A novel and alternative approach to suppress quorum-sensing mechanism. PLoS ONE 2012, 7, e40319. [Google Scholar] [CrossRef]
- Huang, L.; Xu, Q.A.; Liu, C.; Fan, M.W.; Li, Y.H. Anti-caries DNA vaccine-induced secretory immunoglobulin A antibodies inhibit formation of Streptococcus mutans biofilms in vitro. Acta Pharmacol. Sin. 2013, 34, 239–246. [Google Scholar] [CrossRef][Green Version]
- Nomura, H.; Isshiki, Y.; Sakuda, K.; Sakuma, K.; Kondo, S. Effects of oakmoss and its components on biofilm formation of Legionella pneumophila. Biol. Pharm. Bull. 2013, 36, 833–837. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wąsik, T.J. In Vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis against Biofilm Forming Staphylococcus epidermidis Strains. Evid. Based Complement. Altern. Med. Ecam 2013, 2013, 590703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tseng, B.S.; Beckerman, B.; Jin, F.; Gibiansky, M.L.; Harrison, J.J.; Luijten, E.; Parsek, M.R.; Wong, G.C.L. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 2013, 497, 388–391. [Google Scholar] [CrossRef]
- Xiao, J.; Klein, M.I.; Falsetta, M.L.; Lu, B.; Delahunty, C.M.; Yates, J.R., 3rd; Heydorn, A.; Koo, H. The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PLoS Pathog. 2012, 8, e1002623. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, M.; Lei, L.; Li, M.; Yin, J.; Ma, X.; Tao, X.; Yang, Y.; Hu, T. Regulation of water-soluble glucan synthesis by the Streptococcus mutans dexA gene effects biofilm aggregation and cariogenic pathogenicity. Mol. Oral Microbiol. 2019, 34, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Jaykus, L.-A.; Wang, H.H.; Schlesinger, L.S. Food-Borne Microbes: Shaping the Host Ecosystem; ASM Press: Washington, DC, USA, 2009. [Google Scholar]
- Tamura, S.; Yonezawa, H.; Motegi, M.; Nakao, R.; Yoneda, S.; Watanabe, H.; Yamazaki, T.; Senpuku, H. Inhibiting effects of Streptococcus salivarius on competence-stimulating peptide-dependent biofilm formation by Streptococcus mutans. Oral Microbiol. Immunol. 2009, 24, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.J.; Maddocks, S.E.; Larson, M.R.; Forsgren, N.; Persson, K.; Deivanayagam, C.C.; Jenkinson, H.F. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 2010, 77, 276–286. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Strömberg, N.; van Dolleweerd, C.J.; Kelly, C.G.; Jenkinson, H.F. Differential binding specificities of oral streptococcal antigen I/II family adhesins for human or bacterial ligands. Mol. Microbiol. 2005, 55, 1591–1605. [Google Scholar] [CrossRef]
- Larson, M.R.; Rajashankar, K.R.; Crowley, P.J.; Kelly, C.; Mitchell, T.J.; Brady, L.J.; Deivanayagam, C. Crystal structure of the C-terminal region of Streptococcus mutans antigen I/II and characterization of salivary agglutinin adherence domains. J. Biol. Chem. 2011, 286, 21657–21666. [Google Scholar] [CrossRef]
- Sullan, R.M.; Li, J.K.; Crowley, P.J.; Brady, L.J.; Dufrêne, Y.F. Binding forces of Streptococcus mutans P1 adhesin. ACS Nano 2015, 9, 1448–1460. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Pereira, J.C.; Yoshiyama, M.; Pashley, D.H. A review of polymerization contraction: The influence of stress development versus stress relief. Oper. Dent. 1996, 21, 17–24. [Google Scholar]
- Collins, C.J.; Bryant, R.W.; Hodge, K.L. A clinical evaluation of posterior composite resin restorations: 8-year findings. J. Dent. 1998, 26, 311–317. [Google Scholar] [CrossRef]
- Pashley, D.H. Clinical considerations of microleakage. J. Endod. 1990, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1997, 13, 258–269. [Google Scholar]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J. Hydrophobicity of biosurfaces—Origin, quantitative determination and interaction energies. Colloids Surf. B Biointerfaces 1995, 5, 91–110. [Google Scholar] [CrossRef]
- D’Arcangelo, C.; De Angelis, F.; Vadini, M.; Carluccio, F.; Vitalone, L.M.; D’Amario, M. Influence of curing time, overlay material and thickness on three light-curing composites used for luting indirect composite restorations. J. Adhes. Dent. 2012, 14, 377–384. [Google Scholar]
- De Angelis, F.; D’Arcangelo, C.; Buonvivere, M.; Rondoni, G.D.; Vadini, M. Shear bond strength of glass ionomer and resin-based cements to different types of zirconia. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. 2020, 32, 806–814. [Google Scholar] [CrossRef]
- Vadini, M.; D’Amario, M.; De Angelis, F.; Falco, A.; D’Arcangelo, C. No-Prep Rehabilitation of Fractured Maxillary Incisors with Partial Veneers. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. 2016, 28, 351–358. [Google Scholar] [CrossRef]
- Peutzfeldt, A. Resin composites in dentistry: The monomer systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef]
- Amirouche-Korichi, A.; Mouzali, M.; Watts, D.C. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Al-Zain, A.O.; Marghalani, H.Y. Influence of Light-curing Distances on Microflexural Strength of Two Resin-based Composites. Oper. Dent. 2020, 45, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Al-Zain, A.O.; Platt, J.A. Effect of light-curing distance and curing time on composite microflexural strength. Dent. Mater. J. 2021, 40, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.L.; Carvalho, R.F.; Batista, C.H.T.; Siqueira-Júnior, H.M.; Queiroz, J.R.C.; Leite, F.P. Efecto del tratamiento térmico y de fibras de polietileno en la resistencia a la flexión de resinas compuestas. Acta Odontológica Venez. 2014, 52, 3–4. [Google Scholar]
- Grazioli, G.; Francia, A.; Cuevas-Suárez, C.E.; Zanchi, C.H.; Moraes, R.R. Simple and Low-Cost Thermal Treatments on Direct Resin Composites for Indirect Use. Braz. Dent. J. 2019, 30, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lapesqueur, M.; Surriaga, P.; Masache, M.E.; Vásquez, B.; Peña, M.; Gómes, O.M.M.; Domínguez, J.A. Efectos sobre microdureza y grado de conversión de dos tipos de resinas sometidas a tratamientos de pospolimerización. Rec. Nac. Odontol. 2015, 11, 49–56. [Google Scholar] [CrossRef]
- Zamalloa-Quintana, M.; López-Gurreonero, C.; Santander-Rengifo, F.M.; Ladera-Castañeda, M.; Castro-Pérez Vargas, A.; Cornejo-Pinto, A.; Cervantes-Ganoza, L.; Cayo-Rojas, C. Effect of Additional Dry Heat Curing on Microflexural Strength in Three Types of Resin Composite: An In Vitro Study. Crystals 2022, 12, 1045. [Google Scholar] [CrossRef]
- Bağis, Y.H.; Rueggeberg, F.A. Mass loss in urethane/TEGDMA- and Bis-GMA/TEGDMA-based resin composites during post-cure heating. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1997, 13, 377–380. [Google Scholar] [CrossRef]
- Hansel, C.; Leyhausen, G.; Mai, U.E.; Geurtsen, W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. J. Dent. Res. 1998, 77, 60–67. [Google Scholar] [CrossRef]
- Brambilla, E.; Gagliani, M.; Ionescu, A.; Fadini, L.; García-Godoy, F. The influence of light-curing time on the bacterial colonization of resin composite surfaces. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2009, 25, 1067–1072. [Google Scholar] [CrossRef]
- Kraigsley, A.M.; Tang, K.; Lippa, K.A.; Howarter, J.A.; Lin-Gibson, S.; Lin, N.J. Effect of polymer degree of conversion on Streptococcus mutans biofilms. Macromol. Biosci. 2012, 12, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; De Angelis, F.; Biferi, V.; Noviello, C.; Tripodi, D.; Di Lodovico, S.; Cellini, L.; D’Arcangelo, C. Antibacterial and Antibiofilm Properties of Three Resin-Based Dental Composites against Streptococcus mutans. Materials 2022, 15, 1891. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; D’Ercole, S.; Di Giulio, M.; Vadini, M.; Biferi, V.; Buonvivere, M.; Vanini, L.; Cellini, L.; Di Lodovico, S.; D’Arcangelo, C. In Vitro Evaluation of Candida albicans Adhesion on Heat-Cured Resin-Based Dental Composites. Materials 2023, 16, 5818. [Google Scholar] [CrossRef] [PubMed]
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The Bacterial Anti-Adhesive Activity of Double-Etched Titanium (DAE) as a Dental Implant Surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef] [PubMed]
- Di Lodovico, S.; Gasparri, F.; Di Campli, E.; Di Fermo, P.; D’Ercole, S.; Cellini, L.; Di Giulio, M. Prebiotic Combinations Effects on the Colonization of Staphylococcal Skin Strains. Microorganisms 2020, 9, 37. [Google Scholar] [CrossRef]
- Ommen, P.; Zobek, N.; Meyer, R.L. Quantification of biofilm biomass by staining: Non-toxic safranin can replace the popular crystal violet. J. Microbiol. Methods 2017, 141, 87–89. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef]
- Metwalli, K.H.; Khan, S.A.; Krom, B.P.; Jabra-Rizk, M.A. Streptococcus mutans, Candida albicans, and the human mouth: A sticky situation. PLoS Pathog. 2013, 9, e1003616. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colonne, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.H.; Gonzalez-Begne, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Pereira, D.; Seneviratne, C.J.; Koga-Ito, C.Y.; Samaranayake, L.P. Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis. 2018, 24, 518–526. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, X.; Alkhers, N.; Alzamil, H.; Alzoubi, S.; Wu, T.T.; Castillo, D.A.; Campbell, F.; Davis, J.; Herzog, K.; et al. Candida albicans and Early Childhood Caries: A Systematic Review and Meta-Analysis. Caries Res. 2018, 52, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Eskelson, E.; Cavalli, V.; Liporoni, P.C.; Jorge, A.O.; do Rego, M.A. Streptococcus mutans biofilm adhesion on composite resin surfaces after different finishing and polishing techniques. Oper. Dent. 2011, 36, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Uçtaşli, M.B.; Arisu, H.D.; Omürlü, H.; Eligüzeloğlu, E.; Ozcan, S.; Ergun, G. The effect of different finishing and polishing systems on the surface roughness of different composite restorative materials. J. Contemp. Dent. Pract. 2007, 8, 89–96. [Google Scholar] [PubMed]
- Attar, N. The effect of finishing and polishing procedures on the surface roughness of composite resin materials. J. Contemp. Dent. Pract. 2007, 8, 27–35. [Google Scholar] [CrossRef]
- Motevasselian, F.; Zibafar, E.; Yassini, E.; Mirzaei, M.; Pourmirhoseni, N. Adherence of Streptococcus Mutans to Microhybrid and Nanohybrid Resin Composites and Dental Amalgam: An In Vitro Study. J. Dent. 2017, 14, 337–343. [Google Scholar]
- Barbosa, S.H.; Zanata, R.L.; Navarro, M.F.; Nunes, O.B. Effect of different finishing and polishing techniques on the surface roughness of microfilled, hybrid and packable composite resins. Braz. Dent. J. 2005, 16, 39–44. [Google Scholar] [CrossRef]
- Jung, M.; Eichelberger, K.; Klimek, J. Surface geometry of four nanofiller and one hybrid composite after one-step and multiple-step polishing. Oper. Dent. 2007, 32, 347–355. [Google Scholar] [CrossRef]
- Ikeda, M.; Matin, K.; Nikaido, T.; Foxton, R.M.; Tagami, J. Effect of surface characteristics on adherence of S. mutans biofilms to indirect resin composites. Dent. Mater. J. 2007, 26, 915–923. [Google Scholar] [CrossRef]
- Koh, R.; Neiva, G.; Dennison, J.; Yaman, P. Finishing systems on the final surface roughness of composites. J. Contemp. Dent. Pract. 2008, 9, 138–145. [Google Scholar] [CrossRef]
- Ono, M.; Nikaido, T.; Ikeda, M.; Imai, S.; Hanada, N.; Tagami, J.; Matin, K. Surface properties of resin composite materials relative to biofilm formation. Dent. Mater. J. 2007, 26, 613–622. [Google Scholar] [CrossRef]
- Suh, B.I. Oxygen-inhibited layer in adhesion dentistry. J. Esthet. Restor. Dent. Off. Publ. Am. Acad. Esthet. Dent. 2004, 16, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Faria-e-Silva, A.L.; Lima, A.F.; Moraes, R.R.; Piva, E.; Martins, L.R. Degree of conversion of etch-and-rinse and self-etch adhesives light-cured using QTH or LED. Oper. Dent. 2010, 35, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Arrais, C.A.; Pontes, F.M.; Santos, L.P.; Leite, E.R.; Giannini, M. Degree of conversion of adhesive systems light-cured by LED and halogen light. Braz. Dent. J. 2007, 18, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Navarra, C.O.; Cadenaro, M.; Codan, B.; Mazzoni, A.; Sergo, V.; De Stefano Dorigo, E.; Breschi, L. Degree of conversion and interfacial nanoleakage expression of three one-step self-etch adhesives. Eur. J. Oral Sci. 2009, 117, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ahn, S.J.; Wen, Z.T.; Brady, L.J.; Burne, R.A. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect. Immun. 2008, 76, 4259–4268. [Google Scholar] [CrossRef]
- Gajewski, V.E.; Pfeifer, C.S.; Fróes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Asmussen, E. NMR-analysis of monomers in restorative resins. Acta Odontol. Scand. 1975, 33, 129–134. [Google Scholar] [CrossRef]
- Ruyter, I.E.; Sjoevik, I.J. Composition of dental resin and composite materials. Acta Odontol. Scand. 1981, 39, 133–146. [Google Scholar] [CrossRef]
- Vankerckhoven, H.; Lambrechts, P.; van Beylen, M.; Vanherle, G. Characterization of composite resins by NMR and TEM. J. Dent. Res. 1981, 60, 1957–1965. [Google Scholar] [CrossRef]
- Ruyter, I.E.; Oysaed, H. Composites for use in posterior teeth: Composition and conversion. J. Biomed. Mater. Res. 1987, 21, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Azevedo, C.L.; Ferracane, J.L.; Braga, R.R. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 27, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, K.J.; Mariotti, A. BIS-GMA—Based resins in dentistry: Are they safe? J. Am. Dent. Assoc. 1999, 130, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kingman, A.; Hyman, J.; Masten, S.A.; Jayaram, B.; Smith, C.; Eichmiller, F.; Arnold, M.C.; Wong, P.A.; Schaeffer, J.M.; Solanki, S.; et al. Bisphenol A and other compounds in human saliva and urine associated with the placement of composite restorations. J. Am. Dent. Assoc. 2012, 143, 1292–1302. [Google Scholar] [CrossRef]
- Hampe, T.; Liersch, J.; Wiechens, B.; Wassmann, T.; Schubert, A.; Alhussein, M.; Bürgers, R.; Krohn, S. A Pilot Study on Monomer and Bisphenol A (BPA) Release from UDMA-Based and Conventional Indirect Veneering Composites. Polymers 2022, 14, 4580. [Google Scholar] [CrossRef]
- Kim, K.; An, J.S.; Lim, B.S.; Ahn, S.J. Effect of Bisphenol A Glycol Methacrylate on Virulent Properties of Streptococcus mutans UA159. Caries Res. 2019, 53, 84–95. [Google Scholar] [CrossRef]
- Lin, N.J.; Keeler, C.; Kraigsley, A.M.; Ye, J.; Lin-Gibson, S. Effect of dental monomers and initiators on Streptococcus mutans oral biofilms. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 776–785. [Google Scholar] [CrossRef]
- De Angelis, F.; Sarteur, N.; Buonvivere, M.; Vadini, M.; Šteffl, M.; D’Arcangelo, C. Meta-analytical analysis on components released from resin-based dental materials. Clin. Oral Investig. 2022, 26, 6015–6041. [Google Scholar] [CrossRef]
- Lucena-Martín, C.; González-López, S.; Navajas-Rodríguez de Mondelo, J.M. The effect of various surface treatments and bonding agents on the repaired strength of heat-treated composites. J. Prosthet. Dent. 2001, 86, 481–488. [Google Scholar] [CrossRef]
- Uzay, C.; Boztepe, M.H.; Bayramoğlu, M.; Geren, N. Effect of post-curing heat treatment on mechanical properties of fiber reinforced polymer (FRP) composites. Mater. Test. 2017, 59, 366–372. [Google Scholar] [CrossRef]
- Takeshige, F.; Kinomoto, Y.; Torii, M. Additional heat-curing of light-cured composite resin for inlay restoration. J. Osaka Univ. Dent. Sch. 1995, 35, 59–66. [Google Scholar] [PubMed]
- Malta, D.A.; Magne, P.; Monteiro-Junior, S. Bond strength and monomer conversion of indirect composite resin restorations, Part 1: Light vs heat polymerization. J. Adhes. Dent. 2014, 16, 517–522. [Google Scholar] [PubMed]
- Ferracane, J.L.; Condon, J.R. Post-cure heat treatments for composites: Properties and fractography. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1992, 8, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, A.; Gauza-Wlodarczyk, M.; Kubisz, L.; Hedzelek, W. The electrical properties and glass transition of some dental materials after temperature exposure. J. Mater. Sci. Mater. Med. 2017, 28, 186. [Google Scholar] [CrossRef]
- Caughman, W.F.; Caughman, G.B.; Shiflett, R.A.; Rueggeberg, F.; Schuster, G.S. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials 1991, 12, 737–740. [Google Scholar] [CrossRef]
- Rueggeberg, F.A.; Hashinger, D.T.; Fairhurst, C.W. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1990, 6, 241–249. [Google Scholar] [CrossRef]
- Shono, Y.; Ogawa, T.; Terashita, M.; Carvalho, R.M.; Pashley, E.L.; Pashley, D.H. Regional measurement of resin-dentin bonding as an array. J. Dent. Res. 1999, 78, 699–705. [Google Scholar] [CrossRef]
- Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials 2004, 25, 5467–5472. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2000, 11, 333–355. [Google Scholar] [CrossRef]
- Hajishengallis, E.; Parsaei, Y.; Klein, M.I.; Koo, H. Advances in the microbial etiology and pathogenesis of early childhood caries. Mol. Oral Microbiol. 2017, 32, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef] [PubMed]
- Kusuma Yulianto, H.D.; Rinastiti, M.; Cune, M.S.; de Haan-Visser, W.; Atema-Smit, J.; Busscher, H.J.; van der Mei, H.C. Biofilm composition and composite degradation during intra-oral wear. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2019, 35, 740–750. [Google Scholar] [CrossRef]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef]
- Demarco, F.F.; Collares, K.; Coelho-de-Souza, F.H.; Correa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Anterior composite restorations: A systematic review on long-term survival and reasons for failure. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2015, 31, 1214–1224. [Google Scholar] [CrossRef]
- Opdam, N.J.; van de Sande, F.H.; Bronkhorst, E.; Cenci, M.S.; Bottenberg, P.; Pallesen, U.; Gaengler, P.; Lindberg, A.; Huysmans, M.C.; van Dijken, J.W. Longevity of posterior composite restorations: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 943–949. [Google Scholar] [CrossRef]
- Huang, B.; Sadeghinejad, L.; Adebayo, O.I.A.; Ma, D.; Xiao, Y.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Gene expression and protein synthesis of esterase from Streptococcus mutans are affected by biodegradation by-product from methacrylate resin composites and adhesives. Acta Biomater. 2018, 81, 158–168. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lala, H.C.; Shepherd, M.G. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 1990, 58, 1429–1436. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. MMBR 2002, 66, 486–505. [Google Scholar] [CrossRef]
- De Angelis, F.; Mandatori, D.; Schiavone, V.; Melito, F.P.; Valentinuzzi, S.; Vadini, M.; Di Tomo, P.; Vanini, L.; Pelusi, L.; Pipino, C.; et al. Cytotoxic and Genotoxic Effects of Composite Resins on Cultured Human Gingival Fibroblasts. Materials 2021, 14, 5225. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Lee, S.S.; Li, Y.C.; Chang, Y.C. Bisgma stimulates prostaglandin E2 production in macrophages via cyclooxygenase-2, cytosolic phospholipase A2, and mitogen-activated protein kinases family. PLoS ONE 2013, 8, e82942. [Google Scholar] [CrossRef] [PubMed]
- Kuan, Y.H.; Huang, F.M.; Li, Y.C.; Chang, Y.C. Proinflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NFκB activation via PI3K/Akt pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 4003–4009. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.M.; Chang, Y.C.; Lee, S.S.; Yeh, C.H.; Lee, K.G.; Huang, Y.C.; Chen, C.J.; Chen, W.Y.; Pan, P.H.; Kuan, Y.H. BisGMA-induced cytotoxicity and genotoxicity in macrophages are attenuated by wogonin via reduction of intrinsic caspase pathway activation. Environ. Toxicol. 2016, 31, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Emmler, J.; Seiss, M.; Kreppel, H.; Reichl, F.X.; Hickel, R.; Kehe, K. Cytotoxicity of the dental composite component TEGDMA and selected metabolic by-products in human pulmonary cells. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 1670–1675. [Google Scholar] [CrossRef]
- Chang, H.H.; Chang, M.C.; Wang, H.H.; Huang, G.F.; Lee, Y.L.; Wang, Y.L.; Chan, C.P.; Yeung, S.Y.; Tseng, S.K.; Jeng, J.H. Urethane dimethacrylate induces cytotoxicity and regulates cyclooxygenase-2, hemeoxygenase and carboxylesterase expression in human dental pulp cells. Acta Biomater. 2014, 10, 722–731. [Google Scholar] [CrossRef]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit. Rev. Oral Biol. Med. Off. Publ. Am. Assoc. Oral Biol. 2001, 12, 136–151. [Google Scholar] [CrossRef]


| Experimental Group | Curing Protocol | Material | Manufacturer | Lot Number | Composition |
|---|---|---|---|---|---|
| GR | Light-curing | GrandioSO Shade A2 (Nanohybrid) | Voco GmbH, Cuxhaven, Germany | 2213772 | 89% fillers (1 μm glass filler); 20–40 nm (silicon dioxide filler); pigments (iron oxide, titanium dioxide). Bis-GMA, Bis-EMA, TEGDMA |
| Heat-curing | |||||
| VD | Light-curing | Venus Diamond Shade A2 (Nanohybrid) | Kulzer GmbH, Hanau, Germany | K010201 | 64% fillers (5 nm–20 μm), barium aluminum fluoride glass (Ø 0.7 max. <2 μm), discrete nanoparticles. TCD-DI-HEA, UDMA. |
| Heat-curing | |||||
| BF | Light-curing | Enamel Plus HRi Biofuntion Shade BF2 (Nanohybrid) | Micerium SpA, Avegno, Italy | 2023000990 | 74% fillers (0.005 µm-0.05 µm silicon dioxide), (0.2–3.0 µm glassy filler). UDMA, Tricyclodecane dimethanol dimethacrylate. |
| Heat-curing |
| -Planktonic CFU Count (CFU/mL) | |||
| Material | |||
| Curing protocol | GR | VD | BF |
| Light-cured | 7.23 × 106 c1 (8.05 × 105) | 2.14 × 107 b1 (4.03 × 106) | 4.40 ×1 07 a1 (6.13 × 106) |
| Heat-cured | 4.89 × 106 b1 (7.65 × 105) | 4.95 × 106 b2 (9.19 × 105) | 2.80 × 107 a2 (4.33 × 106) |
| -Sessile CFU Count (CFU/mL) | |||
| Material | |||
| Curing protocol | GR | VD | BF |
| Light-cured | 7.49 × 106 b1 (7.88 × 105) | 2.93 × 107 a1 (6.31 × 106) | 6.71 × 106 b1 (8.55 × 105) |
| Heat-cured | 3.97 × 106 a2 (8.70 × 105) | 6.07 × 106 a2 (8.19 × 105) | 6.38 × 106 a1 (4.90 × 105) |
| -Biomass Quantification by Optical Density (OD570nm) | |||
| Material | |||
| Curing protocol | GR | VD | BF |
| Light-cured | 0.1325 b1 (0.0207) | 0.1457 b1 (0.0376) | 0.4280 a1 (0.0907) |
| Heat-cured | 0.1464 a1 (0.0287) | 0.1731 a1 (0.0241) | 0.1931 a2 (0.0490) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, F.; D’Arcangelo, C.; Di Lodovico, S.; Sorrentino, E.; Buonvivere, M.; D’Ercole, S. Influence of Polymerization Protocol on Adhesion and Proliferation of Streptococcus mutans on Three Dental Composite Resins. Biomedicines 2024, 12, 2235. https://doi.org/10.3390/biomedicines12102235
De Angelis F, D’Arcangelo C, Di Lodovico S, Sorrentino E, Buonvivere M, D’Ercole S. Influence of Polymerization Protocol on Adhesion and Proliferation of Streptococcus mutans on Three Dental Composite Resins. Biomedicines. 2024; 12(10):2235. https://doi.org/10.3390/biomedicines12102235
Chicago/Turabian StyleDe Angelis, Francesco, Camillo D’Arcangelo, Silvia Di Lodovico, Edoardo Sorrentino, Matteo Buonvivere, and Simonetta D’Ercole. 2024. "Influence of Polymerization Protocol on Adhesion and Proliferation of Streptococcus mutans on Three Dental Composite Resins" Biomedicines 12, no. 10: 2235. https://doi.org/10.3390/biomedicines12102235
APA StyleDe Angelis, F., D’Arcangelo, C., Di Lodovico, S., Sorrentino, E., Buonvivere, M., & D’Ercole, S. (2024). Influence of Polymerization Protocol on Adhesion and Proliferation of Streptococcus mutans on Three Dental Composite Resins. Biomedicines, 12(10), 2235. https://doi.org/10.3390/biomedicines12102235








