In Silico Investigation of the Interlaminar and Mechanical Fracture of Arteries with Atheromatic Plaque during Angioplasty Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Parts
| Part | Young’s Modulus (MPa) | Poisson’s Ratio (-) | Yield Strength (MPa) | Ultimate Tensile Stress (MPa) | Density (kg/m3) |
|---|---|---|---|---|---|
| Stent [30,31,32,33,34] | 33 × 103 | 0.3 | 507.5 | 1404 | 8430 |
| Artery [38,39,40] | 9.56 × 10−2 | 0.49 | 0.88 × 10−2 | 5.98 × 10−2 | 1050 |
| Plaque [40,41,42] | 1.69 | 0.49 | 62.25 × 10−3 | 1.58 | 2700 |
2.2. Assembled Models
2.3. Methodology
3. Results
3.1. Simulation Phases
3.2. Angioplasty Failures in the Simulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 1 August 2024).
- Migliavacca, F.; Petrini, L.; Colombo, M.; Auricchio, F.; Pietrabissa, R. Mechanical behavior of coronary stents investigated through the finite element method. J. Biomech. 2002, 35, 803–811. [Google Scholar] [CrossRef]
- Martin, D.; Boyle, F. Finite element analysis of balloon-expandable coronary stent deployment: Influence of angioplasty balloon configuration. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 1161–1175. [Google Scholar] [CrossRef]
- Schiavone, A.; Zhao, L.G. A study of balloon type, system constraint and artery constitutive model used in finite element simulation of stent deployment. Mech. Adv. Mater. Mod. Process. 2015, 1, 1. [Google Scholar] [CrossRef]
- Geith, M.; Swidergal, K.; Hochholdinger, B.; Schratzenstaller, T.; Wagner, M.; Holzapfel, G. On the importance of modeling balloon folding, pleating, and stent crimping: An FE study comparing experimental inflation tests. Int. J. Numer. Methods Biomed. Eng. 2019, 35, e3249. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, J.; Zhu, H.; Lu, K.; Dong, P.; Gu, L. Comparative study of tapered versus conventional cylindrical balloon for stent implantation in stenotic tapered artery. Artif. Organs 2020, 44, 727–735. [Google Scholar] [CrossRef]
- Wiesent, L.; Schultheiß, U.; Schmid, C.; Schratzenstaller, T.; Nonn, A. Experimentally validated simulation of coronary stents considering different dogboning ratios and asymmetric stent positioning. PLoS ONE 2019, 14, e0224026. [Google Scholar] [CrossRef]
- Umer, M.; Ali, M.N.; Mubashar, A.; Mir, M. Computational modeling of balloon-expandable stent deployment in coronary artery using the finite element method. Res. Rep. Clin. Cardiol. 2019, 10, 43–56. [Google Scholar] [CrossRef]
- Noble, C.; Carlson, K.D.; Neumann, E.; Doherty, S.; Dragomir-Daescu, D.; Lerman, A.; Erdemir, A.; Young, M. Evaluation of the role of peripheral artery plaque geometry and composition on stent performance. J. Mech. Behav. Biomed. Mater. 2021, 116, 104346. [Google Scholar] [CrossRef]
- Antonini, L.; Mandelli, L.; Berti, F.; Pennati, G.; Petrini, L. Validation of the computational model of a coronary stent: A fundamental step towards in silico trials. J. Mech. Behav. Biomed. Mater. 2021, 122, 104644. [Google Scholar] [CrossRef]
- Cockerill, I.; See, C.W.; Young, M.L.; Wang, Y.; Zhu, D. Designing Better Cardiovascular Stent Materials—A Learning Curve. Adv. Funct. Mater. 2021, 31, 2005361. [Google Scholar] [CrossRef]
- Colombo, M.; Corti, A.; Berceli, S.; Migliavacca, F.; McGinty, S.; Chiastra, C. 3D modelling of drug-coated balloons for the treatment of calcified superficial femoral arteries. PLoS ONE 2021, 16, e0256783. [Google Scholar] [CrossRef]
- Biswas, S.; Sarifuddin; Mandal, P.K. An unsteady analysis of two-phase binding of drug in an asymmetric stenosed vessel. Biomed. Phys. Eng. Express 2021, 8, 015014. [Google Scholar] [CrossRef]
- Jain, A.; McGinty, S.; Pontrelli, G.; Zhou, L. Theoretical modeling of endovascular drug delivery into a multilayer arterial wall from a drug-coated balloon. Int. J. Heat Mass Transf. 2022, 187, 122572. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Zhu, B.; Wu, J.; Wang, X. Drug Release Analysis and Optimization for Drug-Eluting Stents. Sci. World J. 2013, 2013, 827839. [Google Scholar] [CrossRef]
- Loukas, V.S.; Pleouras, D.S.; Karanasiou, G.S.; Kyriakidis, S.; Sakellarios, A.I.; Semertzioglou, A.; Michalis, L.K.; Fotiadis, D.I. Investigation of Drug Eluting Stents Performance Through in silico Modeling; Springer International Publishing: Cham, Switzerland, 2021; pp. 712–721. [Google Scholar]
- Stratakos, E.; Antonini, L.; Poletti, G.; Berti, F.; Tzafriri, A.R.; Petrini, L.; Pennati, G. Investigating Balloon-Vessel Contact Pressure Patterns in Angioplasty: In Silico Insights for Drug-Coated Balloons. Ann. Biomed. Eng. 2023, 51, 2908–2922. [Google Scholar] [CrossRef]
- Escuer, J.; Aznar, I.; McCormick, C.; Peña, E.; McGinty, S.; Martínez, M.A. Influence of vessel curvature and plaque composition on drug transport in the arterial wall following drug-eluting stent implantation. Biomech. Model. Mechanobiol. 2021, 20, 767–786. [Google Scholar] [CrossRef]
- Escuer, J.; Schmidt, A.F.; Peña, E.; Martínez, M.A.; McGinty, S. Mathematical modelling of endovascular drug delivery: Balloons versus stents. Int. J. Pharm. 2022, 620, 121742. [Google Scholar] [CrossRef]
- Wu, W.; Petrini, L.; Gastaldi, D.; Villa, T.; Vedani, M.; Lesma, E.; Previtali, B.; Migliavacca, F. Finite Element Shape Optimization for Biodegradable Magnesium Alloy Stents. Ann. Biomed. Eng. 2010, 38, 2829–2840. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Sheng, K.; Miao, F.; Wang, Y.; Zhang, Y.; Hou, R.; Mei, D.; Sun, Y.; Zheng, Y.; et al. Optimizing structural design on biodegradable magnesium alloy vascular stent for reducing strut thickness and raising radial strength. Mater. Des. 2022, 220, 110843. [Google Scholar] [CrossRef]
- Alihemmati, Z.; Vahidi, B.; Bakhshian Nik, A.; Vaez Ghaemi, R.; Taghizadeh, Y. In Silico Evaluation of Bilinear Elastoplastic Coronary Artery Stents. Adv. Eng. Mater. 2022, 24, 2100800. [Google Scholar] [CrossRef]
- Zhang, H.; Du, T.; Chen, S.; Liu, Y.; Yang, Y.; Hou, Q.; Qiao, A. Finite Element Analysis of the Non-Uniform Degradation of Biodegradable Vascular Stents. J. Funct. Biomater. 2022, 13, 152. [Google Scholar] [CrossRef]
- Vavuranakis, M.; Kalogeras, K.I.; Moldovan, C.; Katsarou, O.; Stefanadis, C. Coronary rupture after stent deployment in a patient under chronic immunosuppressive therapy. J. Cardiol. Cases 2012, 6, e145–e149. [Google Scholar] [CrossRef][Green Version]
- Alfonso, F.; Segovia, J.; Alswies, A. Coronary Rupture During Stent Implantation. Circulation 1998, 98, 2094. [Google Scholar] [CrossRef][Green Version]
- Broadbent, L.P.; Moran, C.J.; Cross, D.T.; Derdeyn, C.P. Management of Ruptures Complicating Angioplasty and Stenting of Supraaortic Arteries: Report of Two Cases and a Review of the Literature. AJNR Am. J. Neuroradiol. 2003, 24, 2057–2061. [Google Scholar]
- Chae, J.K.; Park, S.W.; Kim, Y.H.; Hong, M.K.; Park, S.J. Successful treatment of coronary artery perforation during angioplasty using autologous vein graft-coated stent. Eur. Heart J. 1997, 18, 1030–1032. [Google Scholar] [CrossRef][Green Version]
- Ekici, B.; Erkan, A.F.; Kütük, U.; Töre, H.F. Successful Management of Coronary Artery Rupture with Stent-Graft: A Case Report. Case Rep. Med. 2014, 2014, e391843. [Google Scholar] [CrossRef]
- Mantzaris, M.D.; Siogkas, P.K.; Tsakanikas, V.D.; Potsika, V.T.; Pleouras, D.S.; Sakellarios, A.I.; Karagiannis, G.; Galyfos, G.; Sigala, F.; Liasis, N.; et al. Computational modeling of atherosclerotic plaque progression in carotid lesions with moderate degree of stenosis. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Conference, 1–5 November 2021; pp. 4209–4212. [Google Scholar]
- Auricchio, F.; Constantinescu, A.; Conti, M.; Scalet, G. A computational approach for the lifetime prediction of cardiovascular balloon-expandable stents. Int. J. Fatigue 2015, 75, 69–79. [Google Scholar] [CrossRef]
- Azaouzi, M.; Makradi, A.; Petit, J.; Belouettar, S.; Polit, O. On the numerical investigation of cardiovascular balloon-expandable stent using finite element method. Comput. Mater. Sci. 2013, 79, 326–335. [Google Scholar] [CrossRef]
- Cui, X.; Ren, Q.; Qiao, A.; Li, G.; Li, Z. Fatigue life prediction of stents in a realistic coronary stenosis model. In Proceedings of the 8th International Conference on Computational Methods (ICCM2017), Guilin, China, 25–29 July 2017. [Google Scholar]
- Hsiao, H.-M.; Yeh, C.-T.; Chiu, Y.-H.; Wang, C.; Chen, C.-P. New clinical failure mode triggered by a new coronary stent design. Biomed. Mater. Eng. 2014, 24, 37–43. [Google Scholar] [CrossRef]
- Cui, X.; Ren, Q.; Li, Z.; Peng, K.; Li, G.; Gu, Z.; Qiao, A. Effect of Plaque Composition on Biomechanical Performance of a Carotid Stent: Computational Study. Comput. Model. Eng. Sci. 2018, 116, 455–469. [Google Scholar] [CrossRef]
- Kumar, S.; Hanumantharaju, D. An Investigation on Strength of SS316L and Cobalt-chromium used as Cardiovascular Stent Materials by Non linear FEA. Int. J. Eng. Technol. (IJERT) 2013, 2, 1876–1884. [Google Scholar]
- Fogarotto, F. Finite Element Analysis of Coronary Artery Stenting. Ph.D. Thesis, University of Pavia, Pavia, Italy, 2011. [Google Scholar]
- Amstutz, C.; Weisse, B.; Valet, S.; Haeberlin, A.; Burger, J.; Zurbuchen, A. Temperature-dependent tensile properties of polyamide 12 for the use in percutaneous transluminal coronary angioplasty balloon catheters. BioMed. Eng. OnLine 2021, 20, 110. [Google Scholar] [CrossRef]
- Choudhury, N.; Bouchot, O.; Rouleau, L.; Tremblay, D.; Cartier, R.; Butany, J.; Mongrain, R.; Leask, R.L. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc. Pathol. 2009, 18, 83–91. [Google Scholar] [CrossRef]
- Karimi, A.; Sera, T.; Kudo, S.; Navidbakhsh, M. Experimental verification of the healthy and atherosclerotic coronary arteries incompressibility via Digital Image Correlation. Artery Res. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Reith, S.; Milzi, A.; Lemma, E.D.; Dettori, R.; Burgmaier, K.; Marx, N.; Burgmaier, M. Intrinsic calcification angle: A novel feature of the vulnerable coronary plaque in patients with type 2 diabetes: An optical coherence tomography study. Cardiovasc. Diabetol. 2019, 18, 122. [Google Scholar] [CrossRef]
- Kobielarz, M.; Kozuń, M.; Gąsior-Głogowska, M.; Chwiłkowska, A. Mechanical and structural properties of different types of human aortic atherosclerotic plaques. J. Mech. Behav. Biomed. Mater. 2020, 109, 103837. [Google Scholar] [CrossRef]
- Moraes, M.C.; Cardoso, F.M.; Furuie, S.S. Atherosclerotic plaque characterization using plaque area variation in IVUS images during compression: A computational investigation. Rev. Bras. Eng. Bioméd. 2014, 30, 159–172. [Google Scholar] [CrossRef][Green Version]
- Sarifuddin; Mandal, P.K. Effect of Interstitial Fluid Flow on Drug-Coated Balloon Delivery in a Patient-Specific Arterial Vessel with Heterogeneous Tissue Composition: A Simulation Study. Cardiovasc. Eng. Technol. 2018, 9, 251–267. [Google Scholar] [CrossRef]
- Poletti, G.; Antonini, L.; Mandelli, L.; Tsompou, P.; Karanasiou, G.S.; Papafaklis, M.I.; Michalis, L.K.; Fotiadis, D.I.; Petrini, L.; Pennati, G. Towards a Digital Twin of Coronary Stenting: A Suitable and Validated Image-Based Approach for Mimicking Patient-Specific Coronary Arteries. Electronics 2022, 11, 502. [Google Scholar] [CrossRef]
- Chiastra, C.; Grundeken, M.J.; Collet, C.; Wu, W.; Wykrzykowska, J.J.; Pennati, G.; Dubini, G.; Migliavacca, F. Biomechanical Impact of Wrong Positioning of a Dedicated Stent for Coronary Bifurcations: A Virtual Bench Testing Study. Cardiovasc. Eng. Technol. 2018, 9, 415–426. [Google Scholar] [CrossRef]
- Di Caprio, F.; Saputo, S.; Sellitto, A. Numerical-Experimental Correlation of Interlaminar Damage Growth in Composite Structures: Setting Cohesive Zone Model Parameters. Adv. Mater. Sci. Eng. 2019, 2019, 2150921. [Google Scholar] [CrossRef]
- Alloisio, M.; Chatziefraimidou, M.; Roy, J.; Christian Gasser, T. Fracture of porcine aorta—Part 1: SymconCT fracture testing and DIC. Acta Biomater. 2023, 167, 147–157. [Google Scholar] [CrossRef]
- Alloisio, M.; Gasser, T.C. Fracture of porcine aorta—Part 2: FEM modelling and inverse parameter identification. Acta Biomater. 2023, 167, 158–170. [Google Scholar] [CrossRef]



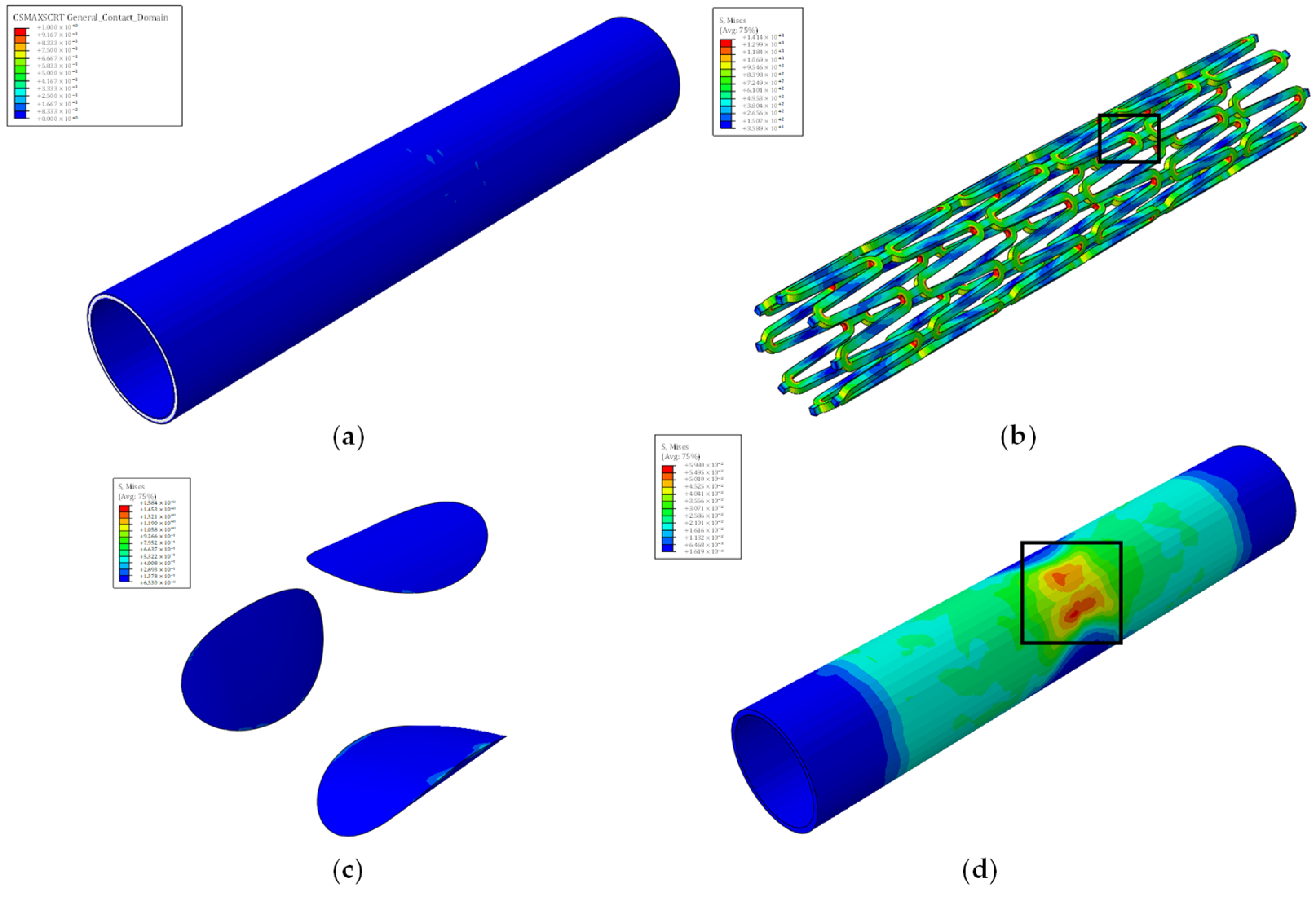
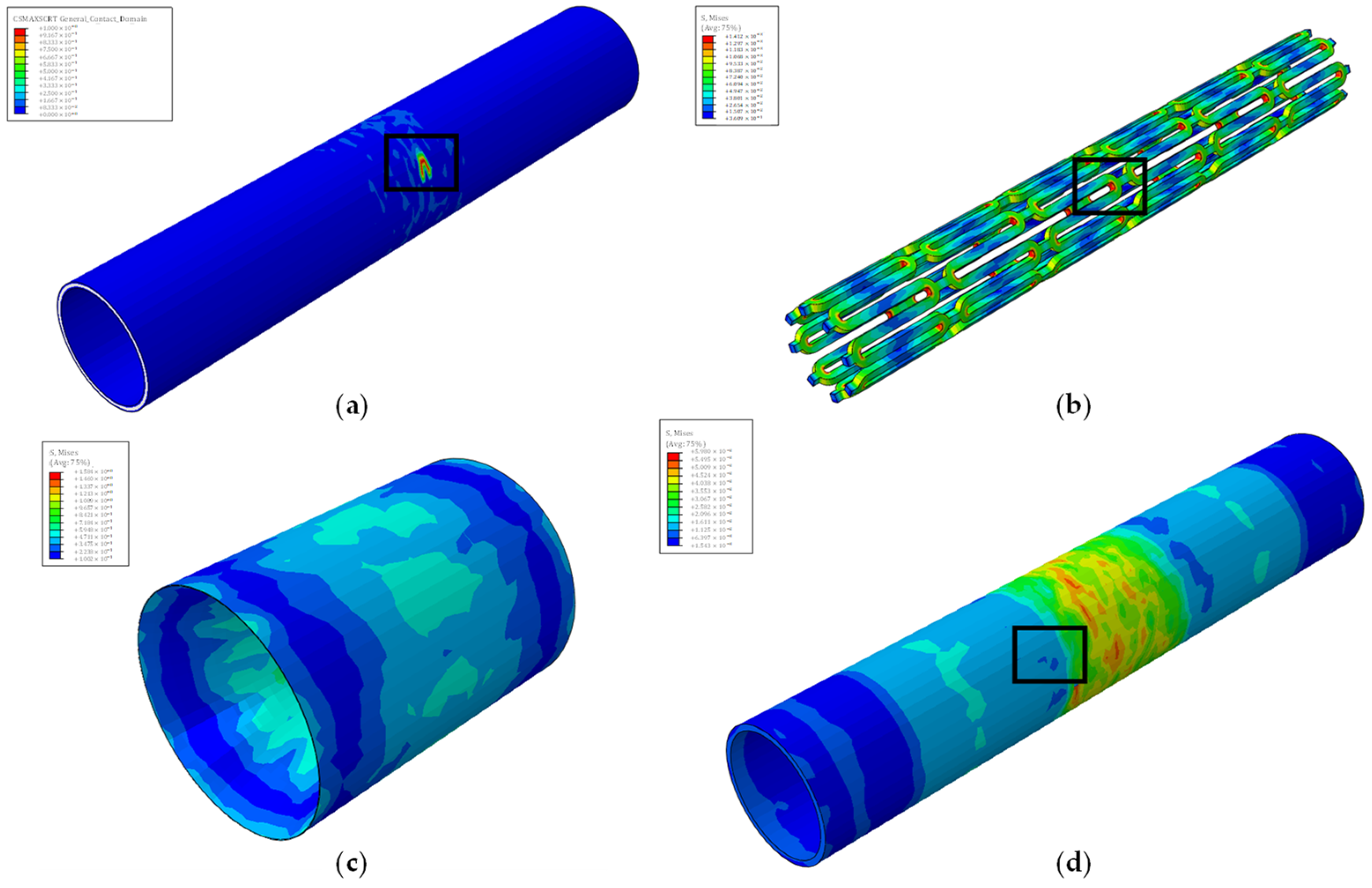
| Traction Elastic Parameters | E/Enn [N/mm] | G1/Ess [N/mm] | G2/Ett [N/mm] | |
|---|---|---|---|---|
| 4 | 4 | 4 | ||
| Damage Initiation Criteria | Normal-only Mode [MPa] | First Direction [MPa] | Second Direction [MPa] | Fracture Energy [kJ/m2] |
| 0.213 | 0.324 | 0.324 | 1.210 |
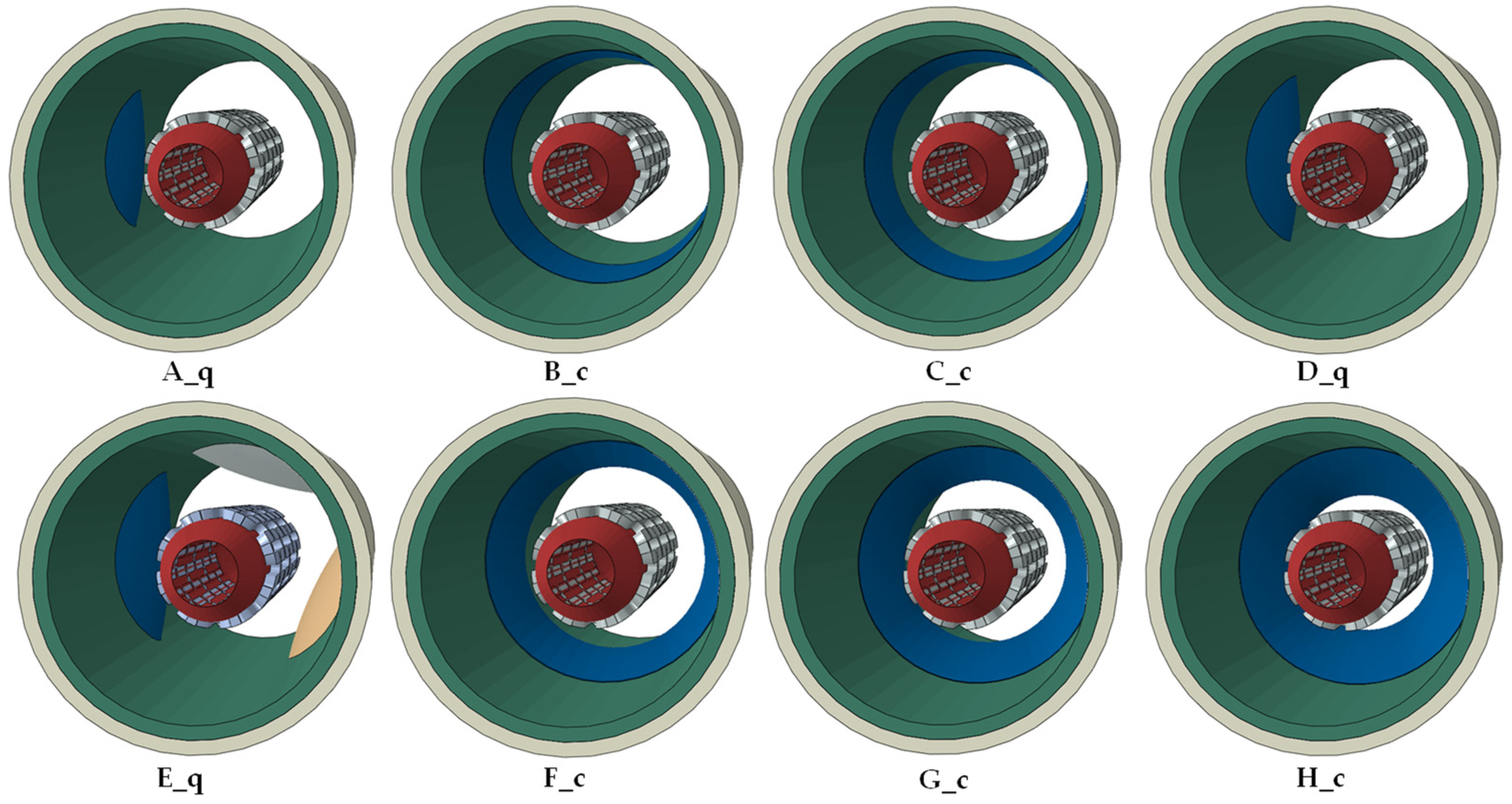
| MODEL * | Volume Stenosis [%] | Stent Radius at the Point of Failure Due to | ||
|---|---|---|---|---|
| Interlaminar Failure [mm] | Artery Mechanical Failure [mm] | Stent Failure [mm] | ||
| A_q | 7.2 | - | 3.1 | 2.5 |
| B_c | 7.8 | 3.2 | 2.7 | 2.5 |
| C_c | 15.4 | 2.6 | 2.0 | 2.5 |
| D_q | 16.8 | - | 1.9 | 2.5 |
| E_q | 34 | - | 1.8 | 2.5 |
| F_c | 36 | 1.5 | 1.8 | 2.5 |
| G_c | 48 | 1.4 | 1.5 | 2.5 |
| H_c | 64 | 0.9 | 1.0 | 2.5 |
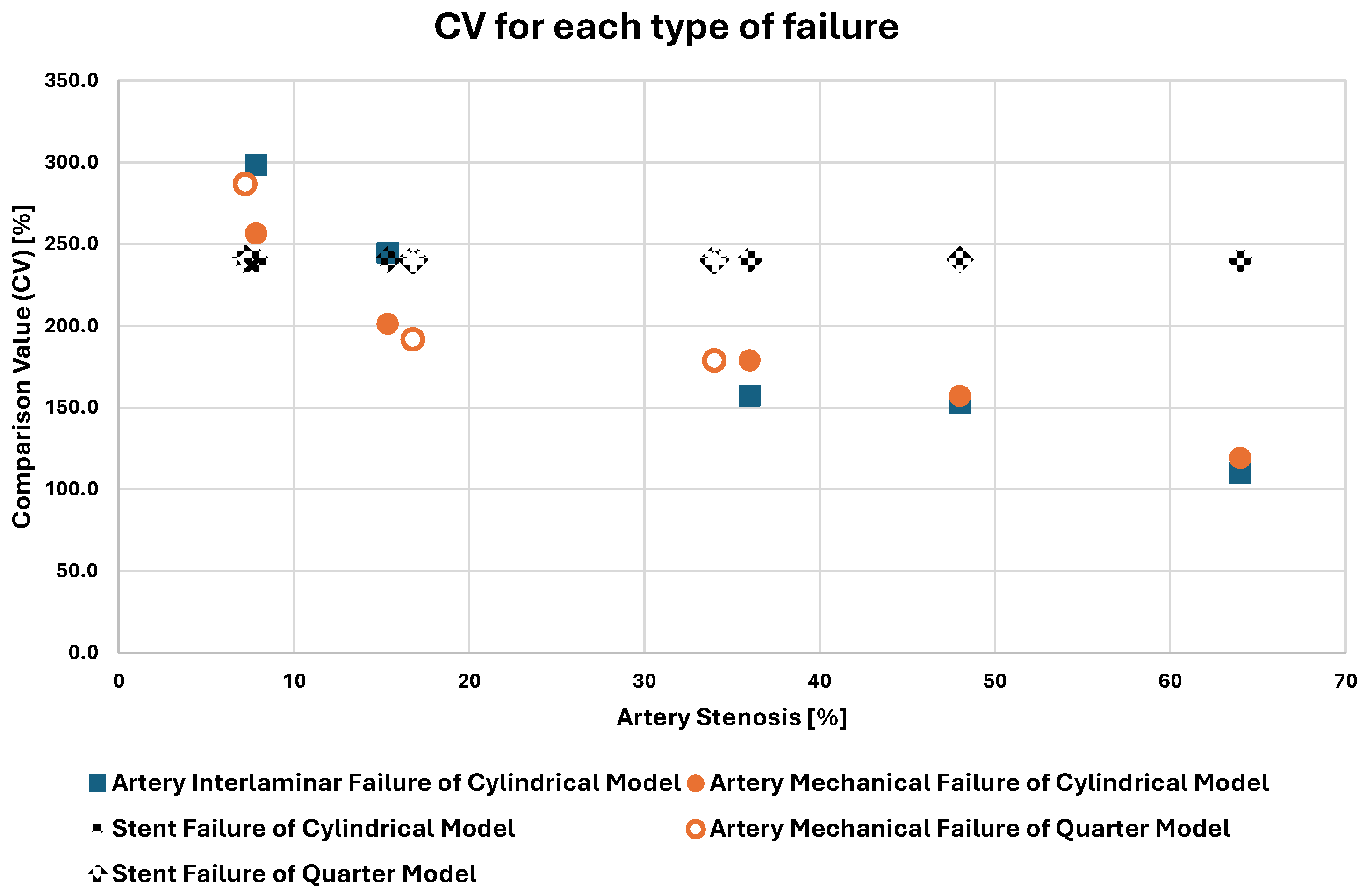
| MODEL * | Volume Stenosis [%] | Comparison Value (CV) for Failure Due to | ||
|---|---|---|---|---|
| Interlaminar Failure [%] | Artery Mechanical Failure [%] | Stent Failure [%] | ||
| A_q | 7.2 | - | 286.5 | 240.5 |
| B_c | 7.8 | 298.4 | 256.5 | 240.5 |
| C_c | 15.4 | 244.6 | 201.2 | 240.5 |
| D_q | 16.8 | - | 191.7 | 240.5 |
| E_q | 34 | - | 178.8 | 240.5 |
| F_c | 36 | 157.1 | 178.8 | 240.5 |
| G_c | 48 | 152.9 | 157.1 | 240.5 |
| H_c | 64 | 109.8 | 119.1 | 240.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psarras, S.; Skafidas, A.-N.; Kostopoulos, V. In Silico Investigation of the Interlaminar and Mechanical Fracture of Arteries with Atheromatic Plaque during Angioplasty Treatment. Biomedicines 2024, 12, 2105. https://doi.org/10.3390/biomedicines12092105
Psarras S, Skafidas A-N, Kostopoulos V. In Silico Investigation of the Interlaminar and Mechanical Fracture of Arteries with Atheromatic Plaque during Angioplasty Treatment. Biomedicines. 2024; 12(9):2105. https://doi.org/10.3390/biomedicines12092105
Chicago/Turabian StylePsarras, Spyridon, Anargyros-Nektarios Skafidas, and Vassilis Kostopoulos. 2024. "In Silico Investigation of the Interlaminar and Mechanical Fracture of Arteries with Atheromatic Plaque during Angioplasty Treatment" Biomedicines 12, no. 9: 2105. https://doi.org/10.3390/biomedicines12092105
APA StylePsarras, S., Skafidas, A.-N., & Kostopoulos, V. (2024). In Silico Investigation of the Interlaminar and Mechanical Fracture of Arteries with Atheromatic Plaque during Angioplasty Treatment. Biomedicines, 12(9), 2105. https://doi.org/10.3390/biomedicines12092105








