Endocrine Disorders in Nephrotic Syndrome—A Comprehensive Review
Abstract
1. Introduction
2. Pineal Gland
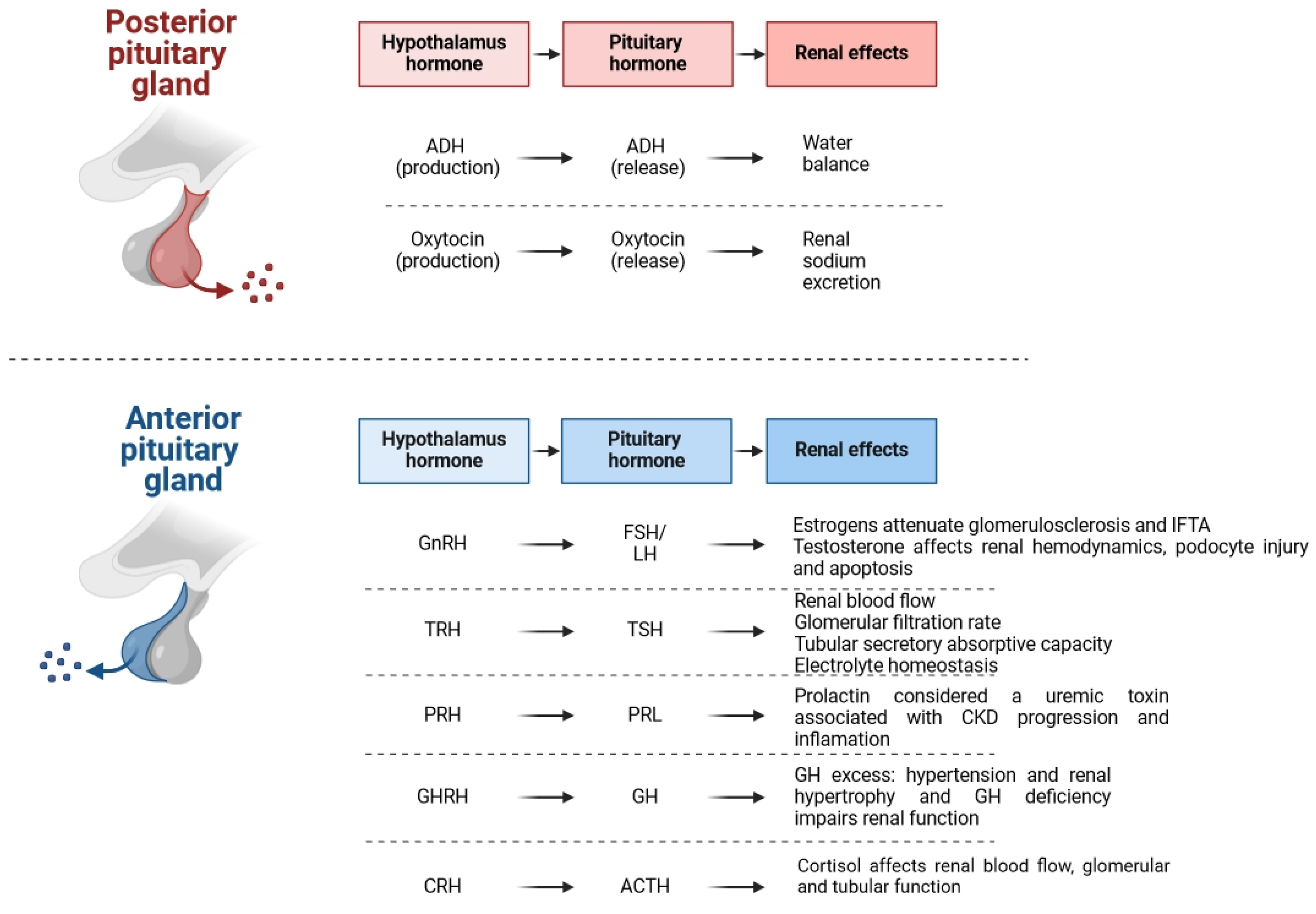
3. Hypothalamus and Hypophysis
3.1. Adrenocorticotropic Hormone
3.2. Growth Hormone
3.3. Prolactin
3.4. Oxytocin
3.5. Vasopressin
4. Pancreas
5. Ovaries and Testicles
6. Thyroid Gland
7. Parathyroid Gland
8. Adrenal Gland
9. Bidirectional Connection of Kidneys and Endocrine Glands—Current Knowledge and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Busuioc, R.M.; Mircescu, G. Nephrotic Syndrome Complications—New and Old. Part 1. Maedica 2022, 17, 153–168. [Google Scholar] [PubMed]
- Frățilă, V.-G.; Lupușoru, G.; Sorohan, B.M.; Obrișcă, B.; Mocanu, V.; Lupușoru, M.; Ismail, G. Nephrotic Syndrome: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2024, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, S.V.; Birn, H.; Jensen, S.K.; Sørensen, H.T.; Nitsch, D.; Christiansen, C.F. Twenty-four-Year Trends in Incidence and Mortality of Nephrotic Syndrome: A Population-Based Cohort Study. Epidemiology 2023, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Zabala Ramirez, M.J.; Stein, E.J.; Jain, K. Nephrotic Syndrome for the Internist. Med. Clin. N. Am. 2023, 107, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar] [CrossRef]
- Politano, S.A.; Colbert, G.B.; Hamiduzzaman, N. Nephrotic Syndrome. Prim. Care 2020, 47, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Claudio, P.; Gabriella, M. Nephrotic syndrome: Pathophysiology and consequences. J. Nephrol. 2023, 36, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M. The interaction between thyroid and kidney disease: An overview of the evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 407–415. [Google Scholar] [CrossRef]
- Gheban, B.A.; Rosca, I.A.; Crisan, M. The morphological and functional characteristics of the pineal gland. Med. Pharm. Rep. 2019, 92, 226–234. [Google Scholar] [CrossRef]
- Kalra, S.; Agrawal, S.; Sahay, M. The reno-pineal axis: A novel role for melatonin. Indian. J. Endocrinol. Metab. 2012, 16, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Raza, Z.; Naureen, Z. Melatonin ameliorates the drug induced nephrotoxicity: Molecular insights. Nefrologia 2020, 40, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, Y.; Ferrebuz, A.; Romero, F.; Vaziri, N.D.; Rodriguez-Iturbe, B. Melatonin ameliorates oxidative stress, inflammation, proteinuria, and progression of renal damage in rats with renal mass reduction. Am. J. Physiol. Renal Physiol. 2008, 294, F336–F344. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.G.; Koomen, G.C.; van Acker, B.A.; Arisz, L. Urinary sodium excretion in patients with nephrotic syndrome, and its circadian variation. Q. J. Med. 1994, 87, 109–117. [Google Scholar] [PubMed]
- Kemp, G.J.; Blumsohn, A.; Morris, B.W. Circadian changes in plasma phosphate concentration, urinary phosphate excretion, and cellular phosphate shifts. Clin. Chem. 1992, 38, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hasan, A.U.; Kobori, H. Melatonin in chronic kidney disease: A promising chronotherapy targeting the intrarenal renin–angiotensin system. Hypertens. Res. 2019, 42, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lu, K.C.; Lin, G.J.; Hsieh, H.Y.; Chu, P.; Lin, S.H.; Sytwu, H.K. Melatonin enhances endogenous heme oxygenase-1 and represses immune responses to ameliorate experimental murine membranous nephropathy. J. Pineal Res. 2012, 52, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Lu, K.C.; Chao, T.K.; Chen, J.S.; Chen, A.; Guo, C.Y.; Hsieh, H.Y.; Shih, H.M.; Sytwu, H.K.; Wu, C.C. Role of melatonin receptor 1A and pituitary homeobox-1 coexpression in protecting tubular epithelial cells in membranous nephropathy. J. Pineal Res. 2018, 65, e12482. [Google Scholar] [CrossRef] [PubMed]
- Aouichat, S.; Navarro-Alarcon, M.; Alarcón-Guijo, P.; Salagre, D.; Ncir, M.; Zourgui, L.; Agil, A. Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat. Pharmaceuticals 2021, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Mehta, A.; Nair, N.; Nemer, L.; Jain, R.; Joshi, H.; Raina, R. ACTH Treatment for Management of Nephrotic Syndrome: A Systematic Review and Reappraisal. Int. J. Nephrol. 2020, 2020, 2597079. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, X.; Wu, X.; Li, Y.; He, Q.; Li, X. Real-word adrenocorticotropic hormone treatment for childhood-onset nephrotic syndrome. Front. Pediatr. 2023, 11, 1044075. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, C.L.; Carrero, J.J. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: The chicken or the egg? Arch. Med. Res. 2013, 44, 591–600. [Google Scholar] [CrossRef]
- Haffner, D.; Grund, A.; Leifheit-Nestler, M. Renal effects of growth hormone in health and in kidney disease. Pediatr. Nephrol. 2021, 36, 2511–2530. [Google Scholar] [CrossRef]
- Feld, S.M.; Hirschberg, R. Insulin-like growth factor-I and insulin-like growth factor-binding proteins in the nephrotic syndrome. Pediatr. Nephrol. 1996, 10, 355–358. [Google Scholar] [CrossRef]
- Mohan, K.R.; Kanitkar, M. Growth in Children with Steroid Sensitive Nephrotic Syndrome. Med. J. Armed Forces India 2009, 65, 4–6. [Google Scholar] [CrossRef][Green Version]
- Loke, K.Y.; Yap, H.K.; Zhou, X.; Tan, S.P.; Chao, S.M.; Lee, K.O. Efficacy and safety of one year of growth hormone therapy in steroid-dependent nephrotic syndrome. J. Pediatr. 1997, 130, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Harambat, J.; Cochat, P. [Long-term steroid therapy in children: Is adjunct therapy relevant in nephrotic syndrome?]. Arch. Pediatr. 2008, 15, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.N.; Hirschberg, R. Tubular epithelial cell activation and interstitial fibrosis. The role of glomerular ultrafiltration of growth factors in the nephrotic syndrome and diabetic nephropathy. Nephrol. Dial. Transpl. 1999, 14, 2072–2074. [Google Scholar] [CrossRef]
- Huang, W.; Molitch, M.E. Prolactin and Other Pituitary Disorders in Kidney Disease. Semin. Nephrol. 2021, 41, 156–167. [Google Scholar] [CrossRef]
- Dourado, M.; Cavalcanti, F.; Vilar, L.; Cantilino, A. Relationship between Prolactin, Chronic Kidney Disease, and Cardiovascular Risk. Int. J. Endocrinol. 2020, 2020, 9524839. [Google Scholar] [CrossRef]
- Heras, M.; Iglesias, P.; Fernández-Reyes, M.J.; Sánchez, R.; Jiménez, M.J.; Muñoz, H.; Tajada, P.; Duarte, J. Nephrotic-range proteinuria in a patient with a giant prolactinoma. Am. J. Kidney Dis. 2008, 51, 1025–1028. [Google Scholar] [CrossRef]
- Horrobin, D.F. The possible role of prolactin in pre-eclampsia. Zentralbl Gynakol. 1977, 99, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.W.; Jeon, U.S.; Kim, G.H.; Park, J.; Oh, Y.K.; Kim, Y.S.; Ahn, C.; Kim, S.; Kim, S.Y.; Lee, J.S.; et al. Antidiuretic action of oxytocin is associated with increased urinary excretion of aquaporin-2. Nephrol. Dial. Transplant. 2004, 19, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Gellai, M.; North, W.G.; Valtin, H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann. N. Y. Acad. Sci. 1993, 689, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Usberti, M.; Federico, S.; Meccariello, S.; Cianciaruso, B.; Balletta, M.; Pecoraro, C.; Sacca, L.; Ungaro, B.; Pisanti, N.; Andreucci, V.E. Role of plasma vasopressin in the impairment of water excretion in nephrotic syndrome. Kidney Int. 1984, 25, 422–429. [Google Scholar] [CrossRef]
- Meena, J.; Sinha, A.; Hari, P.; Bagga, A. Therapy with the Combination of Tolvaptan and Furosemide for Refractory Edema in Nephrotic Syndrome. Indian. J. Nephrol. 2020, 30, 53–55. [Google Scholar] [CrossRef]
- Brovko, M.; Kozlovskaya, L.; Pulin, A.; Moiseev, S.; Sholomova, V.; Shchekochikhin, D.; Gognieva, D.; Milovanova, L.; Fomin, V. Low aquaporin-2 excretion in the nephrotic syndrome: An escape from the vasopressin regulating effect. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Bardoux, P.; Bichet, D.G.; Martin, H.; Gallois, Y.; Marre, M.; Arthus, M.F.; Lonergan, M.; Ruel, N.; Bouby, N.; Bankir, L. Vasopressin increases urinary albumin excretion in rats and humans: Involvement of V2 receptors and the renin-angiotensin system. Nephrol. Dial. Transplant. 2003, 18, 497–506. [Google Scholar] [CrossRef]
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef]
- Hao, S.; Wu, Y.; Kang, Y.; Niu, X.; Zhu, G.; Huang, W. A single-center analysis of primary nephrotic syndrome with acute pancreatitis in children. Medicine 2020, 99, e21056. [Google Scholar] [CrossRef]
- Flint, R.S.; Phillips, A.R.; Farrant, G.J.; McKay, D.; Buchanan, C.M.; Cooper, G.S.; Windsor, J.A. Probing the urinary proteome of severe acute pancreatitis. HPB 2007, 9, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Bekmurzaeva, G.B.; Osmanov, I.M. Pancreatic lesion in children with nephrotic syndrome. Med. Counc. 2021, 1, 134–142. [Google Scholar] [CrossRef]
- Stokes, M.B.; Kwakye, J.; D’Agati, V.D. Nephrotic syndrome and ARF in a diabetic patient. Am. J. Kidney Dis. 2003, 41, 1327–1333. [Google Scholar] [CrossRef]
- Dogra, G.K.; Herrmann, S.; Irish, A.B.; Thomas, M.A.; Watts, G.F. Insulin resistance, dyslipidaemia, inflammation and endothelial function in nephrotic syndrome. Nephrol. Dial. Transplant. 2002, 17, 2220–2225. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Jin, B.; Huang, S.; Yuan, Y.; Ding, G.; Bao, H.; Chen, Y.; Han, Y.; Zhao, F.; Zhang, A. Insulin resistance in children with primary nephrotic syndrome and normal renal function. Pediatr. Nephrol. 2012, 27, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, M.; Czupryniak, A.; Lukamowicz, J.; Ksiazek, E.; Półtorak-Krawczyk, A.; Swiatkowska, E.; Nowicki, M. Insulinowrazliwość i czynność komórki beta trzustki u dzieci z idiopatycznym zespołem nerczycowym [Insulinsensitivity and beta-cell function in children with idiopathic nephrotic syndrome]. Przegl. Lek. 2006, 63, 217–219. [Google Scholar] [PubMed]
- Huang, H.X.; Shen, L.L.; Huang, H.Y.; Zhao, L.H.; Xu, F.; Zhang, D.M.; Zhang, X.L.; Chen, T.; Wang, X.Q.; Xie, Y.; et al. Associations of Plasma Glucagon Levels with Estimated Glomerular Filtration Rate, Albuminuria and Diabetic Kidney Disease in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2021, 45, 868–879. [Google Scholar] [CrossRef]
- Vaziri, N.D. Endocrinological consequences of the nephrotic syndrome. Am. J. Nephrol. 1993, 13, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Elias, A.N.; Carreon, G.; Vaziri, N.D.; Pandian, M.R.; Oveisi, F. The pituitary-gonadal axis in experimental nephrotic syndrome in male rats. J. Lab. Clin. Med. 1992, 120, 949–954. [Google Scholar] [PubMed]
- Menjívar, M.; Vilchis, F.; Cárdenas, M.; Cruz, C.; Merchant, H.; Pérez-Palacios, G.; Pedraza-Chaverri, J. Pituitary-ovarian dysfunction in rats with induced nephrotic syndrome. Eur. J. Endocrinol. 1995, 132, 502–506. [Google Scholar] [CrossRef]
- De Castro, I.; Easterling, T.R.; Bansal, N.; Jefferson, J.A. Nephrotic syndrome in pregnancy poses risks with both maternal and fetal complications. Kidney Int. 2017, 91, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Bhaduaria, D.; Pradhan, M.; Jain, M.; Prasad, N.; Patel, M.; Gupta, A.; Sharma, R.K. Feto-maternal and renal outcomes of nephrotic syndrome in pregnancy. Saudi J. Kidney Dis. Transpl. 2021, 32, 1397–1406. [Google Scholar] [PubMed]
- Siligato, R.; Gembillo, G.; Cernaro, V.; Torre, F.; Salvo, A.; Granese, R.; Santoro, D. Maternal and Fetal Outcomes of Pregnancy in Nephrotic Syndrome Due to Primary Glomerulonephritis. Front. Med. 2020, 7, 563094. [Google Scholar] [CrossRef] [PubMed]
- Tomarelli, R.G.; Ampuero, A.C.; Hevia, J.P.; Donoso, F.A.; Arriagada, S.D. Desarrollo de Síndrome nefrótico en paciente con Tiroiditis de Hashimoto [Development of nephrotic syndrome in a patient with Hashimoto’s Thyroiditis]. Andes Pediatr. 2022, 93, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Alsaiqali, M.; Narayanaswamy, M.; McFarlane, I. The Vicious Cycle of Hypothyroidism and Severe Proteinuria: A Case Report. Cureus 2022, 14, e28674. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mishra, O.P.; Mandal, P.P.; Patel, P.S.; Sharma, S.S.; Saini, H.; Rani, K.; Chandrasekhar, S.; Singh, M.P. Thyroid function in patients with idiopathic nephrotic syndrome. Int. Urol. Nephrol. 2021, 53, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Fukata, S.; Ito, M.; Nishikawa, M.; Kasahara, T.; Nishihara, E.; Akamiuzu, T.; Miyauchi, A. Hypothyroidism due to nephrotic syndrome: A notable clinical entity. Endocr. J. 2022, 69, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Thapa Karki, S.; Khatun, N.; Chapagain, R.H.; Shrestha, N.J.; Agrawal, S. Hypothyroidism among Children with Nephrotic Syndrome Admitted to a Tertiary Care Centre. JNMA J. Nepal. Med. Assoc. 2024, 62, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, N.; Chen, X.; Xing, C. Low triiodothyronine syndrome is associated with platelet function in patients with nephrotic syndrome. Rev. Assoc. Med. Bras. (1992) 2019, 65, 988–992. [Google Scholar] [CrossRef]
- Benido Silva, V.; Pereira, M.T.; Moreira, C.L.; Santos Monteiro, S.; Inácio, I.; Cardoso, M.H. Nephrotic Syndrome as a Cause of Transient Clinical Hypothyroidism. Case Rep. Endocrinol. 2021, 2021, 5523929. [Google Scholar] [CrossRef]
- Gu, Q.H.; Cao, X.; Mao, X.M.; Jia, J.Y.; Yan, T.K. Significance of thyroid dysfunction in the patients with primary membranous nephropathy. BMC Nephrol. 2022, 23, 398. [Google Scholar] [CrossRef]
- Kwong, N.; Medici, M.; Marqusee, E.; Wassner, A.J. Severity of Proteinuria Is Directly Associated with Risk of Hypothyroidism in Adults. J. Clin. Endocrinol. Metab. 2021, 106, e757–e762. [Google Scholar] [CrossRef]
- Yuasa, R.; Muramatsu, M.; Saito, A.; Osuka, H.; Morita, T.; Hamasaki, Y.; Sakai, K. Urinary excretion of thyroid hormone in CKD patients: A proof-of-concept of nephrogenic hypothyroidism. Ren. Fail. 2023, 45, 2293224. [Google Scholar] [CrossRef]
- Iwazu, Y.; Kotani, K.; Sugase, T.; Nagata, D.; Yamada, T. Relationship of Thyroid Function with Renal Hemodynamics and Cholesterol Metabolism in Proteinuric Kidney Disease: A Pilot Study. Metabolites 2024, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Z.; Hu, Y.; Ai, S.L.; Cheng, L.; Liu, J.; Morris, E.; Li, Y.; Gou, S.J.; Fu, P. The relationship between thyroid dysfunction and nephrotic syndrome: A clinicopathological study. Sci. Rep. 2019, 9, 6421. [Google Scholar] [CrossRef]
- Jain, D.; Aggarwal, H.K.; Pavan Kumar, Y.M.; Jain, P. Evaluation of thyroid dysfunction in patients with nephrotic syndrome. Med. Pharm. Rep. 2019, 92, 139–144. [Google Scholar] [CrossRef]
- Koopman, T.; Niedlich-den Herder, C.; Stegeman, C.A.; Links, T.P.; Bijzet, J.; Hazenberg, B.P.C.; Diepstra, A. Kidney Involvement in Systemic Calcitonin Amyloidosis Associated With Medullary Thyroid Carcinoma. Am. J. Kidney Dis. 2017, 69, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Ong, L.; Loh, T.P.; Chua, H.R.; Tham, C.; Meng, K.C.; Pin, L. Calcium, Vitamin D, and Bone Derangement in Nephrotic Syndrome. J. ASEAN Fed. Endocr. Soc. 2021, 36, 50–55. [Google Scholar] [CrossRef]
- Maji, M.; Kumar, M.; Chacham, S.; Mirza, A.A.; Bhat, N.K.; Mandal, S. Severity of Vitamin D Deficiency in Children with Nephrotic Syndrome: A Study from Tertiary Care Center in Northern India. Saudi J. Kidney Dis. Transpl. 2022, 33, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Gembillo, G.; Siligato, R.; Amatruda, M.; Conti, G.; Santoro, D. Vitamin D and Glomerulonephritis. Medicina 2021, 57, 186. [Google Scholar] [CrossRef]
- Thakor, J.M.; Mistry, K.N.; Gang, S. Association between serum calcium and biochemical parameters among nephrotic syndrome patients: A case-control study. Egypt. Pediatr. Assoc. Gaz. 2022, 70, 18. [Google Scholar] [CrossRef]
- Malluche, H.H.; Goldstein, D.A.; Massry, S.G. Osteomalacia and hyperparathyroid bone disease in patients with nephrotic syndrome. J. Clin. Investig. 1979, 63, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Cetin, N.; Gencler, A.; Sivrikoz, I.A. Bone mineral density and vitamin D status in children with remission phase of steroid-sensitive nephrotic syndrome. Saudi J. Kidney Dis. Transpl. 2019, 30, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Basu, S.; Akhtar, S.; Sinha, R.; Sen, A.; Sengupta, J. Free vitamin D levels in steroid-sensitive nephrotic syndrome and healthy controls. Pediatr. Nephrol. 2020, 35, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Chesney, R.W.; Hamstra, A.; Rose, P.; DeLuca, H.F. Vitamin D and parathyroid hormone status in children with the nephrotic syndrome and chronic mild glomerulonephritis. Int. J. Pediatr. Nephrol. 1984, 5, 1–4. [Google Scholar] [PubMed]
- Pukajło-Marczyk, A.; Jakubowska, A.; Bargenda-Lange, A.; Kiliś-Pstrusińska, K.; Zwolińska, D. Assessment of the Concentration of Bone Metabolism Markers: Sclerostin and FGF-23 in Children with Idiopathic Nephrotic Syndrome Treated with Glucocorticosteroids. Dis. Markers 2019, 2019, 9698367. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Zhang, S.; Guo, Z.; Peng, S. Significance of changes in FGF23 levels in childhood primary nephrotic syndrome and children who progress to end-stage renal disease. Exp. Ther. Med. 2023, 26, 390. [Google Scholar] [CrossRef]
- Tomo, S.; Birdi, A.; Yadav, D.; Chaturvedi, M.; Sharma, P. Klotho: A Possible Role in the Pathophysiology of Nephrotic Syndrome. EJIFCC 2022, 33, 3–10. [Google Scholar] [PubMed]
- Lenoir, O.; Tharaux, P.L. Should we consider calcimimetics as a therapeutic option for nephrotic syndrome? Kidney Int. 2022, 101, 1110–1112. [Google Scholar] [CrossRef]
- Mizdrak, M.; Ticinovic Kurir, T.; Mizdrak, I.; Kumric, M.; Krnic, M.; Bozic, J. The Role of the Gap Junction Protein Connexin in Adrenal Gland Tumorigenesis. Int. J. Mol. Sci. 2024, 25, 5399. [Google Scholar] [CrossRef]
- Rascher, W.; Tulassay, T. Hormonal regulation of water metabolism in children with nephrotic syndrome. Kidney Int. Suppl. 1987, 21, S83–S89. [Google Scholar] [PubMed]
- de Sousa, M.V.; Guida, J.P.; do Valle, C.F.; Camargo, L.F.; Rivelli, G.G.; Mazzali, M. Spironolactone in Post-Transplant Proteinuria: A Safe Alternative Therapy. Transplant. Proc. 2017, 49, 813–816. [Google Scholar] [CrossRef]
- Fujii, W.; Shibata, S. Mineralocorticoid Receptor Antagonists for Preventing Chronic Kidney Disease Progression: Current Evidence and Future Challenges. Int. J. Mol. Sci. 2023, 24, 7719. [Google Scholar] [CrossRef] [PubMed]
- Asao, T.; Oki, K.; Yoneda, M.; Tanaka, J.; Kohno, N. Hypothalamic-pituitary-adrenal axis activity is associated with the prevalence of chronic kidney disease in diabetic patients. Endocr. J. 2016, 63, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Y. Relationship between cortisol and diabetic microvascular complications: A retrospective study. Eur. J. Med. Res. 2023, 28, 391. [Google Scholar] [CrossRef] [PubMed]
- Smets, P.; Meyer, E.; Maddens, B.; Daminet, S. Cushing’s syndrome, glucocorticoids and the kidney. Gen. Comp. Endocrinol. 2010, 169, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sagmeister, M.S.; Harper, L.; Hardy, R.S. Cortisol excess in chronic kidney disease—A review of changes and impact on mortality. Front. Endocrinol. 2023, 13, 1075809. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Chen, Y.; Su, J.; Gao, Q.; Fan, Y.; Feng, J.; Liu, M.; He, Q. Correlation of dehydroepiandrosterone with diabetic nephropathy and its clinical value in early detection. J. Diabetes Investig. 2022, 13, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Kitagawa, Y.; Kamiuchi, K.; Hasegawa, G.; Yoshikawa, T.; Nakamura, N. Low serum dehydroepiandrosterone sulfate concentration is a predictor for deterioration of urinary albumin excretion in male patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2006, 73, 47–50. [Google Scholar] [CrossRef]
- Moyer, J.M.; Handley, C.A. Norepinephrine and epinephrine effect on renal hemodynamics, with particular reference to the possibility of vascular shunting and decreasing the active glomeruli. Circulation 1952, 5, 91–97. [Google Scholar] [CrossRef]
- Szokol, M.; Soltész, M.B.; Nagy, A.; Lengyel, Z.; Gomba, S. The effect of adrenalectomy on the proteinuria of spontaneously hypertensive rats and normotensive controls. Exp. Pathol. 1986, 30, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bilha, S.C.; Hogas, S.; Hogas, M.; Marcu, S.; Leustean, L.; Ungureanu, M.-C.; Branisteanu, D.D.; Preda, C. Thyroid, Gonadal and Adrenal Dysfunction in Kidney Transplant Recipients: A Review for the Clinician. Biomolecules 2023, 13, 920. [Google Scholar] [CrossRef] [PubMed]
- Meyrier, A.; Niaudet, P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018, 94, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Kuczera, P.; Adamczak, M.; Wiecek, A. Endocrine Abnormalities in Patients with Chronic Kidney Disease. Pril (Makedon. Akad. Nauk. Umet. Odd. Med. Nauki) 2015, 36, 109–118. [Google Scholar] [CrossRef]
- Krysiak, R.; Kędzia, A.; Krupej-Kędzierska, J.; Kowalska, B.; Okopień, B. Zaburzenia endokrynologiczne u chorych z przewlekłą niewydolnością nerek-część II [Endocrine abnormalities in patients with chronic renal failure-part II]. Pol. Merkur. Lekarski 2015, 38, 293–299. [Google Scholar] [PubMed]
- Nagami, G.T.; Kraut, J.A. The Role of the Endocrine System in the Regulation of Acid–Base Balance by the Kidney and the Progression of Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 2420. [Google Scholar] [CrossRef] [PubMed]

| Endocrine Gland | Hormones | Roles in Physiological Settings |
|---|---|---|
| Pineal gland (epiphysis) | -Serotonin -N, N-dimethyltryptamine -Melatonin | -Sleep patterns following diurnal cycles -Reproduction -Thermoregulation |
| Posterior pituitary gland | ADH | Water balance |
| Oxytocin | Behavior, such as social binding, reproduction, and childbirth | |
| Anterior pituitary gland | FSH/LH | -Trigger ovaries to estrogen production -Breast development -Menstruation/ovulation -Stimulate Sertoli cells to produce androgen-binding protein, thereby stimulating spermatogenesis |
| TSH | Thyroid hormone regulation | |
| Prolactin | Breastfeeding and lactation | |
| Growth hormone | Postnatal growth, metabolism, and homeostasis | |
| ACTH | Cortisol homeostasis | |
| Pancreas | Insulin Glucagon Somatostatin Pancreatic polypeptide | Glucose and metabolism homeostasis |
| Ovaries | -Estrogen -Androgen -Inhibin -Progesterone | -Menstrual cycle -Fertility |
| Testicles | Androgens (testosterone) | Fertility |
| Thyroid gland | -Triiodothyronine (T3) -Thyroxine (T4) | -Metabolic rate -Protein synthesis -Growth -Development |
| Calcitonin | Calcium homeostasis | |
| Parathyroid gland | Parathyroid hormone | Calcium homeostasis |
| Adrenal gland | Cortex: aldosterone, glucocorticoids, and androgens | -Electrolyte and water homeostasis -Stress response and glucose metabolism -Reproductive health and body development |
| Medulla: catecholamines | Rapid reaction to stress situations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizdrak, M.; Smajic, B.; Mizdrak, I.; Ticinovic Kurir, T.; Kumric, M.; Paladin, I.; Batistic, D.; Bozic, J. Endocrine Disorders in Nephrotic Syndrome—A Comprehensive Review. Biomedicines 2024, 12, 1860. https://doi.org/10.3390/biomedicines12081860
Mizdrak M, Smajic B, Mizdrak I, Ticinovic Kurir T, Kumric M, Paladin I, Batistic D, Bozic J. Endocrine Disorders in Nephrotic Syndrome—A Comprehensive Review. Biomedicines. 2024; 12(8):1860. https://doi.org/10.3390/biomedicines12081860
Chicago/Turabian StyleMizdrak, Maja, Bozo Smajic, Ivan Mizdrak, Tina Ticinovic Kurir, Marko Kumric, Ivan Paladin, Darko Batistic, and Josko Bozic. 2024. "Endocrine Disorders in Nephrotic Syndrome—A Comprehensive Review" Biomedicines 12, no. 8: 1860. https://doi.org/10.3390/biomedicines12081860
APA StyleMizdrak, M., Smajic, B., Mizdrak, I., Ticinovic Kurir, T., Kumric, M., Paladin, I., Batistic, D., & Bozic, J. (2024). Endocrine Disorders in Nephrotic Syndrome—A Comprehensive Review. Biomedicines, 12(8), 1860. https://doi.org/10.3390/biomedicines12081860







