Current Perspectives on Olfactory Loss in Atypical Parkinsonisms—A Review Article
Abstract
1. Introduction
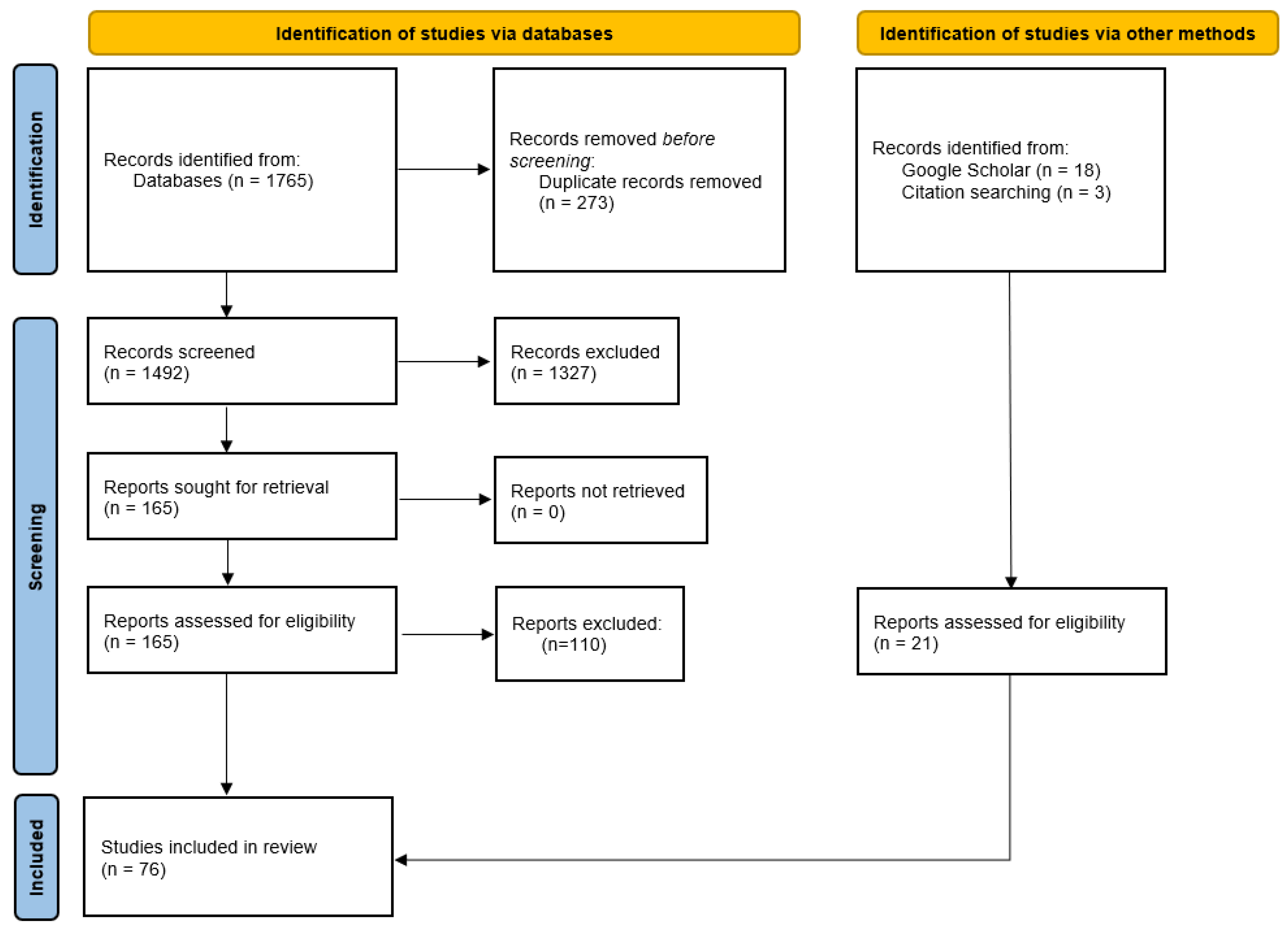
2. Methodology
2.1. Search Strategy
2.2. Study Screening and Selection
2.3. Study Eligibility Criteria
3. State of the Art
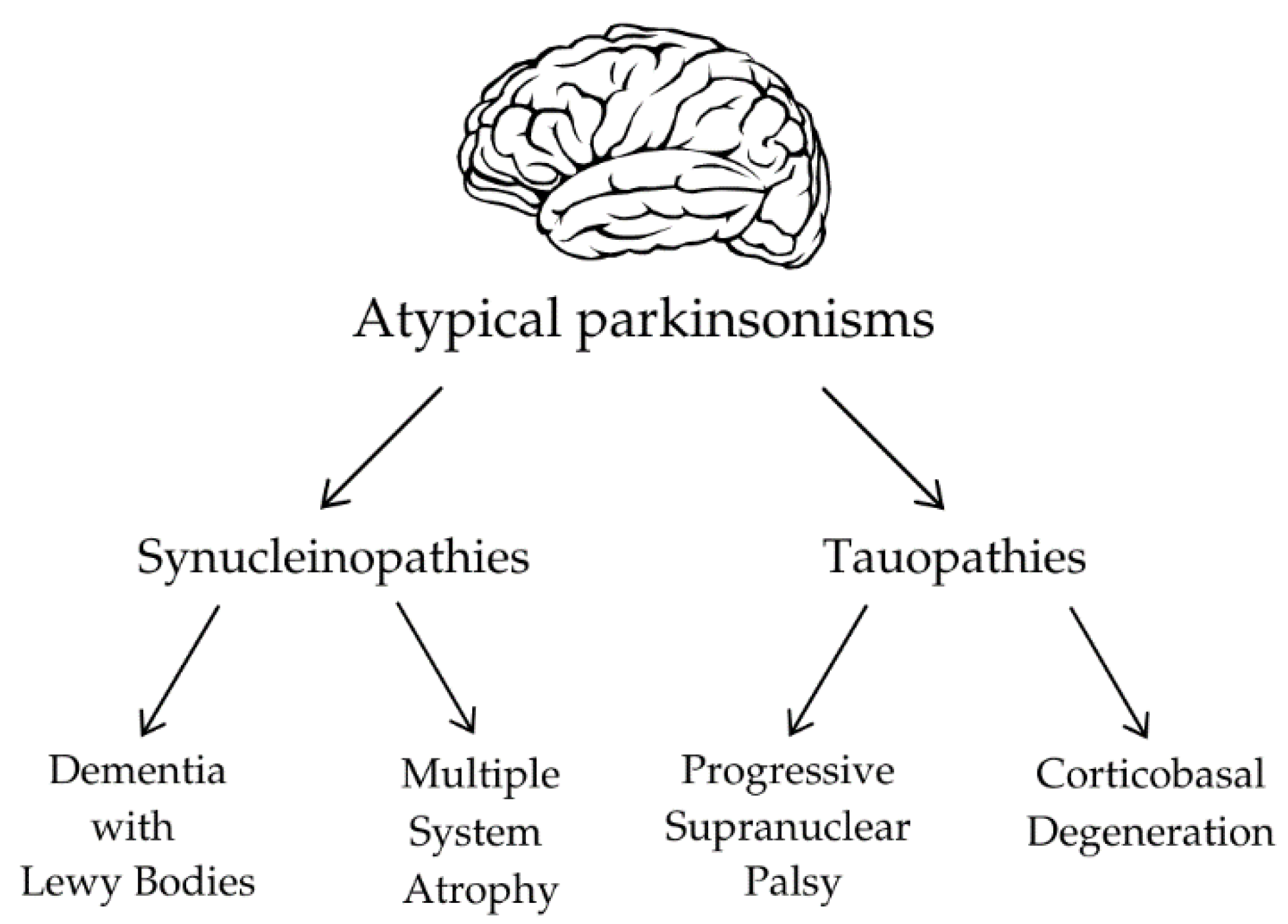
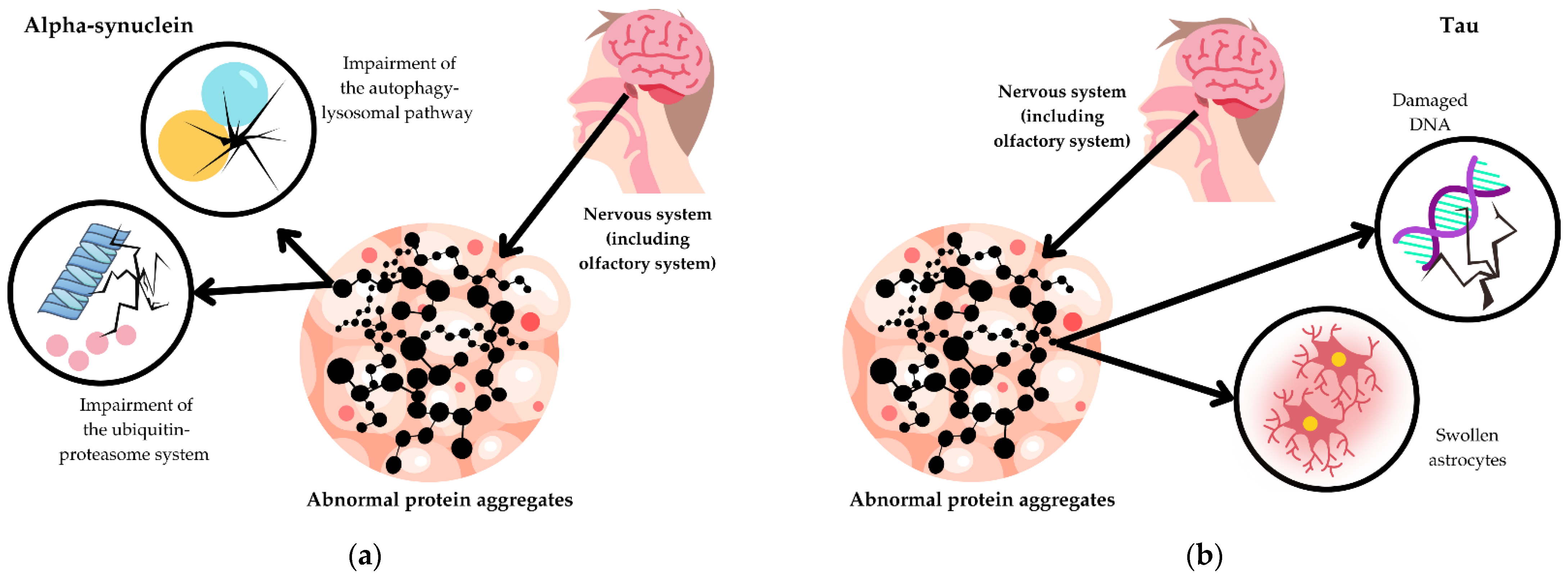
3.1. Pathophysiology of Atypical Parkinsonisms
3.2. Olfactory Dysfunction in Synucleinopathies
3.2.1. Dementia with Lewy Bodies
3.2.2. Multiple System Atrophy
3.3. Olfactory Dysfunction in Tauopathies
3.3.1. Progressive Supranuclear Palsy
3.3.2. Corticobasal Degeneration
4. Perspectives and Conclusions
4.1. Limitations
4.2. Clinical Significance
4.3. Background and Future Directions in the Analysis of Olfactory Loss
4.4. Differential Diagnosis
4.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, R.Y. Epidemiology of atypical parkinsonian syndromes. Tzu Chi Med. J. 2022, 34, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Chen, W.; Su, C.H.; Liu, C.-H. Incidence and Comorbidity of Dementia with Lewy Bodies: A Population-Based Cohort Study. Behav. Neurol. 2018, 2018, 7631951. [Google Scholar] [CrossRef] [PubMed]
- Vann Jones, S.A.; O’Brien, J.T. The prevalence and incidence of dementia with Lewy bodies: A systematic review of population and clinical studies. Psychol. Med. 2014, 44, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B.; Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Dykeman, J.; Pringsheim, T.; Steeves, T.; Smith, E.E.; Pearson, D.; Jetté, N. The Prevalence and Incidence of Dementia with Lewy Bodies: A Systematic Review. Can. J. Neurol. Sci. 2016, 43 (Suppl. S1), S83–S95. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Ben-Shlomo, Y.; Quinn, N.P. Prevalence of progressive supranuclear palsy and multiple system atrophy: A cross-sectional study. Lancet 1999, 354, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, V.C.; Paraskevas, G.P.; Paraskevas, P.G.; Stefanis, L.; Kapaki, E. Corticobasal degenerati on and corticobasal syndrome: A review. Clin. Park. Relat. Disord. 2019, 1, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Backstrom, D.; Granasen, G.; Domellof, M.E.; Linder, J.; Mo, S.J.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L. Early predictors of mortality in parkinsonism and Parkinson disease: A population-based study. Neurology 2018, 91, e2045–e2056. [Google Scholar] [CrossRef] [PubMed]
- Siuda, J. Importance of non-motor symptoms in PD and atypical parkinsonism. Neurol. Neurochir. Pol. 2021, 55, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, C.; Morgante, L.; Antonini, A.; Barone, P.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, M.G.; Ceravolo, R.; Cicarelli, G.; et al. Non-motor symptoms in atypical and secondary parkinsonism: The PRIAMO study. J. Neurol. 2010, 257, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko-Alster, N.; Migda, A.; Migda, B.; Kutyłowski, M.; Królicki, L.; Friedman, A. Sleep disturbances in progressive supranuclear palsy syndrome (PSPS) and corticobasal syndrome (CBS). Neurol. Neurochir. Pol. 2023, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bak, T.H.; Caine, D.; Hearn, V.C.; Hodges, J.R. Visuospatial functions in atypical parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2006, 77, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Cheon, S.M.; Kim, J.W. Autonomic dysfunctions in parkinsonian disorders. J. Mov. Disord. 2009, 2, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, M.; Schinwelski, M.; Sitek, E.J.; Muraszko-Klaudel, A.; Brockhuis, B.; Jamrozik, Z.; Sławek, J. The role of neuroimaging in the diagnosis of the atypical parkinsonian syndromes in clinical practice. Neurol. Neurochir. Pol. 2015, 49, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Hummel, T.; Hummel, C.; Sommer, U.; Junghanns, S.; Reichmann, H. Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov. Disord. 2007, 22, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Shephard, B.C.; Daniel, S.E. Olfactory dysfunction in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Deems, D.A.; Stellar, S. Olfactory dysfunction in parkinsonism: A general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 1988, 38, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Mungersdorf, M.; Reichmann, H.; Strehle, G.; Hummel, T. Olfactory function in Parkinsonian syndromes. J. Clin. Neurosci. 2002, 9, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, K.; Chase, B.A.; Robowski, P.; Strongosky, A.; Narożańska, E.; Sitek, E.J.; Berdynski, M.; Barcikowska, M.; Baker, M.C.; Rademakers, R.; et al. Assessment of Olfactory Function in MAPT-Associated Neurodegenerative Disease Reveals Odor-Identification Irreproducibility as a Non-Disease-Specific, General Characteristic of Olfactory Dysfunction. PLoS ONE 2016, 11, e0165112. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari, D.; Cantuti-Castelvetri, I.; Fan, Z.; Rockenstein, E.; Masliah, E.; Hyman, B.T.; McLean, P.J.; Unni, V.K. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of alpha-synuclein. J. Neurosci. 2011, 31, 14508–14520. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.W.; Yen, S.H.; Suzuki, K.I.; Davies, P.; Garcia, J.H. Ballooned neurons in select neurodegenerative diseases contain phosphorylated neurofilament epitopes. Acta Neuropathol. 1986, 71, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Fiock, K.L.; Hook, J.N.; Hefti, M.M. Determinants of astrocytic pathology in stem cell models of primary tauopathies. Acta Neuropathol. Commun. 2023, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Violet, M.; Delattre, L.; Tardivel, M.; Sultan, A.; Chauderlier, A.; Caillierez, R.; Talahari, S.; Nesslany, F.; Lefebvre, B.; Bonnefoy, E.; et al. A major role for Tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front. Cell. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, W.; Liu, P.; Wang, Z.; Zhou, Y.; Liu, X.; Li, A. alpha-Synuclein aggregation in the olfactory bulb induces olfactory deficits by perturbing granule cells and granular-mitral synaptic transmission. NPJ Parkinsons Dis. 2021, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, W.; Wu, X.; Li, J.; Yang, J.; Tu, C.; Ye, X.; Ling, S. Olfactory deficit is associated with mitral cell dysfunction in the olfactory bulb of P301S tau transgenic mice. Brain Res. Bull. 2019, 148, 34–45. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.-F.; Postuma, R.B. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Kasanuki, K.; Iseki, E.; Ota, K.; Kondo, D.; Ichimiya, Y.; Sato, K.; Arai, H. (123)I-FP-CIT SPECT findings and its clinical relevance in prodromal dementia with Lewy bodies. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Yokogi, M.; Kusano, K.; Matsubara, R.; Sato, M.; Tomita, I.; Seto, M.; Satoh, A.; Tsujihata, M. Evaluation of olfactory impairment using a simple test kit “The Odor Stick Identification test for the Japanese” (OSIT-J) in neurodegenerative diseases. Acta Med. Nagasaki. 2022, 65, 29–36. [Google Scholar] [CrossRef]
- Fujishiro, H.; Iseki, E.; Nakamura, S.; Kasanuki, K.; Chiba, Y.; Ota, K.; Murayama, N.; Sato, K. Dementia with Lewy bodies: Early diagnostic challenges. Psychogeriatrics 2013, 13, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Miyamoto, M. Odor identification predicts the transition of patients with isolated RBD: A retrospective study. Ann. Clin. Transl. Neurol. 2022, 9, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shoji, Y.; Yanagimoto, H.; Morita, K.; Kodama, H.; Tsuruhisa, Y.; Ookawa, J. A characteristic of olfactory function in four types of dementia and non-dementia subjects using smell identification test. Psychogeriatrics 2024, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, K.; Fukatsu, R.; Yamada, R.; Takamaru, Y.; Hara, Y.; Yasumura, S. Characteristics of initial symptoms and symptoms at diagnosis in probable dementia with Lewy body disease: Incidence of symptoms and gender differences. Psychogeriatrics 2020, 20, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Gelpi, E.; Navarro-Otano, J.; Tolosa, E.; Gaig, C.; Compta, Y.; Rey, M.J.; Martí, M.J.; Hernández, I.; Valldeoriola, F.; Reñé, R.; et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. 2014, 29, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Dues, D.J.; Nguyen, A.P.T.; Becker, K.; Ma, J.; Moore, D.J. Hippocampal subfield vulnerability to alpha-synuclein pathology precedes neurodegeneration and cognitive dysfunction. NPJ Parkinsons Dis. 2023, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.H.; Cheshire, W.P. First symptoms in multiple system atrophy. Clin. Auton. Res. 2018, 28, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Borghammer, P.; Knudsen, K.; Ostergaard, K.; Danielsen, E.H.; Pavese, N.; Arveschoug, A.; Bluhme, H.; Bode, M.; Morsing, A. Combined DaT imaging and olfactory testing for differentiating parkinsonian disorders. Int. J. Clin. Pract. 2014, 68, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Holmes, C.; Sharabi, Y.; Wu, T. Survival in synucleinopathies: A prospective cohort study. Neurology 2015, 85, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Serradell, M.; Vilaseca, I.; Valldeoriola, F.; Salamero, M.; Molina, C.; Santamaria, J.; Tolosa, E. Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder. Parkinsonism Relat. Disord. 2013, 19, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hashimoto, M.; Yoshioka, M.; Murakami, M.; Kawasaki, K.; Urashima, M. The odor stick identification test for Japanese differentiates Parkinson’s disease from multiple system atrophy and progressive supra nuclear palsy. BMC Neurol. 2011, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Valbuena, I.; Visanji, N.P.; Kim, A.; Lau, H.H.C.; So, R.W.L.; Alshimemeri, S.; Gao, A.; Seidman, M.A.; Luquin, M.R.; Watts, J.C.; et al. Alpha-synuclein seeding shows a wide heterogeneity in multiple system atrophy. Transl. Neurodegener. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Karthik, K.; Holla, V.V.; Kamble, N.; Yadav, R.; Pal, P.K.; Mahale, R.R. Olfactory Bulb Volume, Olfactory Sulcus Depth in Parkinson’s Disease, Atypical Parkinsonism. Mov. Disord. Clin. Pract. 2023, 10, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, J.; Han, J.; Ji, Y. Clinical outcomes and cognitive impairments between progressive supranuclear palsy and multiple system atrophy. Brain Behav. 2022, 12, e2827. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Pechlaner, R.; Boesveldt, S.; Volc, D.; Pinter, B.; Reiter, E.; Müller, C.; Krismer, F.; Berendse, H.W.; van Hilten, J.J.; et al. Optimizing odor identification testing as quick and accurate diagnostic tool for Parkinson’s disease. Mov. Disord. 2016, 31, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Shill, H.A.; Zhang, N.; Driver-Dunckley, E.; Mehta, S.; Adler, C.H.; Beach, T.G. Olfaction in Neuropathologically Defined Progressive Supranuclear Palsy. Mov. Disord. 2021, 36, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Stutzbach, L.D.; Xie, S.X.; Naj, A.C.; Albin, R.; Gilman, S.; PSP Genetics Study Group; Lee, V.M.Y.; Trojanowski, J.Q.; Devlin, B.; Schellenberg, G.D. The unfolded protein response is activated in disease-affected brain regions in progressive supranuclear palsy and Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.E.; Kouri, N.; Lin, W.L.; Jack, C.R., Jr.; Dickson, D.W.; Vemuri, P. Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimer’s Res Ther. 2014, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, L.; Rawal, R.; Ghosh, S.; Pauly, C.; Pauly, L.; Hanff, A.-M.; Kolber, P.L.; Jónsdóttir, S.R.; Mcintyre, D.; Azaiz, K.; et al. Luxembourg Parkinson’s study -comprehensive baseline analysis of Parkinson’s disease and atypical parkinsonism. Front. Neurol. 2023, 14, 1330321. [Google Scholar] [CrossRef] [PubMed]
- Sengoku, R.; Matsushima, S.; Bono, K.; Sakutaa, K.; Yamazakia, M.; Miyagawaa, S.; Komatsua, T.; Mitsumuraa, H.; Konoa, Y.; Kamiyama, T.; et al. Olfactory function combined with morphology distinguishes Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Snowden, J.S.; Neary, D.; Coccia, M.; Provinciali, L.; Ralph, M.A.L. Distinct patterns of olfactory impairment in Alzheimer’s disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 2007, 45, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Pardini, M.; Huey, E.D.; Cavanagh, A.L.; Grafman, J. Olfactory function in corticobasal syndrome and frontotemporal dementia. Arch. Neurol. 2009, 66, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Mundinano, I.C.; Caballero, M.C.; Ordonez, C.; Hernandez, M.; DiCaudo, C.; Marcilla, I.; Erro, M.-E.; Tuñon, M.-T.; Luquin, M.-R. Increased dopaminergic cells and protein aggregates in the olfactory bulb of patients with neurodegenerative disorders. Acta Neuropathol. 2011, 122, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Magerova, H.; Vyhnalek, M.; Laczo, J.; Andel, R.; Rektorova, I.; Kadlecova, A.; Bojar, M.; Hort, J. Odor identification in frontotemporal lobar degeneration subtypes. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Krzyzanowska, E.; Koziorowski, D.; Szlufik, S.; Różański, D.; Noskowska, J.; Mianowicz, J.; Michno, A.; Królicki, L.; Friedman, A. Difficulties in the diagnosis of four repeats (4R) tauopathic parkinsonian syndromes. Neurol. Neurochir. Pol. 2018, 52, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Respondek, G.; Grimm, M.J.; Piot, I.; Arzberger, T.; Compta, Y.; Englund, E.; Ferguson, L.W.; Gelpi, E.; Roeber, S.; Giese, A.; et al. Validation of the movement disorder society criteria for the diagnosis of 4-repeat tauopathies. Mov. Disord. 2020, 35, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Marrero-Gonzalez, P.; Serradell, M.; Gaig, C.; Santamaria, J.; Vilaseca, I. Significance of hyposmia in isolated REM sleep behavior disorder. J. Neurol. 2021, 268, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Ferdenzi, C.; Roberts, S.C.; Schirmer, A.; Delplanque, S.; Cekic, S.; Porcherot, C.; Cayeux, I.; Sander, D.; Grandjean, D. Variability of affective responses to odors: Culture, gender, and olfactory knowledge. Chem. Senses 2013, 38, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Millar Vernetti, P.; Rossi, M.; Cerquetti, D.; Lloret, S.P.; Merello, M. Comparison of Olfactory Identification Patterns among Parkinson’s Disease Patients from Different Countries. Chem. Senses 2016, 41, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Sorokowski, P.; Hummel, T. Cross-Cultural Administration of an Odor Discrimination Test. Chemosens. Percept. 2014, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Przewodowska, D.; Marzec, W.; Madetko, N. Novel Therapies for Parkinsonian Syndromes–Recent Progress and Future Perspectives. Front. Mol. Neurosci. 2021, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Alster, P.; Madetko, N.; Friedman, A. Neutrophil-to-lymphocyte ratio (NLR) at boundaries of Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol. Neurochir. Pol. 2021, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, P.L. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Rydbirk, R.; Elfving, B.; Folke, J.; Pakkenberg, B.; Winge, K.; Brudek, T.; Aznar, S. Increased prefrontal cortex interleukin-2 protein levels and shift in the peripheral T cell population in progressive supranuclear palsy patients. Sci. Rep. 2019, 9, 7781. [Google Scholar] [CrossRef] [PubMed]
- Iravani, B.; Arshamian, A.; Lundstrom, J.N. Loss of olfactory sensitivity is an early and reliable marker for COVID-19. Chem. Senses 2022, 47, bjac022. [Google Scholar] [CrossRef] [PubMed]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell dysfunction: A biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Hummel, T.; Invitto, S.; Spinato, G.; Tomasoni, M.; Emanuelli, E.; Tofanelli, M.; Cavicchia, A.; Grill, V.; Vaira, L.A.; et al. Psychophysical assessment of olfactory and gustatory function in post-mild COVID-19 patients: A matched case-control study with 2-year follow-up. Int. Forum Allergy Rhinol. 2023, 13, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Denaro, C.A.; Haloush, Y.I.; Hsiao, S.Y.; Orgera, J.J.; Osorio, T.; Riggs, L.M.; Sassaman, J.W.; Williams, S.A.; Carlo, A.R.M.; Da Costa, R.T.; et al. COVID-19 and neurodegeneration: The mitochondrial connection. Aging Cell 2022, 21, e13727. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Morphological differences between the two major subtypes of multiple system atrophy with cognitive impairment. Parkinsonism Relat. Disord. 2023, 107, 105273. [Google Scholar] [CrossRef] [PubMed]
- Mahale, R.R.; Krishnan, S.; Divya, K.P.; Jisha, V. Subtypes of PSP and Prognosis: A Retrospective Analysis. Ann. Indian Acad. Neurol. 2021, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hanyu, H.; Sato, T.; Hirao, K.; Kanetaka, H.; Sakurai, H.; Iwamoto, T. Differences in clinical course between dementia with Lewy bodies and Alzheimer’s disease. Eur. J. Neurol. 2009, 16, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Kim, H.S.; Cheon, S.M.; Cha, J.-K.; Kim, S.-H.; Kim, J.W. Dementia with Lewy Bodies versus Alzheimer’s Disease and Parkinson’s Disease Dementia: A Comparison of Cognitive Profiles. J. Clin. Neurol. 2011, 7, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Červený, K.; Janoušková, K.; Vaněčková, K.; Zavázalová, Š.; Funda, D.; Astl, J.; Holy, R. Olfactory Evaluation in Clinical Medical Practice. J. Clin. Med. 2022, 11, 6628. [Google Scholar] [CrossRef]
- Nag, S.; Barnes, L.L.; Yu, L.; Buchman, A.S.; Bennett, D.A.; Schneider, J.A.; Wilson, R.S. Association of Lewy Bodies with Age-Related Clinical Characteristics in Black and White Decedents. Neurology 2021, 97, e825–e835. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Iranzo, A.; Hogl, B.; Frauscher, B.; Müller, C.; Santamaría, J.; Tolosa, E.; Serradell, M.; Mitterling, T.; Gschliesser, V.; et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology 2015, 84, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; You, H.; Liu, J.F.; Ni, D.-F.; Zhang, Z.-X.; Guan, J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef] [PubMed]
| Disease | Prevalence of OL | Severity of OL | Reference to OL in Diagnostic Criteria | |
|---|---|---|---|---|
| Synucleinopathies | DLB | +++ | profound OL | supportive feature |
| MSA | +/− | usually, normosmia | anosmia as an exclusion criterion | |
| Tauopathies | PSP | + | mild OL | - |
| CBD | +/− | single studies report hyposmia | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bochniak, K.; Soszyński, M.; Madetko-Alster, N.; Alster, P. Current Perspectives on Olfactory Loss in Atypical Parkinsonisms—A Review Article. Biomedicines 2024, 12, 2257. https://doi.org/10.3390/biomedicines12102257
Bochniak K, Soszyński M, Madetko-Alster N, Alster P. Current Perspectives on Olfactory Loss in Atypical Parkinsonisms—A Review Article. Biomedicines. 2024; 12(10):2257. https://doi.org/10.3390/biomedicines12102257
Chicago/Turabian StyleBochniak, Katarzyna, Mateusz Soszyński, Natalia Madetko-Alster, and Piotr Alster. 2024. "Current Perspectives on Olfactory Loss in Atypical Parkinsonisms—A Review Article" Biomedicines 12, no. 10: 2257. https://doi.org/10.3390/biomedicines12102257
APA StyleBochniak, K., Soszyński, M., Madetko-Alster, N., & Alster, P. (2024). Current Perspectives on Olfactory Loss in Atypical Parkinsonisms—A Review Article. Biomedicines, 12(10), 2257. https://doi.org/10.3390/biomedicines12102257






