An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes
Abstract
1. Introduction and General Aspects of Nephrotic Syndrome
2. Diseases Associated with Nephrotic Syndromes
2.1. Membranous Nephropathy
2.2. MN Classification and Pathophysiology
2.3. MN Diagnosis
2.4. MN Treatment
2.5. New Treatment Strategies
3. Renal Manifestations of Systemic Autoimmune Diseases
3.1. Nephrotic Syndrome in Systemic Autoimmune Disease
3.2. Pathogenesis of Membranous LN
3.3. Diagnostic Workup
3.4. Treatment
Prognostic Markers
3.5. Other Systemic Autoimmune Diseases
4. IgA Nephropathy
4.1. Pathophysiology
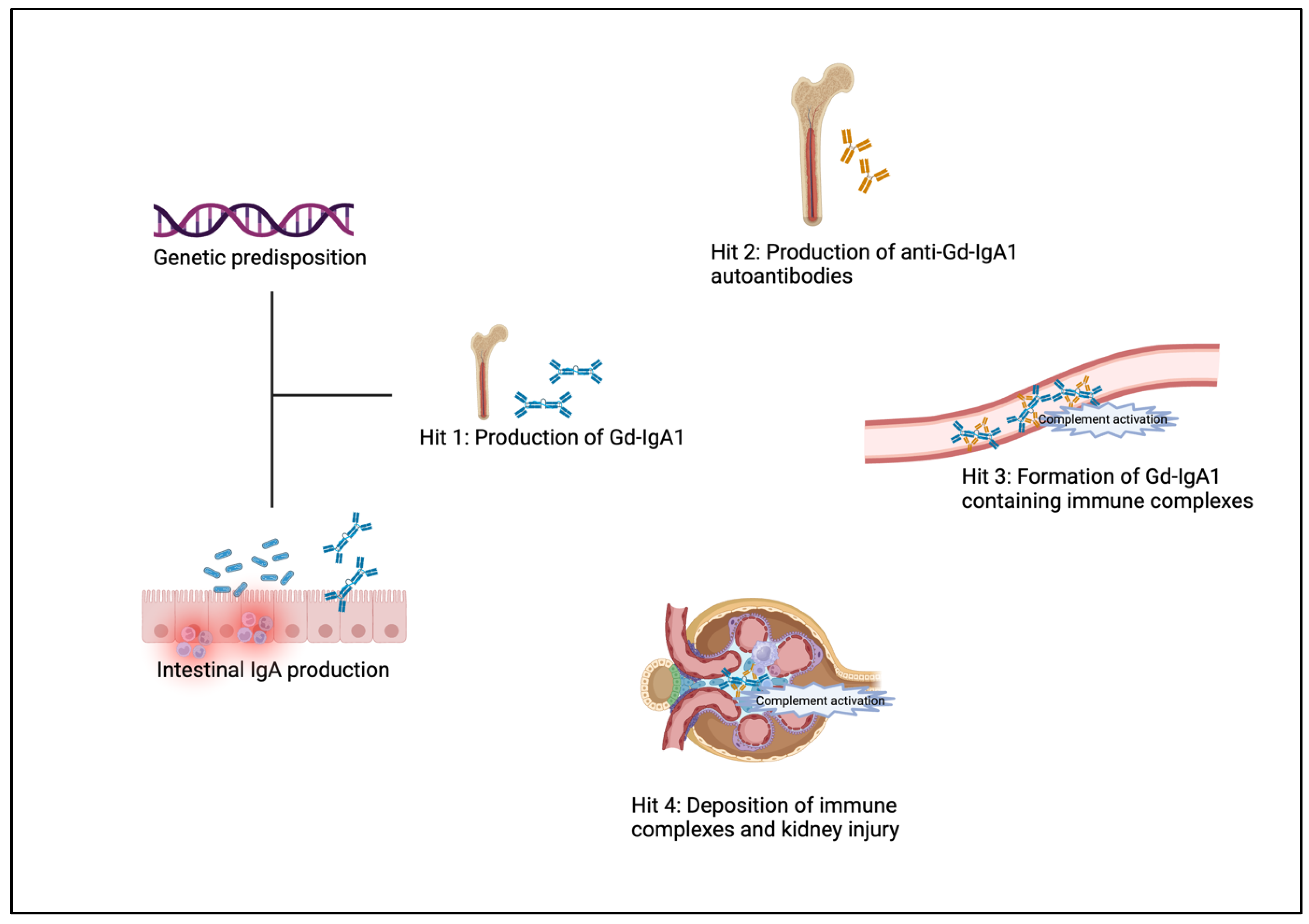
4.2. Pathology
4.3. Clinical Features
4.4. IgAN as a Cause of Nephrotic Syndrome
4.5. Diagnosis
4.6. Therapy
4.6.1. Supportive Care
4.6.2. Immunosuppressive Therapy
4.6.3. New Forms of Immunosuppressive Therapy
4.6.4. Investigational Agents
4.6.5. Therapy of Variant and Secondary Forms
4.7. Summary
5. Membranoproliferative Glomerulonephritis
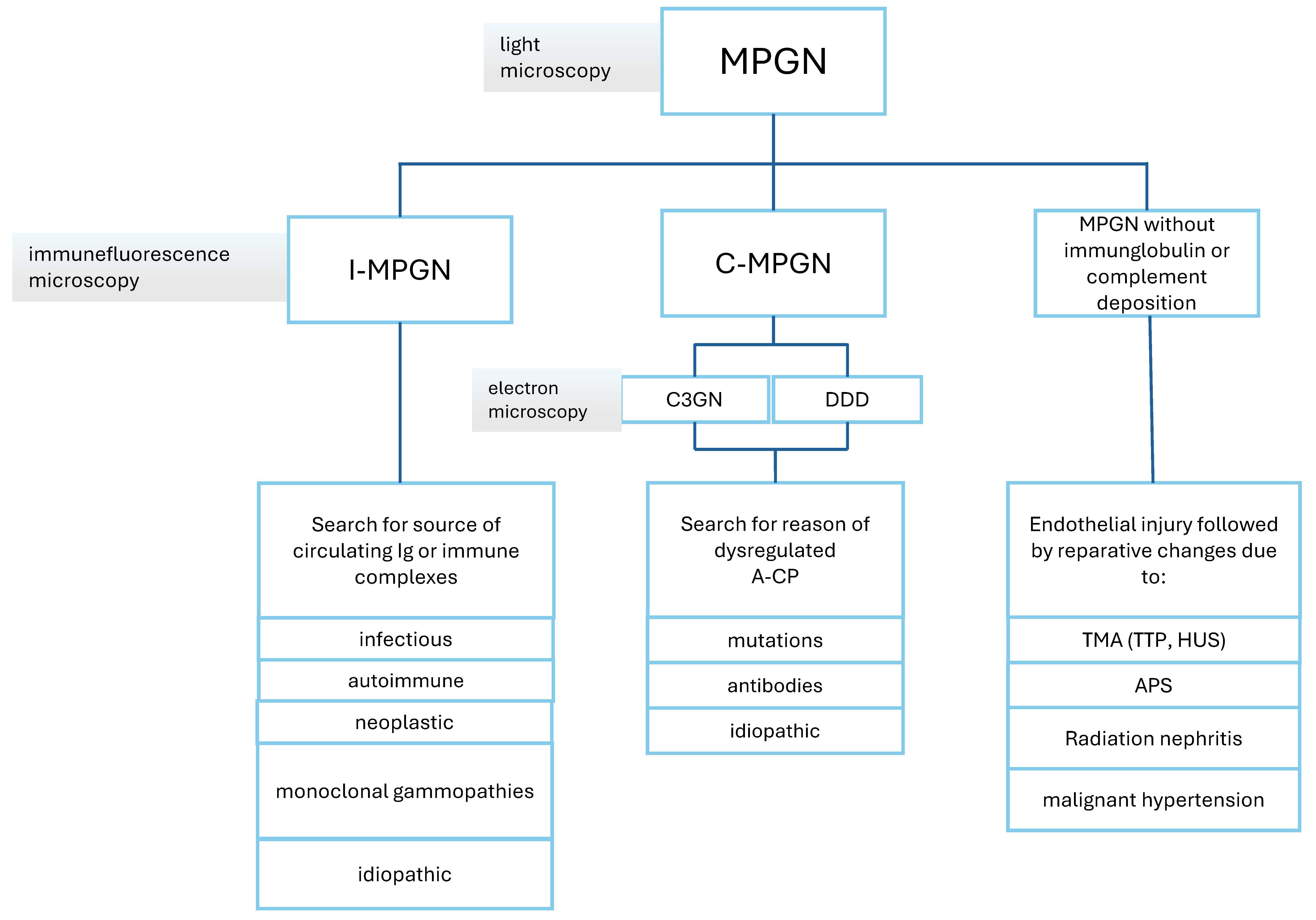
5.1. MPGN Classification and Pathophysiology
5.2. MPGN Diagnosis
5.3. MPGN Treatment
5.4. What’s New?
6. Podocytopathies: Focal Segmental Glomerulosclerosis and Minimal-Change Disease
6.1. Pathophysiology
6.1.1. Minimal Change Disease
6.1.2. Focal Segmental Glomerulosclerosis
6.2. Pathology
6.2.1. Minimal Change Disease
6.2.2. Focal Segmental Glomerulosclerosis
6.3. MCD and Primary FSGS—Different Manifestations of the Same Progressive Disease?
6.4. Clinical Presentation
6.4.1. Minimal Change Disease
6.4.2. Focal Segmental Glomerulosclerosis
6.5. Diagnostic
6.5.1. Minimal Change Disease
6.5.2. Focal Segmental Glomerulosclerosis
6.6. Therapy
6.6.1. Minimal Change Disease
6.6.2. Focal Segmental Glomerulosclerosis
Investigational Therapies in FSGS
6.7. What’s New?
6.8. Summary
7. Amyloidosis and Monoclonal Gammopathy of Renal Significance
7.1. MGRS Classification
7.2. MGRS Diagnosis
7.3. MGRS Treatment
7.4. Renal Amyloidosis as Cause of the Nephrotic Syndrome
| Name/Abbreviation | Etiology | Amyloidogenic Protein | Kidney Involvement |
|---|---|---|---|
| AL/AH | acquired | Immunoglobulin light or heavy chains | Yes |
| AA | acquired/hereditary | Serum amyloid A (SAA) | Yes |
| ATTRv | hereditary | Mutant transthyretin | Yes (PU in 1/3 of all patients, ESRD in 10%) [146] |
| ATTRwt | acquired | Wild-type transthyretin | Yes |
| ALect2 | unknown | Leucocyte chemotactic factor 2 (LECT2) | Yes |
| AGel | hereditary | Gelsolin | Yes |
| AApoAI | hereditary | Apolipoprotein AI | Yes |
| AApoAII | hereditary | Apolipoprotein AII | Yes |
| AApoAIV | acquired/hereditary | Apolipoprotein AIV | Yes |
| AApoCII | hereditary | Apolipoprotein CII | Yes |
| AApoCIII | hereditary | Apolipoprotein CIII | Yes |
| AFib | hereditary | Fibrinogen A alpha chain | Yes |
| Aß2M | iatrogenic | Beta-2 microglobulin | Yes |
| ALys | hereditary | Lysozyme | Yes |
| ACal | malignant | Calcitonin | Yes |
| Aß2m | hereditary, iatrogenic | Beta-2-microglobulin | no |
| ASom | malignant | Somatostatin | no |
| AGluc | malignant | Glucagon | no |
| AGLP1 | iatrogenic | Glucagon-like peptide analog | no |
| APTH | acquired, malignant | Parathyroid hormone | no |
| AIns | iatrogenic | Insulin | no |
| APro | acquired, malignant | Prolactin | no |
| AIAPP | acquired, malignant | Islet amyloid Amylin | no |
| AANP | acquired | Atrial natriuretic peptide | no |
| AKer | hereditary | Keratoepithelin | no |
| Abeta | acquired/hereditary | Amyloid precursor protein (APP) | no |
| APrP | acquired/hereditary | Prion protein (PRP) | no |
| ABri/ADan | hereditary | BRI gene product | no |
| ACys | hereditary | Cystatin C | no |
| ATMEM106B | acquired | Transmembrane 106B (TMEM106B) | no |
| ASPC | acquired | Lung surfactant protein | no |
| AIL1RAP | iatrogenic | Interleukin 1 receptor antagonist protein | no |
| LGMD D3 | hereditary | Human heterogeneous ribonucleoprotein D-like (hnRNPDL) | no |
| ANO5 | hereditary | Anoctamin5 | no |
| DYSF | hereditary | Dysferlin | no |
| ALac | acquired | Lactoferrin | no |
| AOAAP | malignant | Odontogenic ameloblast-associated protein | no |
| ASem1 | acquired | Semenogelin 1 | no |
| AMed | acquired | Lactadherin | no |
| ACor | acquired | Corneodesmosin | no |
| AEnf | iatrogenic | Enfuvirtide | no |
| ACatK | malignant | Cathepsin K | no |
| AEFEMP1 | acquired | Epithelial growth factor (EGF)-containing fibulin-like extracellular matrix protein 1 (EFEMP1) | no |
| Clinicaltrials.gov Identifier | Entity | Drug |
|---|---|---|
| NCT06397001 | AA | nL-SAA1-01 (antisense oligonucleotide) |
| NCT05199337 | AL | ZN-d5 |
| NCT05145816 | AL | Belantamab Mafodotin |
| NCT02312206 | AL | Birtamimab |
| NCT04973137 | AL | Birtamimab |
| NCT06342466 | AL | Pomalidomid |
| NCT05451771 | AL | Venetoclax |
| NCT03236792 | AL | Ixazomib |
| NCT05898646 | AL | Daratumumab maintenance |
| NCT01789242 | AL | Carfilzomib |
| NCT04298372 | AL | Lenalidomid |
| NCT05066607 | AL | Isatuximab |
| NCT03618537 | AL | Ixazomib maintenance |
| NCT06420167 | AL | Dapagliflozin |
| NCT05978661 | AL | FKC288 (CAR-T-cells) |
| NCT06158854 | AL | ABBV-383 (BCMA-directed) |
| NCT06292780 | AL | Linvoseltamab (BCMA-bispecific) |
| NCT05839626 | AL | SAR445514 (NKp46/CD16-based BCMA-targeted NK cell engager) |
| NCT05652335 | AL | JNJ-79635322, a tri-specific antibody (BCMA-GPRC5D-CD3) |
8. Diabetic Kidney Disease
9. Fibrillary Glomerulonephritis
10. Drug-Induced Nephrotic Syndrome
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hull, R.P.; Goldsmith, D.J.A. Nephrotic syndrome in adults. BMJ 2008, 336, 1185. [Google Scholar] [CrossRef] [PubMed]
- Frățilă, V.-G.; Lupușoru, G.; Sorohan, B.M.; Obrișcă, B.; Mocanu, V.; Lupușoru, M.; Ismail, G. Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 569. [Google Scholar] [CrossRef]
- Koomans, H.A.; Kortlandt, W.; Geers, A.B.; Mees, E.J.D. Lowered Protein Content of Tissue Fluid in Patients with the Nephrotic Syndrome: Observations during Disease and Recovery. Nephron 1985, 40, 391–395. [Google Scholar] [CrossRef]
- Gupta, S.; Pepper, R.J.; Ashman, N.; Walsh, S.B. Nephrotic Syndrome: Oedema Formation and Its Treatment With Diuretics. Front. Physiol. 2019, 9, 1868. [Google Scholar] [CrossRef] [PubMed]
- Siddall, E.C.; Radhakrishnan, J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012, 82, 635–642. [Google Scholar] [CrossRef]
- Kerlin, B.A.; Ayoob, R.; Smoyer, W.E. Epidemiology and Pathophysiology of Nephrotic Syndrome–Associated Thromboembolic Disease. Clin. J. Am. Soc. Nephrol. 2012, 7, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Venous Thrombosis in the Nephrotic Syndrome. N. Engl. J. Med. 2013, 368, 956–958. [Google Scholar] [CrossRef]
- Gordon-Cappitelli, J.; Choi, M.J. Prophylactic Anticoagulation in Adult Patients with Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2019, 15, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar] [CrossRef]
- Go, A.S.; Tan, T.C.; Chertow, G.M.; Ordonez, J.D.; Fan, D.; Law, D.; Yankulin, L.; Wojcicki, J.M.; Zheng, S.; Chen, K.K.; et al. Primary Nephrotic Syndrome and Risks of ESKD, Cardiovascular Events, and Death: The Kaiser Permanente Nephrotic Syndrome Study. J. Am. Soc. Nephrol. 2021, 32, 2303–2314. [Google Scholar] [CrossRef]
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-Type Phospholipase A2 Receptor as Target Antigen in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Tomas, N.M.; Beck, L.H., Jr.; Meyer-Schwesinger, C.; Seitz-Polski, B.; Ma, H.; Zahner, G.; Dolla, G.; Hoxha, E.; Helmchen, U.; Dabert-Gay, A.S.; et al. Thrombospondin Type-1 Domain-Containing 7A in Idiopathic Membranous Nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C.; Tomas, N.M.; Dehde, S.; Seifert, L.; Hermans-Borgmeyer, I.; Wiech, T.; Koch-Nolte, F.; Huber, T.B.; Zahner, G. A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int. 2020, 97, 913–919. [Google Scholar] [CrossRef]
- Tomas, N.M.; Hoxha, E.; Reinicke, A.T.; Fester, L.; Helmchen, U.; Gerth, J.; Bachmann, F.; Budde, K.; Koch-Nolte, F.; Zahner, G.; et al. Autoantibodies against thrombospondin type 1 domain–containing 7A induce membranous nephropathy. J. Clin. Investig. 2016, 126, 2519–2532. [Google Scholar] [CrossRef]
- Sethi, S.; Beck, L.H.; Glassock, R.J.; Haas, M.; Vriese, A.S.D.; Caza, T.N.; Hoxha, E.; Lambeau, G.; Tomas, N.M.; Madden, B.; et al. Mayo Clinic consensus report on membranous nephropathy: Proposal for a novel classification. Kidney Int. 2023, 104, 1092–1102. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, H.; Tang, D. Mechanisms of Primary Membranous Nephropathy. Biomolecules 2021, 11, 513. [Google Scholar] [CrossRef] [PubMed]
- Fervenza, F.C.; Sethi, S.; Specks, U. Idiopathic Membranous Nephropathy: Diagnosis and Treatment. Clin. J. Am. Soc. Nephrol. 2008, 3, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Ponticelli, C. Secondary Membranous Nephropathy. A Narrative Review. Front. Med. 2020, 7, 611317. [Google Scholar] [CrossRef]
- Bobart, S.A.; Vriese, A.S.D.; Pawar, A.S.; Zand, L.; Sethi, S.; Giesen, C.; Lieske, J.C.; Fervenza, F.C. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019, 95, 429–438. [Google Scholar] [CrossRef]
- Ronco, P.; Plaisier, E.; Debiec, H. The role of PLA2R antibody monitoring: What we know and what we do not know. Nephrol. Dial. Transplant. 2021, 38, 826–833. [Google Scholar] [CrossRef]
- Sethi, S.; Fervenza, F.C. Membranous nephropathy—Diagnosis and identification of target antigens. Nephrol. Dial. Transplant. 2023, 39, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Kalay, Z.; Sahin, O.E.; Copur, S.; Danacı, S.; Ortiz, A.; Yau, K.; Cherney, D.Z.; Kanbay, M. SGLT-2 inhibitors in nephrotic-range proteinuria: Emerging clinical evidence. Clin. Kidney J. 2022, 16, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Heerspink, H.L.; Mark, P.B.; Dwyer, J.P.; Nowicki, M.; Wheeler, D.C.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; Langkilde, A.M.; et al. Effects of Dapagliflozin in Patients with Membranous Nephropathy. Glomerular Dis. 2024, 4, 137–145. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Dahan, K.; Debiec, H.; Plaisier, E.; Cachanado, M.; Rousseau, A.; Wakselman, L.; Michel, P.-A.; Mihout, F.; Dussol, B.; Matignon, M.; et al. Rituximab for Severe Membranous Nephropathy: A 6-Month Trial with Extended Follow-Up. J. Am. Soc. Nephrol. 2017, 28, 348–358. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Delbarba, E.; Santoro, D.; Gesualdo, L.; Pani, A.; Dallera, N.; Mani, L.-Y.; Santostefano, M.; Feriozzi, S.; Quaglia, M.; et al. Rituximab or Cyclophosphamide in the Treatment of Membranous Nephropathy: The RI-CYCLO Randomized Trial. J. Am. Soc. Nephrol. 2021, 32, 972–982. [Google Scholar] [CrossRef]
- Fernández-Juárez, G.; Rojas-Rivera, J.; van de Logt, A.-E.; Justino, J.; Sevillano, A.; Caravaca-Fontán, F.; Ávila, A.; Rabasco, C.; Cabello, V.; Varela, A.; et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021, 99, 986–998. [Google Scholar] [CrossRef]
- Sethi, S.; Kumar, S.; Lim, K.; Jordan, S.C. Obinutuzumab is Effective for the Treatment of Refractory Membranous Nephropathy. Kidney Int. Rep. 2020, 5, 1515–1518. [Google Scholar] [CrossRef]
- Geara, A.S.; Bhoj, V.; Hogan, J.J. Bortezomib Treatment for Refractory PLA2R-Positive Membranous Nephropathy. Glomerular Dis. 2021, 1, 40–43. [Google Scholar] [CrossRef]
- Vink, C.H.; van Cranenbroek, B.; van der Heijden, J.W.; Koenen, H.P.J.M.; Wetzels, J.F.M. Daratumumab for multidrug-resistant phospholipase-A2 receptor–related membranous nephropathy. Kidney Int. 2022, 101, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Riecken, K.; Zahner, G.; Hambach, J.; Hagenstein, J.; Dubberke, G.; Huber, T.B.; Koch-Nolte, F.; Fehse, B.; Tomas, N.M. An antigen-specific chimeric autoantibody receptor (CAAR) NK cell strategy for the elimination of anti-PLA2R1 and anti-THSD7A antibody-secreting cells. Kidney Int. 2024, 105, 886–889. [Google Scholar] [CrossRef]
- Mahajan, A.; Amelio, J.; Gairy, K.; Kaur, G.; Levy, R.A.; Roth, D.; Bass, D. Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: A pragmatic review mapping disease severity and progression. Lupus 2020, 29, 1011–1020. [Google Scholar] [CrossRef]
- Hanly, J.G.; O’Keeffe, A.G.; Su, L.; Urowitz, M.B.; Romero-Diaz, J.; Gordon, C.; Bae, S.-C.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J.; et al. The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatology 2016, 55, 252–262. [Google Scholar] [CrossRef]
- Anders, H.-J.; Saxena, R.; Zhao, M.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.N.; Hassen, S.I.; Kenan, D.J.; Storey, A.; Arthur, J.M.; Herzog, C.; Edmondson, R.D.; Bourne, T.D.; Beck, L.H.; Larsen, C.P. Transforming Growth Factor Beta Receptor 3 (TGFBR3)–Associated Membranous Nephropathy. Kidney360 2021, 2, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Caza, T.N.; Hassen, S.I.; Kuperman, M.; Sharma, S.G.; Dvanajscak, Z.; Arthur, J.; Edmondson, R.; Storey, A.; Herzog, C.; Kenan, D.J.; et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 2021, 100, 171–181. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, M.C.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P. Exostosin 1/Exostosin 2–Associated Membranous Nephropathy. J. Am. Soc. Nephrol. 2019, 30, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Rosa, M.D.; Rocha, A.S.; Rosa, G.D.; Dubinsky, D.; Almaani, S.J.; Rovin, B.H. Low-Grade Proteinuria Does Not Exclude Significant Kidney Injury in Lupus Nephritis. Kidney Int. Rep. 2020, 5, 1066–1068. [Google Scholar] [CrossRef]
- Chedid, A.; Rossi, G.M.; Peyronel, F.; Menez, S.; Atta, M.G.; Bagnasco, S.M.; Arend, L.J.; Rosenberg, A.Z.; Fine, D.M. Low-Level Proteinuria in Systemic Lupus Erythematosus. Kidney Int. Rep. 2020, 5, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Huong, T.D.L.; Papo, T.; Beaufils, H.; Wechsler, B.; Blétry, O.; Baumelou, A.; Godeau, P.; Piette, J. Renal Involvement in Systemic Lupus Erythematosus: A Study of 180 Patients from a Single Center. Medicine 1999, 78, 148–166. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Lupus Nephritis Work Group; Rovin, B.H.; Ayoub, I.M.; Chan, T.M.; Liu, Z.-H.; Mejía-Vilet, J.M.; Floege, J. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024, 105, S1–S69. [Google Scholar] [CrossRef]
- Dall’Era, M.; Stone, D.; Levesque, V.; Cisternas, M.; Wofsy, D. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res. 2011, 63, 351–357. [Google Scholar] [CrossRef]
- Mok, C.C.; Teng, Y.K.O.; Saxena, R.; Tanaka, Y. Treatment of lupus nephritis: Consensus, evidence and perspectives. Nat. Rev. Rheumatol. 2023, 19, 227–238. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, H.; Ying, M.; Xie, Z.; Li, X.; Wang, H.; Zhao, J.; Lin, C.; Wang, Y.; Feng, S.; et al. Calcineurin inhibitors cyclosporin A and tacrolimus protect against podocyte injury induced by puromycin aminonucleoside in rodent models. Sci. Rep. 2016, 6, 32087. [Google Scholar] [CrossRef]
- Cattran, D.C.; Greenwood, C.; Ritchie, S.; Bernstein, K.; Churchill, D.N.; Clark, W.F.; Morrin, P.A.; Lavoie, S. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Kidney Int. 1995, 47, 1130–1135. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Liu, Z.; Xing, C.; Fu, P.; Ni, Z.; Chen, J.; Lin, H.; Liu, F.; He, Y.; et al. Multitarget Therapy for Induction Treatment of Lupus Nephritis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 18–26. [Google Scholar] [CrossRef]
- Frangou, E.; Anders, H.-J.; Bajema, I.M.; Teng, Y.K.O.; Malvar, A.; Rovin, B.H.; Kronbichler, A. Immunosuppression Withdrawal in Patients with Lupus Nephritis. J. Am. Soc. Nephrol. 2024, 35, 955–958. [Google Scholar] [CrossRef]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Ginzler, E.M.; Gibson, K.; Satirapoj, B.; Santillán, A.E.Z.; Levchenko, O.; Navarra, S.; Atsumi, T.; Yasuda, S.; Chavez-Perez, N.N.; et al. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis Rheumatol. 2024, 76, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Furie, R.; Teng, Y.K.O.; Contreras, G.; Malvar, A.; Yu, X.; Ji, B.; Green, Y.; Gonzalez-Rivera, T.; Bass, D.; et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2022, 101, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Chang, A.; Brandt, D.; Guttikonda, R.; Utset, T.O.; Clark, M.R. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 2011, 63, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.R.; Trotter, K.; Chang, A. The Pathogenesis and Therapeutic Implications of Tubulointerstitial Inflammation in Human Lupus Nephritis. Semin. Nephrol. 2015, 35, 455–464. [Google Scholar] [CrossRef]
- Tamirou, F.; Lauwerys, B.R.; Dall’Era, M.; Mackay, M.; Rovin, B.; Cervera, R.; Houssiau, F.A. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: Data from the MAINTAIN Nephritis Trial. Lupus Sci. Med. 2015, 2, e000123. [Google Scholar] [CrossRef]
- Korbet, S.M.; Lewis, E.J.; Collaborative Study Group. Severe lupus nephritis: The predictive value of a ≥50% reduction in proteinuria at 6 months. Nephrol. Dial. Transplant. 2013, 28, 2313–2318. [Google Scholar] [CrossRef]
- Bertolo, M.; Baumgart, S.; Durek, P.; Peddinghaus, A.; Mei, H.; Rose, T.; Enghard, P.; Grützkau, A. Deep Phenotyping of Urinary Leukocytes by Mass Cytometry Reveals a Leukocyte Signature for Early and Non-Invasive Prediction of Response to Treatment in Active Lupus Nephritis. Front. Immunol. 2020, 11, 256. [Google Scholar] [CrossRef]
- Parikh, S.V.; Malvar, A.; Song, H.; Shapiro, J.; Mejia-Vilet, J.M.; Ayoub, I.; Almaani, S.; Madhavan, S.; Alberton, V.; Besso, C.; et al. Molecular profiling of kidney compartments from serial biopsies differentiate treatment responders from non-responders in lupus nephritis. Kidney Int. 2022, 102, 845–865. [Google Scholar] [CrossRef]
- Santoro, D.; Vadalà, C.; Siligato, R.; Buemi, M.; Benvenga, S. Autoimmune Thyroiditis and Glomerulopathies. Front. Endocrinol. 2017, 8, 119. [Google Scholar] [CrossRef]
- Sasaki, K.; Yasuda, K.; Nakanishi, K.; Rakugi, H.; Isaka, Y.; Yamato, M. Membranous nephropathy secondary to Graves’ disease with deposits of thyroid peroxidase in an adult. CEN Case Rep. 2014, 3, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Dalivand, M.M.; Hadjiabbasi, A.; Ramezanzadeh, E.; Habibzadeh, S.M.; Goudarzi, K.; Shahriarirad, R.; Zayeni, H. Nephrotic syndrome due to focal segmental glomerulosclerosis complicating scleroderma: A case report. J. Med. Case Rep. 2024, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Hirohata, S.; Ohse, T.; Takeuchi, A.; Hashimoto, T. Systemic sclerosis complicated with nephrotic syndrome: Relevance with antiribosomal P antibody. Rheumatol. Int. 2001, 21, 40–43. [Google Scholar] [CrossRef]
- Stehlé, T.; Joly, D.; Vanhille, P.; Boffa, J.-J.; Rémy, P.; Mesnard, L.; Hoffmann, M.; Grimbert, P.; Choukroun, G.; Vrtovsnik, F.; et al. Clinicopathological study of glomerular diseases associated with sarcoidosis: A multicenter study. Orphanet J. Rare Dis. 2013, 8, 65. [Google Scholar] [CrossRef]
- Ion, O.; Obrișcă, B.; Ismail, G.; Sorohan, B.; Bălănică, S.; Mircescu, G.; Sinescu, I. Kidney Involvement in Hypocomplementemic Urticarial Vasculitis Syndrome—A Case-Based Review. J. Clin. Med. 2020, 9, 2131. [Google Scholar] [CrossRef]
- Kurihara, S.; Harada, M.; Ichikawa, T.; Ehara, T.; Kobayashi, M. Nephrotic Syndrome due to Focal Segmental Glomerulosclerosis Complicating Sjögren’s Syndrome: A Case Report and Literature Review. Case Rep. Rheumatol. 2019, 2019, 1749795. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, R.; Hara, S.; Kawahara, H.; Ito, K.; Mizushima, I.; Hirata, M.; Nagata, M.; Kawano, M. Glomerulonephritis with severe nephrotic syndrome induced by immune complexes composed of galactose-deficient IgA1 in primary Sjögren’s syndrome: A case report. BMC Nephrol. 2021, 22, 108. [Google Scholar] [CrossRef]
- Gentile, M.; Sanchez-Russo, L.; Riella, L.V.; Verlato, A.; Manrique, J.; Granata, S.; Fiaccadori, E.; Pesce, F.; Zaza, G.; Cravedi, P. Immune abnormalities in IgA nephropathy. Clin. Kidney J. 2023, 16, 1059–1070. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.R.; Cook, H.T.; Pickering, M.C. Complement activation in IgA nephropathy. Semin. Immunopathol. 2021, 43, 679–690. [Google Scholar] [CrossRef]
- Floege, J.; Daha, M.R. IgA nephropathy: New insights into the role of complement. Kidney Int. 2018, 94, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Caillard, S.; Frémeaux-Bacchi, V. The complement system in IgAN: Mechanistic context for therapeutic opportunities. Nephrol. Dial. Transplant. 2023, 38, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Caravaca-Fontán, F.; Gutiérrez, E.; Sevillano, Á.M.; Praga, M. Targeting complement in IgA nephropathy. Clin. Kidney J. 2023, 16, ii28–ii39. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2021, 361, 176–194. [Google Scholar] [CrossRef]
- Suzuki, H.; Kiryluk, K.; Novak, J.; Moldoveanu, Z.; Herr, A.B.; Renfrow, M.B.; Wyatt, R.J.; Scolari, F.; Mestecky, J.; Gharavi, A.G.; et al. The Pathophysiology of IgA Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Pattrapornpisut, P.; Avila-Casado, C.; Reich, H.N. IgA Nephropathy: Core Curriculum 2021. Am. J. Kidney Dis. 2021, 78, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Knoppova, B.; Reily, C.; Maillard, N.; Rizk, D.V.; Moldoveanu, Z.; Mestecky, J.; Raska, M.; Renfrow, M.B.; Julian, B.A.; Novak, J. The Origin and Activities of IgA1-Containing Immune Complexes in IgA Nephropathy. Front. Immunol. 2016, 7, 117. [Google Scholar] [CrossRef]
- Gleeson, P.J.; O’Shaughnessy, M.M.; Barratt, J. IgA nephropathy in adults—Treatment standard. Nephrol. Dial. Transplant. 2023, 38, 2464–2473. [Google Scholar] [CrossRef]
- Hassler, J.R. IgA nephropathy: A brief review. Semin. Diagn. Pathol. 2020, 37, 143–147. [Google Scholar] [CrossRef]
- Herlitz, L.C.; Bomback, A.S.; Stokes, M.B.; Radhakrishnan, J.; D’Agati, V.D.; Markowitz, G.S. IgA Nephropathy with Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 1033–1039. [Google Scholar] [CrossRef]
- Li, H.; Lu, W.; Li, H.; Liu, X.; Zhang, X.; Xie, L.; Lan, P.; Yu, X.; Dai, Y.; Xie, X.; et al. Immune Characteristics of IgA Nephropathy with Minimal Change Disease. Front. Pharmacol. 2021, 12, 793511. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-H.; Park, S.-H.; Choi, S.-K.; Jung, S.W.; Jeong, K.H.; Kim, Y.-G.; Moon, J.-Y.; Lim, S.-J.; Sung, J.-Y.; Jhee, J.H.; et al. Characterization of IgA Deposition in the Kidney of Patients with IgA Nephropathy and Minimal Change Disease. J. Clin. Med. 2020, 9, 2619. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Er, L.; Espino-Hernandez, G.; Kim, S.J.; et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern. Med. 2019, 179, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.-H.; Suzuki, Y.; Matsuzaki, K.; Er, L.; Reich, H.N.; Barratt, J.; Cattran, D.C.; et al. Application of the International IgA Nephropathy Prediction Tool one or two years post-biopsy. Kidney Int. 2022, 102, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Siwy, J.; Wendt, R.; Lipphardt, M.; Koziolek, M.J.; Maixnerova, D.; Peters, B.; Kerschbaum, J.; Leierer, J.; Neprasova, M.; et al. Urine proteomics for prediction of disease progression in patients with IgA nephropathy. Nephrol. Dial. Transplant. 2022, 37, 42–52. [Google Scholar] [CrossRef]
- Pitcher, D.; Braddon, F.; Hendry, B.; Mercer, A.; Osmaston, K.; Saleem, M.A.; Steenkamp, R.; Wong, K.; Turner, A.N.; Wang, K.; et al. Long-Term Outcomes in IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2023, 18, 727–738. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- Judge, P.; Staplin, N.; Mayne, K.; Wanner, C.; Green, J.; Hauske, S.; Emberson, J.; Preiss, D.; Ng, S.; Roddick, A.; et al. Impact of primary kidney disease on the effects of empagliflozin in patients with chronic kidney disease: Secondary analyses of the EMPA-KIDNEY trial. Lancet Diabetes Endocrinol. 2024, 12, 51–60. [Google Scholar] [CrossRef]
- Rovin, B.H.; Barratt, J.; Heerspink, H.J.L.; Alpers, C.E.; Bieler, S.; Chae, D.-W.; Diva, U.A.; Floege, J.; Gesualdo, L.; Inrig, J.K.; et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 2023, 402, 2077–2090. [Google Scholar] [CrossRef]
- Rauen, T.; Eitner, F.; Fitzner, C.; Sommerer, C.; Zeier, M.; Otte, B.; Panzer, U.; Peters, H.; Benck, U.; Mertens, P.R.; et al. Intensive Supportive Care plus Immunosuppression in IgA Nephropathy. N. Engl J Medicine 2015, 373, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wong, M.G.; Hladunewich, M.A.; Jha, V.; Hooi, L.S.; Monaghan, H.; Zhao, M.; Barbour, S.; Jardine, M.J.; Reich, H.N.; et al. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy. JAMA 2022, 327, 1888–1898. [Google Scholar] [CrossRef] [PubMed]
- Lafayette, R.; Kristensen, J.; Stone, A.; Floege, J.; Tesař, V.; Trimarchi, H.; Zhang, H.; Eren, N.; Paliege, A.; Reich, H.N.; et al. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet 2023, 402, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-H.; Le, W.-B.; Chen, N.; Wang, W.-M.; Liu, Z.-S.; Liu, D.; Chen, J.-H.; Tian, J.; Fu, P.; Hu, Z.-X.; et al. Mycophenolate Mofetil Combined With Prednisone Versus Full-Dose Prednisone in IgA Nephropathy With Active Proliferative Lesions: A Randomized Controlled Trial. Am. J. Kidney Dis. 2017, 69, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-J.; Yang, Y.; Shi, S.-F.; Bao, Y.-F.; Yang, C.; Zhu, S.-N.; Sui, G.-L.; Chen, Y.-Q.; Lv, J.-C.; Zhang, H. Effects of Hydroxychloroquine on Proteinuria in IgA Nephropathy: A Randomized Controlled Trial. Am. J. Kidney Dis. 2019, 74, 15–22. [Google Scholar] [CrossRef]
- Zhang, H.; Rizk, D.V.; Perkovic, V.; Maes, B.; Kashihara, N.; Rovin, B.; Trimarchi, H.; Sprangers, B.; Meier, M.; Kollins, D.; et al. Results of a randomized double-blind placebo-controlled Phase 2 study propose iptacopan as an alternative complement pathway inhibitor for IgA nephropathy. Kidney Int. 2024, 105, 189–199. [Google Scholar] [CrossRef]
- Mathur, M.; Barratt, J.; Chacko, B.; Chan, T.M.; Kooienga, L.; Oh, K.-H.; Sahay, M.; Suzuki, Y.; Wong, M.G.; Yarbrough, J.; et al. A Phase 2 Trial of Sibeprenlimab in Patients with IgA Nephropathy. N. Engl. J. Med. 2023, 390, 20–31. [Google Scholar] [CrossRef]
- Bomback, A.S.; Appel, G.B. Pathogenesis of the C3 glomerulopathies and reclassification of MPGN. Nat. Rev. Nephrol. 2012, 8, 634–642. [Google Scholar] [CrossRef]
- Alexander, M.P.; Sethi, S. Glomerulonephritis; Springer: Cham, Switzerland, 2019; pp. 403–419. [Google Scholar] [CrossRef]
- Alchi, B.; Jayne, D. Membranoproliferative glomerulonephritis. Pediatr. Nephrol. 2010, 25, 1409–1418. [Google Scholar] [CrossRef]
- Sethi, S.; Fervenza, F.C.; Zhang, Y.; Zand, L.; Vrana, J.A.; Nasr, S.H.; Theis, J.D.; Dogan, A.; Smith, R.J.H. C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012, 82, 465–473. [Google Scholar] [CrossRef]
- Xiao, X.; Pickering, M.; Smith, R. C3 Glomerulopathy: The Genetic and Clinical Findings in Dense Deposit Disease and C3 Glomerulonephritis. Semin. Thromb. Hemost. 2014, 40, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Remuzzi, G. C3G and Ig-MPGN—Treatment standard. Nephrol. Dial. Transplant. 2023, 39, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Stea, E.D.; D’Ettorre, G.; Mitrotti, A.; Gesualdo, L. The complement system in the pathogenesis and progression of kidney diseases: What doesn’t kill you makes you older. Eur. J. Intern. Med. 2024, 124, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Gamez, J.D.; Vrana, J.A.; Theis, J.D.; Bergen, H.R.; Zipfel, P.F.; Dogan, A.; Smith, R.J.H. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009, 75, 952–960. [Google Scholar] [CrossRef]
- Sethi, S.; Fervenza, F.C. Membranoproliferative Glomerulonephritis—A New Look at an Old Entity. N. Engl. J. Med. 2012, 366, 1119–1131. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Sethi, S.; Glassock, R.J. Idiopathic membranoproliferative glomerulonephritis: Does it exist? Nephrol. Dial. Transplant. 2012, 27, 4288–4294. [Google Scholar] [CrossRef]
- Pickering, M.C.; D’Agati, V.D.; Nester, C.M.; Smith, R.J.; Haas, M.; Appel, G.B.; Alpers, C.E.; Bajema, I.M.; Bedrosian, C.; Braun, M.; et al. C3 glomerulopathy: Consensus report. Kidney Int. 2013, 84, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Marinozzi, M.-C.; Chauvet, S.; Quintrec, M.L.; Mignotet, M.; Petitprez, F.; Legendre, C.; Cailliez, M.; Deschenes, G.; Fischbach, M.; Karras, A.; et al. C5 nephritic factors drive the biological phenotype of C3 glomerulopathies. Kidney Int. 2017, 92, 1232–1241. [Google Scholar] [CrossRef]
- Ohi, H.; Watanabe, S.; Fujita, T.; Seki, M.; Hatano, M. Detection of C3bBb-stabilizing activity (C3 nephritic factor) in the serum from patients with membranoproliferative glomerulonephritis. J. Immunol. Methods 1990, 131, 71–76. [Google Scholar] [CrossRef]
- Chen, Q.; Müller, D.; Rudolph, B.; Hartmann, A.; Kuwertz-Bröking, E.; Wu, K.; Kirschfink, M.; Skerka, C.; Zipfel, P.F. Combined C3b and Factor B Autoantibodies and MPGN Type II. N. Engl. J. Med. 2011, 365, 2340–2342. [Google Scholar] [CrossRef]
- Józsi, M.; Reuter, S.; Nozal, P.; López-Trascasa, M.; Sánchez-Corral, P.; Prohászka, Z.; Uzonyi, B. Autoantibodies to complement components in C3 glomerulopathy and atypical hemolytic uremic syndrome. Immunol. Lett. 2014, 160, 163–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Somers, M.; Kertesz, S.; Rosen, S.; Herrin, J.; Colvin, R.; de Carreta, N.P.; Kim, M. Non-nephrotic children with membranoproliferative glomerulonephritis: Are steroids indicated? Pediatr. Nephrol. 1995, 9, 140–144. [Google Scholar] [CrossRef]
- Licht, C.; Heinen, S.; Józsi, M.; Löschmann, I.; Saunders, R.E.; Perkins, S.J.; Waldherr, R.; Skerka, C.; Kirschfink, M.; Hoppe, B.; et al. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int. 2006, 70, 42–50. [Google Scholar] [CrossRef]
- Bomback, A.S.; Smith, R.J.; Barile, G.R.; Zhang, Y.; Heher, E.C.; Herlitz, L.; Stokes, M.B.; Markowitz, G.S.; D’Agati, V.D.; Canetta, P.A.; et al. Eculizumab for Dense Deposit Disease and C3 Glomerulonephritis. Clin. J. Am. Soc. Nephrol. 2012, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Quintrec, M.L.; Lapeyraque, A.-L.; Lionet, A.; Sellier-Leclerc, A.-L.; Delmas, Y.; Baudouin, V.; Daugas, E.; Decramer, S.; Tricot, L.; Cailliez, M.; et al. Patterns of Clinical Response to Eculizumab in Patients With C3 Glomerulopathy. Am. J. Kidney Dis. 2018, 72, 84–92. [Google Scholar] [CrossRef]
- Vivarelli, M.; Bomback, A.S.; Meier, M.; Wang, Y.; Webb, N.J.A.; Veldandi, U.K.; Smith, R.J.H.; Kavanagh, D. Iptacopan in Idiopathic Immune Complex–Mediated Membranoproliferative Glomerulonephritis: Protocol of the APPARENT Multicenter, Randomized Phase 3 Study. Kidney Int. Rep. 2024, 9, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Madden, B.; Singh, R.D.; Haas, M.; Palma, L.M.P.; Sharma, A.; Vargas, M.J.; Gross, L.; Negron, V.; Nate, T.; Charlesworth, M.C.; et al. Apolipoprotein E is enriched in dense deposits and is a marker for dense deposit disease in C3 glomerulopathy. Kidney Int. 2024, 105, 1077–1087. [Google Scholar] [CrossRef]
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Prim. 2020, 6, 68. [Google Scholar] [CrossRef]
- Watts, A.J.B.; Keller, K.H.; Lerner, G.; Rosales, I.; Collins, A.B.; Sekulic, M.; Waikar, S.S.; Chandraker, A.; Riella, L.V.; Alexander, M.P.; et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J. Am. Soc. Nephrol. 2022, 33, 238–252. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Kopp, J.B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017, 12, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Vriese, A.S.D.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef]
- D’Agati, V. Pathologic classification of focal segmental glomerulosclerosis. Semin. Nephrol. 2003, 23, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; Deegens, J.K.; Smeets, B.; Moeller, M.J.; Wetzels, J.F. Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat. Rev. Nephrol. 2016, 12, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Catanese, L.; Siwy, J.; Wendt, R.; Amann, K.; Beige, J.; Hendry, B.; Mischak, H.; Mullen, W.; Paterson, I.; Schiffer, M.; et al. Differentiating primary and secondary FSGS using non-invasive urine biomarkers. Clin. Kidney J. 2023, 17, sfad296. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.R.; Lawrence, C.; Condon, M.; Sood, B.; Warwicker, P.; Brown, H.; Pattison, J.; Bhandari, S.; Barratt, J.; Turner, N.; et al. Randomized, Controlled Trial of Tacrolimus and Prednisolone Monotherapy for Adults with De Novo Minimal Change Disease: A Multicenter, Randomized, Controlled Trial. Clin. J. Am. Soc. Nephrol. 2020, 15, 209–218. [Google Scholar] [CrossRef]
- Randone, P.; Sanna, E.; Dolla, C.; Gallo, E.; Mingozzi, S.; Tarragoni, R.; Torazza, M.C.; Niarchos, A.; Mella, A.; Manzione, A.M.; et al. Rescue with Obinutuzumab and Daratumumab as Combined B-cell/Plasma Cell Targeting Approach in Severe Post-Transplant FSGS Recurrence. Am. J. Transplant. 2024, 24, 1896–1900. [Google Scholar] [CrossRef]
- Rheault, M.N.; Alpers, C.E.; Barratt, J.; Bieler, S.; Canetta, P.; Chae, D.-W.; Coppock, G.; Diva, U.; Gesualdo, L.; Heerspink, H.J.L.; et al. Sparsentan versus Irbesartan in Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2023, 389, 2436–2445. [Google Scholar] [CrossRef]
- Soler, M.J.; Glassock, R.J.; Fervenza, F.C. Inaxaplin for Proteinuric Kidney Disease in Persons with Two APOL1 Variants. N. Engl. J. Med. 2023, 388, 2490–2491. [Google Scholar]
- Hengel, F.E.; Dehde, S.; Lassé, M.; Zahner, G.; Seifert, L.; Schnarre, A.; Kretz, O.; Demir, F.; Pinnschmidt, H.O.; Grahammer, F.; et al. Autoantibodies Targeting Nephrin in Podocytopathies. N. Engl. J. Med. 2024, 391, 422–433. [Google Scholar] [CrossRef]
- Sanders, P.W. Pathogenesis and treatment of myeloma kidney. J. Lab. Clin. Med. 1994, 124, 484–488. [Google Scholar] [PubMed]
- Solomon, A.; Weiss, D.T.; Kattine, A.A. Nephrotoxic Potential of Bence Jones Proteins. N. Engl. J. Med. 1991, 324, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Kapoulas, S.; Raptis, V.; Papaioannou, M. New aspects on the pathogenesis of renal disorders related to monoclonal gammopathies. Néphrologie Thérapeutique 2015, 11, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Soubrier, M.; Sauron, C.; Souweine, B.; Larroche, C.; Wechsler, B.; Guillevin, L.; Piette, J.C.; Rousset, H.; Deteix, P. Growth factors and proinflammatory cytokines in the renal involvement of POEMS syndrome. Am. J. Kidney Dis. 1999, 34, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Merlini, G.; Bridoux, F.; Leung, N.; Mikhael, J.; Harrison, S.J.; Kastritis, E.; Garderet, L.; Gozzetti, A.; van de Donk, N.W.; et al. Management of multiple myeloma-related renal impairment: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2023, 24, e293–e311. [Google Scholar] [CrossRef]
- Hundemer, G.L.; Imsirovic, H.; Visram, A.; McCurdy, A.; Knoll, G.; Biyani, M.; Canney, M.; Massicotte-Azarniouch, D.; Tanuseputro, P.; McCudden, C.; et al. The Association Between the Urine Protein-to-Albumin Gap and the Diagnosis of Multiple Myeloma: A Population-Based Retrospective Cohort Study. Am. J. Kidney Dis. 2023, 81, 732–734. [Google Scholar] [CrossRef]
- Leung, N.; Gertz, M.; Kyle, R.A.; Fervenza, F.C.; Irazabal, M.V.; Eirin, A.; Kumar, S.; Cha, S.S.; Rajkumar, S.V.; Lacy, M.Q.; et al. Urinary Albumin Excretion Patterns of Patients with Cast Nephropathy and Other Monoclonal Gammopathy–Related Kidney Diseases. Clin. J. Am. Soc. Nephrol. 2012, 7, 1964–1968. [Google Scholar] [CrossRef]
- Kastritis, E.; Palladini, G.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Sanchorawala, V.; Gibbs, S.; Mollee, P.; Venner, C.P.; et al. Daratumumab-Based Treatment for Immunoglobulin Light-Chain Amyloidosis. N. Engl. J. Med. 2021, 385, 46–58. [Google Scholar] [CrossRef]
- Cohen, C.; Royer, B.; Javaugue, V.; Szalat, R.; Karoui, K.E.; Caulier, A.; Knebelmann, B.; Jaccard, A.; Chevret, S.; Touchard, G.; et al. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015, 88, 1135–1143. [Google Scholar] [CrossRef]
- Palladini, G.; Dispenzieri, A.; Gertz, M.A.; Kumar, S.; Wechalekar, A.; Hawkins, P.N.; Schönland, S.; Hegenbart, U.; Comenzo, R.; Kastritis, E.; et al. New Criteria for Response to Treatment in Immunoglobulin Light Chain Amyloidosis Based on Free Light Chain Measurement and Cardiac Biomarkers: Impact on Survival Outcomes. J. Clin. Oncol. 2012, 30, 4541–4549. [Google Scholar] [CrossRef]
- Said, S.M.; Sethi, S.; Valeri, A.M.; Leung, N.; Cornell, L.D.; Fidler, M.E.; Hernandez, L.H.; Vrana, J.A.; Theis, J.D.; Quint, P.S.; et al. Renal Amyloidosis: Origin and Clinicopathologic Correlations of 474 Recent Cases. Clin. J. Am. Soc. Nephrol. 2013, 8, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Picken, M.M. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol. 2020, 143, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Cohen, A.D.; Comenzo, R.L.; Kastritis, E.; Landau, H.J.; Libby, E.N.; Liedtke, M.; Sanchorawala, V.; Schönland, S.; Wechalekar, A.; et al. Birtamimab plus standard of care in light-chain amyloidosis: The phase 3 randomized placebo-controlled VITAL trial. Blood 2023, 142, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Taubmann, J.; Bucci, L.; Wilhelm, A.; Bergmann, C.; Völkl, S.; Aigner, M.; Rothe, T.; Minopoulou, I.; Tur, C.; et al. CD19 CAR T-Cell Therapy in Autoimmune Disease—A Case Series with Follow-up. N. Engl. J. Med. 2024, 390, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Lobato, L. Portuguese-type amyloidosis (transthyretin amyloidosis, ATTR V30M). J. Nephrol. 2003, 16, 438–442. [Google Scholar]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef]
- Chemouny, J.M.; Bobot, M.; Sannier, A.; Maisons, V.; Jourde-Chiche, N.; Ferriere, E.; Joly, D.; Vigneau, C.; Rioux-Leclercq, N.; Barba, C.; et al. Kidney Biopsy in Type 2 Diabetes: A Multicenter Cross-Sectional Study. Am. J. Nephrol. 2021, 52, 131–140. [Google Scholar] [CrossRef]
- Catanese, L.; Rupprecht, H.; Huber, T.B.; Lindenmeyer, M.T.; Hengel, F.E.; Amann, K.; Wendt, R.; Siwy, J.; Mischak, H.; Beige, J. Non-Invasive Biomarkers for Diagnosis, Risk Prediction, and Therapy Guidance of Glomerular Kidney Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3519. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Marcovecchio, M.L. Biomarkers of diabetic kidney disease. Diabetologia 2018, 61, 996–1011. [Google Scholar] [CrossRef]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- EMPA-Kidney Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2022, 388, 117–127. [Google Scholar] [PubMed]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Ruilope, L.M.; Pitt, B.; Anker, S.D.; Rossing, P.; Kovesdy, C.P.; Pecoits-Filho, R.; Pergola, P.; Joseph, A.; Lage, A.; Mentenich, N.; et al. Kidney outcomes with finerenone: An analysis from the FIGARO-DKD study. Nephrol. Dial. Transplant. 2022, 38, 372–383. [Google Scholar] [CrossRef]
- Agarwal, R.; Filippatos, G.; Pitt, B.; Anker, S.D.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Gebel, M.; Ruilope, L.M.; et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2021, 43, 474–484. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Alpers, C.E.; Rennke, H.G.; Hopper, J.; Biava, C.G. Fibrillary glomerulonephritis: An entity with unusual immunofluorescence features. Kidney Int. 1987, 31, 781–789. [Google Scholar] [CrossRef]
- Devaney, K.; Sabnis, S.G.; Antonovych, T.T. Nonamyloidotic fibrillary glomerulopathy, immunotactoid glomerulopathy, and the differential diagnosis of filamentous glomerulopathies. Mod. Pathol. 1991, 4, 36–45. [Google Scholar]
- Nasr, S.H.; Valeri, A.M.; Cornell, L.D.; Fidler, M.E.; Sethi, S.; Leung, N.; Fervenza, F.C. Fibrillary Glomerulonephritis: A Report of 66 Cases from a Single Institution. Clin. J. Am. Soc. Nephrol. 2011, 6, 775–784. [Google Scholar] [CrossRef]
- Payan Schober, F.; Jobson, M.A.; Poulton, C.J.; Singh, H.K.; Nickeleit, V.; Falk, R.J.; Jennette, J.C.; Nachman, P.H.; Pendergraft, W.F., III. Clinical Features and Outcomes of a Racially Diverse Population with Fibrillary Glomerulonephritis. Am. J. Nephrol. 2017, 45, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Javaugue, V.; Karras, A.; Glowacki, F.; McGregor, B.; Lacombe, C.; Goujon, J.-M.; Ragot, S.; Aucouturier, P.; Touchard, G.; Bridoux, F. Long-term Kidney Disease Outcomes in Fibrillary Glomerulonephritis: A Case Series of 27 Patients. Am. J. Kidney Dis. 2013, 62, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Vrana, J.A.; Dasari, S.; Bridoux, F.; Fidler, M.E.; Kaaki, S.; Quellard, N.; Rinsant, A.; Goujon, J.M.; Sethi, S.; et al. DNAJB9 Is a Specific Immunohistochemical Marker for Fibrillary Glomerulonephritis. Kidney Int. Rep. 2018, 3, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Alexander, M.P.; Vrana, J.A.; Theis, J.D.; Mills, J.R.; Negron, V.; Sethi, S.; Dispenzieri, A.; Highsmith, W.E.; Nasr, S.H.; et al. DnaJ Heat Shock Protein Family B Member 9 Is a Novel Biomarker for Fibrillary GN. J. Am. Soc. Nephrol. 2018, 29, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Andeen, N.K.; Yang, H.-Y.; Dai, D.-F.; MacCoss, M.J.; Smith, K.D. DnaJ Homolog Subfamily B Member 9 Is a Putative Autoantigen in Fibrillary GN. J. Am. Soc. Nephrol. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Avasare, R.S.; Robinson, B.A.; Nelson, J.; Woltjer, R.; Krajbich, V.; Nguyen, V.; Garcia, D.; Setthavongsack, N.; Kizzar, C.; Raess, P.W.; et al. DNAJB9 Is Not Transcriptionally Upregulated in the Glomerulus in Fibrillary Glomerulonephritis. Kidney Int. Rep. 2020, 5, 368–372. [Google Scholar] [CrossRef]
- Nasr, S.H.; Dasari, S.; Lieske, J.C.; Benson, L.M.; Vanderboom, P.M.; Holtz-Heppelmann, C.J.; Giesen, C.D.; Snyder, M.R.; Erickson, S.B.; Fervenza, F.C.; et al. Serum levels of DNAJB9 are elevated in fibrillary glomerulonephritis patients. Kidney Int. 2019, 95, 1269–1272. [Google Scholar] [CrossRef]
- Andeen, N.K.; Troxell, M.L.; Riazy, M.; Avasare, R.S.; Lapasia, J.; Jefferson, J.A.; Akilesh, S.; Najafian, B.; Nicosia, R.F.; Alpers, C.E.; et al. Fibrillary Glomerulonephritis: Clinicopathologic Features and Atypical Cases from a Multi-Institutional Cohort. Clin. J. Am. Soc. Nephrol. 2019, 14, 1741–1750. [Google Scholar] [CrossRef]
- Erickson, S.B.; Zand, L.; Nasr, S.H.; Alexander, M.P.; Leung, N.; Drosou, M.E.; Fervenza, F.C. Treatment of fibrillary glomerulonephritis with rituximab: A 12-month pilot study. Nephrol. Dial. Transplant. 2020, 36, 104–110. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Bomback, A.S.; Perazella, M.A. Drug-Induced Glomerular Disease: Direct Cellular Injury. Clin. J. Am. Soc. Nephrol. 2015, 10, 1291–1299. [Google Scholar] [CrossRef]
- Romagnani, P.; Kitching, A.R.; Leung, N.; Anders, H.-J. The five types of glomerulonephritis classified by pathogenesis, activity and chronicity (GN-AC). Nephrol. Dial. Transplant. 2023, 38, ii3–ii10. [Google Scholar] [CrossRef] [PubMed]


| Target Antigen | Podocyte Expressed | TM vs. Secreted | Clinical/Disease Association | Distinctive Histopathologic Features | Incidence |
|---|---|---|---|---|---|
| PLA2R | Yes | TM | None | Global, granular, subepithelial deposits; IgG4 predominant | 55% |
| THSD7A | Yes | TM | Malignancy | Similar to PLA2R | 2% |
| NELL1 | No | secreted | Malignancy, Drugs, AID | IgG1 predominant, deposits may be a segmental or incomplete-loop pattern | 10% |
| SEMA3B | Yes | secreted | Pediatric | IgG1 predominant, may have additional mesangial deposits; TBM deposits may be present | 2% |
| PCDH7 | No | TM | Older | C3 absent or weak | 2% |
| HTRA1 | Yes | secreted | None | IgG4 predominant, similar to PLA2R | <1% |
| NTNG1 | Yes | secreted | None | IgG4 predominant, similar to PLA2R | <1% |
| EXT1/EXT2 | No | TM | AID, Lupus | IgG1 predominant, IgA, IgM often present, mesangial deposits, may coexist with class III/IV lupus | 7% |
| NCAM1 | No | TM | Lupus | Similar to EXT1/EXT2 | 2% |
| TGFBR3 | Yes | TM | Lupus | Similar to EXT1/EXT2 | |
| CNTN1 | No | secreted | CIDP | IgG4 predominant | 1% |
| FAT1 | Yes | TM | HSCT | TBM deposits can be present | 1% |
| NDNF | Yes | secreted | Syphilis | Lumpy deposits, superficial hump-like by EM, IgG1 | 1% |
| PCSK6 | No | secreted | NSAID | IgG1 and 4 | 2% |
| Low Risk | Moderate Risk | High Risk | Very High Risk |
|---|---|---|---|
| eGFR60 mL/min/1.73 m2 Proteinuria <3.5 g/d Serum albumin >3 g/dL Or eGFR 60 mL/min/1.73 m2 and 50% reduction in proteinuria in <6 months under therapy with RAASi | eGFR > 60mL/min/1.73 m2 Proteinuria > 3.5 g/d and no decline > 50% after 6 month of RAASi And Not fullfilling high-risk criteria | eGFR < 60mL/min/1.73 m2 and/or proteinuria > 8 g/d for >6 month Or eGFR > 60 mL/min/1.73 m2, Proteinuria > 3.5g/d and no decline > 50% after 6 months of RAASi and at least one of the following: -serum albumin < 2.5g/dL -anti-PLAR2ab > 50 RU/mL -urinary a1-microglobulin > 40 µg/min -urinary IgG > 1 µg/min -urinary b2 microglobulin > 250 mg/d -selectivity index > 0.2 | Life-threatening nephrotic syndrome or Rapidly deteriorating kidney function |
| Study | N | Intervention | Results |
|---|---|---|---|
| GEMRITUX 2017 [25] | 77 | Two doses of 375 mg/m2 RTX vs. SOC | RTX + SOC: 65% at 17 months (CR 19%) SOC: 34% at 17 months (CR 3%) |
| MENTOR 2019 [26] | 130 | 1000 mg RTX d1 + d14, repeated at 6 months if partial response vs. CyA | RTX: 62% at 18 months (CR 28%) CyA 33% (CR 2%) |
| RI-CYCLO 2021 [27] | 74 | 1000 mg RTX d1 + d14 vs. 6 m cyclic regimen GC alternated with CYP every other month | RTX: 66% at 18 months (CR 31%) CYP-GC: 79% at 18 months (CR 21%) |
| STARMEN 2021 [28] | 86 | 6 m cyclic regimen with GC alternated with CYP every other month vs. Tac 0.05 mg/kg/d first 6 months, followed by RTX 1 g | CYP-GC: 84% at 18 months (CR 44%) Tac-RTX: 53% at 18 months (CR 16%) |
| Histological Finding on 1st Biopsy | |
|---|---|
| Class I | 0–6% |
| Class II | 1–20% |
| Class III | 10–25% |
| Class IV | 35–60% |
| Class V | 5–30% |
| Class VI | <5% |
| Group | Disease |
|---|---|
| Gastrointestinal and liver diseases | Inflammatory bowel disease, celiac disease, cirrhosis |
| Infection | HBV, HCV, HIV, tuberculosis, leprosy |
| Autoimmune diseases | Ankylosing spondylitis, rheumatoid arthritis, Sjögren syndrome |
| Malignancy | Lung cancer, renal cell carcinoma, non-Hodgkin and Hodgkin lymphoma, IgA myeloma |
| Respiratory tract | Sarcoidosis, bronchioloitis obliterans, pulmonary hemosiderosis, cystic fibrosis, pulmonary fibrosis |
| Skin | Dermatitis herpetiformis, psoriasis |
| Supportive Care in IgAN |
|---|
| Blood pressure control |
| Reduction in proteinuria with RAAS-inhibitors and SGLT2i |
| Treatment of dyslipidemia |
| Lifestyle modification (dietary sodium restriction, smoking cessation, weight control, exercise) |
| Investigational Agent | Mechanism of Action | Clinicaltrials.gov Identifier |
|---|---|---|
| Complement inhibitors | ||
| Narsoplimab | MASP-2 inhibition (lectin pathway) | NCT02682407, NCT03608033 |
| Iptacopan | Complement factor B inhibitor (alternative pathway) | NCT03373461, NCT04578834 |
| Vemircopan | Complement factor D inhibitor (alternative pathway) | NCT05097989 |
| Cemdisiran | Complement factor 5 inhibitor (common pathway) | NCT03841448 |
| Ravulizumab | Complement factor 5 inhibitor (common pathway) | NCT06291376, NCT04564339 |
| Pegcetacoplan | Complement factor 3 inhibitor (common pathway) | NCT03453619 |
| Avacopan | C5aR1/inhibition of anaphylatoxin (common pathway) | NCT02384317 |
| BAFF/APRIL inhibitors | ||
| Sibeprenlimab | Antibody targeting APRIL | NCT05248646 |
| Zigakibart | Antibody targeting APRIL | NCT05852938 |
| Atacicept | Neutralizes activity of APRIL and BAFF | NCT04716231 |
| Telitacicept | Neutralizes activity of APRIL and BAFF | Coming |
| Povetacicept | Neutralizes activity of APRIL and BAFF | NCT05732402 |
| Endothelin-1 antagonists | ||
| Atrasentan | Antagonist of endothelin A receptor | NCT05834738 |
| Sparsentan | Inhibition of angiotensin II and endothelin A receptors | NCT05003986 (children) |
| Disease Group | Disease |
|---|---|
| infectious diseases | hepatitis C (with or without cryoglobulinemia) |
| hepatitis B | |
| HIV, EBV | |
| bacterial endocarditis | |
| visceral abscess | |
| ventriculoatrial shunt infection | |
| protozoa infection | |
| mycoplasm infection | |
| tuberculosis | |
| brucellosis | |
| malaria | |
| schistosomiasis | |
| echinococcosis | |
| autoimmune/rheumatologic disorder | systemic lupus erythematosus |
| cryoglobulinemia | |
| sjögren’s syndrome | |
| rheumatoid arthritis | |
| mixed connective tissue disease | |
| monoclonal gammopathies and neoplasia | monoclonal gammopathy of unknown significance |
| chronic lymphocytic leukemia | |
| lymphoma | |
| leukemia | |
| multiple myeloma | |
| Waldenstrom macroglobulinemia | |
| carcinoma | |
| malignant melanoma | |
| other | alpha 1 antitrypsin deficiency |
| sickle cell disease | |
| thrombotic microangiopathy | |
| transplant glomerulopathy |
| Complement Analysis | CH50 |
|---|---|
| APH50 | |
| C3 | |
| C4 | |
| C3d | |
| sC5b-9 | |
| factor H | |
| factor I | |
| factor B | |
| autoantibodies | C3NeF |
| anti-factor B | |
| anti-C3 convertase | |
| anti-factor H | |
| genetic analysis | factor H |
| factor I | |
| CFHR1-5 | |
| MCP/CD46 | |
| C3 | |
| exclusion of differential diagnosis | ANA |
| ANCA | |
| anti-GBM-antibodies | |
| HIV serology | |
| HBV serology | |
| HCV serology | |
| serum and urine protein electrophoresis |
| Group | Disease |
|---|---|
| Drugs | NSAID, salazopyrin, D-penicillamine, mercury exposure, gold, tiopronin, lithium, tyrosine-kinase inhibitors |
| Malignancies | Hodgkin lymphoma, non-Hodgkin lymphoma, leukemia, multiple myeloma |
| Infections | Syphilis, tuberculosis, mycoplasma, ehrlichiosis, hepatitis C, echinococcus, borreliosis |
| Allergy | Fungi, pollen, dust, bee stings, cat fur, food allergens |
| Autoimmune disorders | systemic lupus erythematosus, type 1 diabetes mellitus, myasthenia gravis, autoimmune pancreatitis, celiac disease |
| Secondary to Alterations of Glomerular Epithelial Cells | |
|---|---|
| Viral infections | HIV, CMV, Parvovirus B12, EBV, HCV, Hemophagocytic syndrome, SARS-CoV-2 |
| Drug-induced | Direct-acting antiviral therapy, mTOR inhibitors, Calcineurin inhibitors, anthracyclines, heroin, lithium, interferon, anabolic steroids, NSAIDs |
| Secondary to adaptive changes with glomerular hypertension | |
| Reduced nephron number | Reflux nephropathy, renal dysplasia, oligomeganephronia, sickle cell disease, age-related FSGS |
| Normal nephron number | Obesity-related glomerulopathy, primary glomerular diseases, systemic conditions (e.g., diabetic nephropathy, hypertensive nephrosclerosis) |
| Complete remission | Reduction in proteinuria to ˂0.3 g/d or PCR < 300 mg/g, stable serum creatinine and serum albumin > 3.5 g/dL |
| Partial remission | Reduction in proteinuria to 0.3g–3.5/d or PCR < 300–3500 mg/g and a decrease > 50% from baseline |
| Relapse | Proteinuria > 3.5g/d or PCR > 3500 mg/g after complete remission has been achieved or an increase in proteinuria > 50% in patients who had undergone partial remission |
| Steroid-resistant MCD/FSGS | Persistence of proteinuria > 3.5 g/d or PCR > 3500 mg/g with <50% reduction from baseline despite prednisone 1 mg/kg/d for >16 weeks |
| Steroid-dependent MCD/FSGS | Relapse occurring during or within 2 weeks of completing glucocorticoid therapy |
| Frequently relapsing MCD | Two or more relapses per 6 months |
| Manifestation | Albuminuria/Nephrotic Syndrome | Ig-Subtype Association |
|---|---|---|
| AL/AH/AHL amyloidosis | +++/> 60% | lambda >> kappa |
| Monoclonal immunoglobulin deposition disease MIDD (LCDD, LHCDD, HCDD) | ++/~ 20% | kappa >> lambda |
| Proliferative glomerulonephritis with monoclonal immunoglobuline deposits (PGNMID) | +++/~ 50% | 2/3: no detectable circulating monoclonal protein. 1/3: detectable, often IgG3kappa |
| Type 1 Cryoglobulinemic glomerulonephritis | ++/~ 35–40% | renal: IgG >> IgM articular: IgG3 |
| Light-chain proximal tubulopathy | +(+)/rare | kappa >> lambda |
| C3 glomerulopathy with monoclonal gammopathy | ++/~ 40% | |
| Thrombotic microangiopathy with monoclonal gammopathy | +(+)/~40% | |
| Monoclonal immunotactoid glomerulonephritis | +++/~70% | lambda >> kappa |
| Light chain crystalline podocytopathy | ++/~ 30% | kappa >> lambda |
| Drugs | Associated Disease |
|---|---|
| IFN-α and-β | MCD, FSGS, MN |
| IFN-γ | FSGS |
| bisphosphonates (e.g., pamidronate, zoledronate) | MCD, FSGS |
| anabolic steroids | FSGS |
| lithium | MCD, FSGS, MN |
| NSAIDs | MCD, MN |
| cyclooxygenase-2 inhibitors | MCD, MN |
| mTOR inhibitors (e.g., sirolomis, everolimus) | FSGS |
| antibiotics (e.g., Ampicillin, rifampicin, cefixime) | MCD |
| tamoxifen | MCD |
| penacillamine | MCD, MN |
| bucillamine | MCD, MN |
| etanercept | MCD |
| gold | MCD, MN |
| methimazole | MCD |
| enalapril | MCD |
| mercury | MCD |
| heroin | FSGS |
| fluconazole | MN |
| probenecid | MN |
| captopril | MN |
| mercury | MN |
| clarithromycin | MN |
| VEGF pathway inhibitors (e.g., bevacizumab, Sunitinib/Sorafenib, Aflibercept, Ramucirumab) | MCD, FSGS, MN |
| Src family kinase inhibitors (e.g., dasatinib) | MCD, FSGS, endotheliosis |
| EGFR pathway inhibitors (e.g., erlotinib, gefitinib, cetuximab, panitumumab | MCD, MN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wendt, R.; Sobhani, A.; Diefenhardt, P.; Trappe, M.; Völker, L.A. An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes. Biomedicines 2024, 12, 2259. https://doi.org/10.3390/biomedicines12102259
Wendt R, Sobhani A, Diefenhardt P, Trappe M, Völker LA. An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes. Biomedicines. 2024; 12(10):2259. https://doi.org/10.3390/biomedicines12102259
Chicago/Turabian StyleWendt, Ralph, Alina Sobhani, Paul Diefenhardt, Moritz Trappe, and Linus Alexander Völker. 2024. "An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes" Biomedicines 12, no. 10: 2259. https://doi.org/10.3390/biomedicines12102259
APA StyleWendt, R., Sobhani, A., Diefenhardt, P., Trappe, M., & Völker, L. A. (2024). An Updated Comprehensive Review on Diseases Associated with Nephrotic Syndromes. Biomedicines, 12(10), 2259. https://doi.org/10.3390/biomedicines12102259





