Safety Profiles Related to Dosing Errors of Rapid-Acting Insulin Analogs: A Comparative Analysis Using the EudraVigilance Database
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Designs
2.2. Material
2.3. Descriptive Analysis
2.4. Disproportionality Analysis
2.5. Ethics
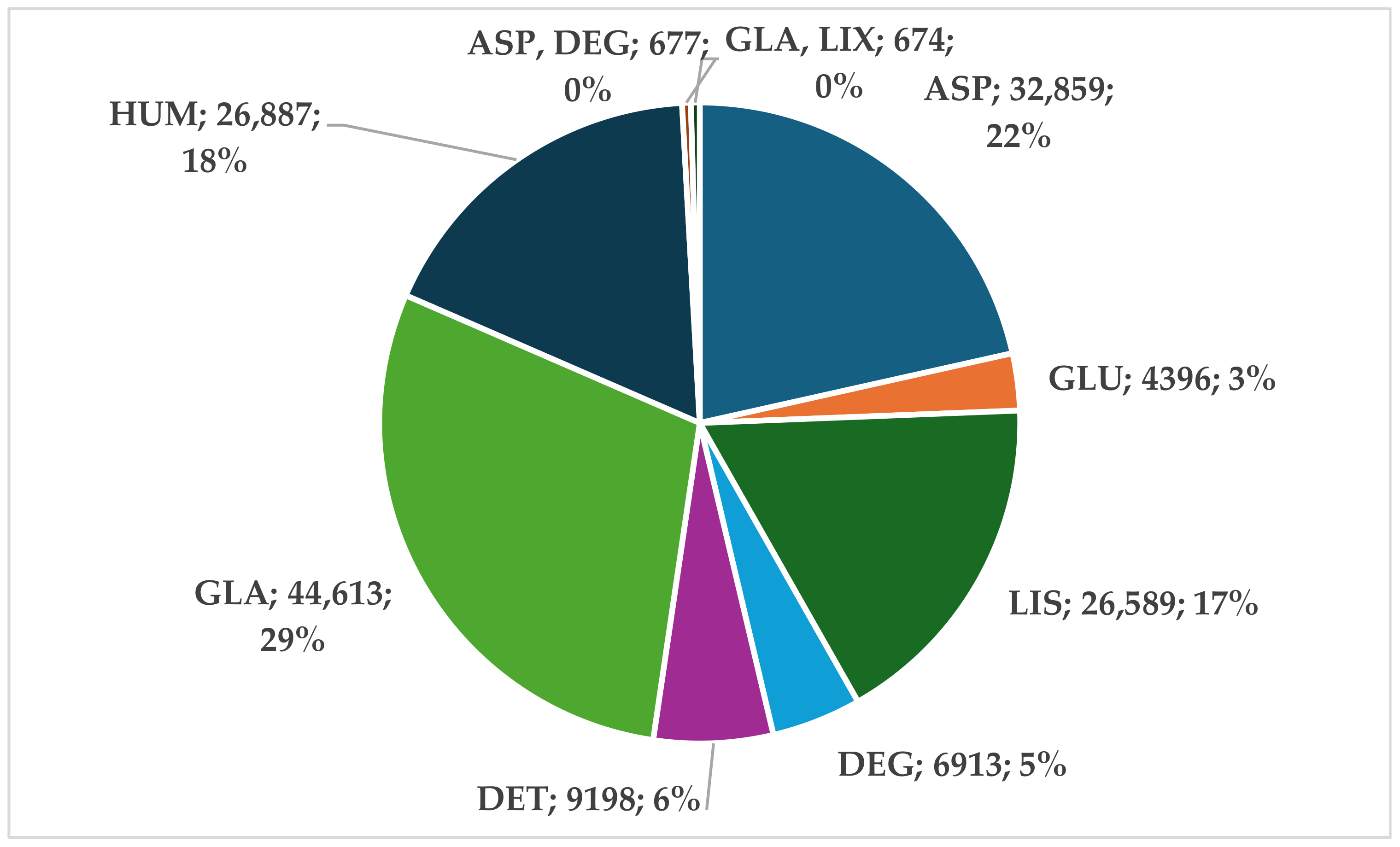
3. Results
3.1. Descriptive Analysis
3.1.1. Analysis of the Entire Insulin Group
3.1.2. Analysis of Rapid-Acting Insulin Analogs (RAIAs)
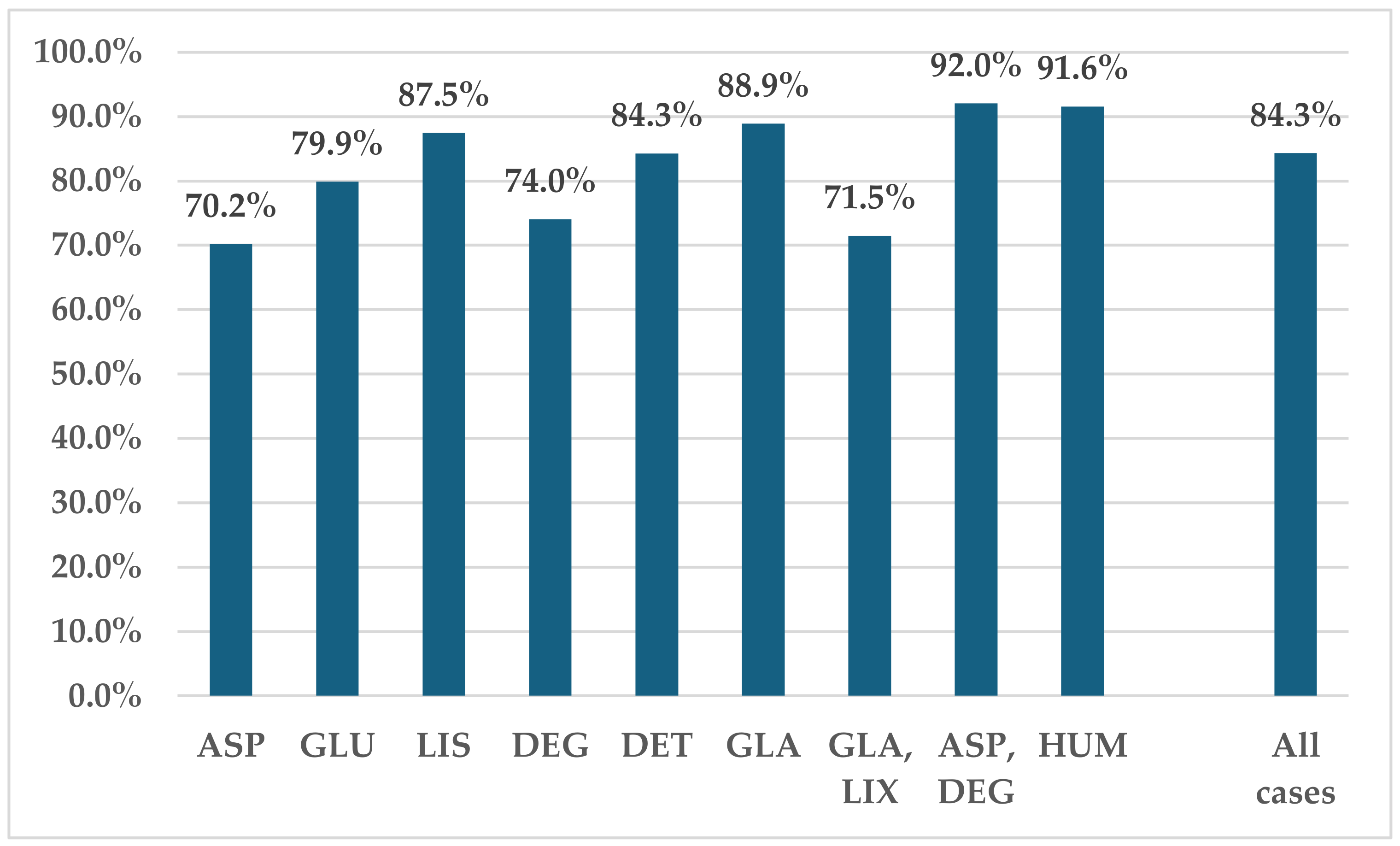
3.2. Disproportionality Analysis
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Diabetes Atlas, 10th ed. 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 12 August 2023).
- Ghibu, S.; Ilie, I.; Mureșan, A.; Mogoșan, C. Perspecives in the Experimental Study of the Metabolic Syndrome. Farmacia 2015, 63, 4. [Google Scholar]
- Pop, C.; Ștefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 7 September 2024).
- Bolli, G.B.; Porcellati, F.; Lucidi, P.; Fanelli, C.G.; Owens, D.R. One-Hundred Year Evolution of Prandial Insulin Preparations: From Animal Pancreas Extracts to Rapid-Acting Analogs. Metab.-Clin. Exp. 2022, 126, 154935. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.; Kroon, L. Ultra-Rapid-Acting Insulins: How Fast Is Really Needed? Clin. Diabetes 2021, 39, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Blumer, I.; Hadar, E.; Hadden, D.R.; Jovanovič, L.; Mestman, J.H.; Murad, M.H.; Yogev, Y. Diabetes and Pregnancy: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4227–4249. [Google Scholar] [CrossRef]
- Bolli, G.B.; Luzio, S.; Marzotti, S.; Porcellati, F.; Sert-Langeron, C.; Charbonnel, B.; Zair, Y.; Owens, D.R. Comparative Pharmacodynamic and Pharmacokinetic Characteristics of Subcutaneous Insulin Glulisine and Insulin Aspart Prior to a Standard Meal in Obese Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2011, 13, 251–257. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes: A Patient-Centered ApproachPosition Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012, 35, 1364–1379. [Google Scholar] [CrossRef]
- Sharma, A.K.; Taneja, G.; Kumar, A.; Sahu, M.; Sharma, G.; Kumar, A.; Sardana, S.; Deep, A. Insulin Analogs: Glimpse on Contemporary Facts and Future Prospective. Life Sci. 2019, 219, 90–99. [Google Scholar] [CrossRef]
- Brunelle, R.L.; Llewelyn, J.; Anderson, J.H.; Gale, E.A.M.; Koivisto, V.A. Meta-Analysis of the Effect of Insulin Lispro on Severe Hypoglycemia in Patients With Type 1 Diabetes. Diabetes Care 1998, 21, 1726–1731. [Google Scholar] [CrossRef]
- Kramer, C.K.; Retnakaran, R.; Zinman, B. Insulin and Insulin Analogs as Antidiabetic Therapy: A Perspective from Clinical Trials. Cell Metab. 2021, 33, 740–747. [Google Scholar] [CrossRef]
- Nicolucci, A.; Ceriello, A.; Di Bartolo, P.; Corcos, A.; Orsini Federici, M. Rapid-Acting Insulin Analogues Versus Regular Human Insulin: A Meta-Analysis of Effects on Glycemic Control in Patients with Diabetes. Diabetes Ther. 2020, 11, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Chakraborty, P.P. Errors of Insulin Therapy: Real-Life Experiences from Developing World. J. Fam. Med. Prim. Care 2017, 6, 724. [Google Scholar] [CrossRef]
- Wei, E.T.; Koh, E.; Kelly, M.S.; Wright, L.A.; Tylee, T.S. Patient Errors in Use of Injectable Antidiabetic Medications: A Need for Improved Clinic-Based Education. J. Am. Pharm. Assoc. 2020, 60, e76–e80. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Raveendran, A.V.; Jayakrishnan, B.; Sreelakshmi, R.; Jose, R.; Chandran, V.; Raj, S.K.; Basanth, A.; Kesavadev, J. How Common Are the Errors in Insulin Injection Techniques—A Real World Study. Int. J. Diabetes Technol. 2023, 2, 109–111. [Google Scholar] [CrossRef]
- Buse, J.B.; Davies, M.J.; Frier, B.M.; Philis-Tsimikas, A. 100 Years on: The Impact of the Discovery of Insulin on Clinical Outcomes. BMJ Open Diabetes Res. Care 2021, 9, e002373. [Google Scholar] [CrossRef]
- Truong, T.H.; Nguyen, T.T.; Armor, B.L.; Farley, J.R. Errors in the Administration Technique of Insulin Pen Devices: A Result of Insufficient Education. Diabetes Ther. 2017, 8, 221–226. [Google Scholar] [CrossRef]
- Cousins, D.; Rosario, C.; Scarpello, J. Insulin, Hospitals and Harm: A Review of Patient Safety Incidents Reported to the National Patient Safety Agency. Clin. Med. 2011, 11, 28–30. [Google Scholar] [CrossRef]
- Ashley-Fenn, W.; Janjua, Z.; Monir, S.; Yaqub, H.; Swaray, A.; Maltese, G.; Malik, I. An Audit of Inpatient Insulin Prescriptions—How Error Relates to Information Source. Futur. Healthc. J. 2024, 11, 100146. [Google Scholar] [CrossRef]
- Frid, A.H.; Hirsch, L.J.; Menchior, A.R.; Morel, D.R.; Strauss, K.W. Worldwide Injection Technique Questionnaire Study: Population Parameters and Injection Practices. Mayo Clin. Proc. 2016, 91, 1212–1223. [Google Scholar] [CrossRef]
- Leese, G.P.; Wang, J.; Broomhall, J.; Kelly, P.; Marsden, A.; Morrison, W.; Frier, B.M.; Morris, A.D. Frequency of Severe Hypoglycemia Requiring Emergency Treatment in Type 1 and Type 2 DiabetesA Population-Based Study of Health Service Resource Use. Diabetes Care 2003, 26, 1176–1180. [Google Scholar] [CrossRef]
- Anderson, J.H.; Brunelle, R.L.; Koivisto, V.A.; Pfützner, A.; Trautmann, M.E.; Vignati, L.; DiMarchi, R. Reduction of Postprandial Hyperglycemia and Frequency of Hypoglycemia in IDDM Patients on Insulin-Analog Treatment. Diabetes 1997, 46, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; Amiel, S.A.; Mansell, P. Effect of the Fast-Acting Insulin Analog Lispro on the Risk of Nocturnal Hypoglycemia during Intensified Insulin Therapy. U.K. Lispro Study Group. Diabetes Care 1999, 22, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; Colagiuri, S.; Vaaler, S.; Wolffenbuttel, B.H.R.; Koelendorf, K.; Friberg, H.H.; Windfeld, K.; Lindholm, A. Hypoglycaemia with Insulin Aspart: A Double-Blind, Randomised, Crossover Trial in Subjects with Type 1 Diabetes. Diabet. Med. 2004, 21, 769–775. [Google Scholar] [CrossRef]
- Kalambokis, G.N.; Tsatsoulis, A.A.; Tsianos, E.V. The Edematogenic Properties of Insulin. Am. J. Kidney Dis. 2004, 44, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Kuroe, A.; Taniuguchi, A.; Fukushima, M.; Nakai, Y.; Ohgushi, M.; Ohya, M.; Seino, Y. Early and Late Onset Side Effects of Short-Acting Insulin Analogue in Seven Japanese Diabetic Patients. Diabetes Res. Clin. Pract. 2007, 77, 412–413. [Google Scholar] [CrossRef]
- Bliss, M. The History of Insulin. Diabetes Care 1993, 16, 4–7. [Google Scholar] [CrossRef]
- Baza Europeană de Date Privind Rapoartele Despre Reacţiile Adverse Suspectate La Medicamente. Available online: https://www.adrreports.eu/ro/index.html (accessed on 15 August 2024).
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. [Google Scholar] [CrossRef]
- European Medicines Agency Good Practice Guide Medication Error Recording Coding Reporting Assessment. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/good-practice-guide-recording-coding-reporting-and-assessment-medication-errors_en.pdf (accessed on 8 September 2024).
- European Medicines Agency Serious Adverse Reaction. Available online: https://www.ema.europa.eu/en/glossary-terms/serious-adverse-reaction (accessed on 7 September 2024).
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the Noise in Signal Detection of Adverse Drug Reactions by Standardizing the Background: A Pilot Study on Analyses of Proportional Reporting Ratios-by-Therapeutic Area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef]
- European Medicines Agency Screening for Adverse Reactions in EudraVigilance. European Medicine Agency. Available online: https://www.ema.europa.eu/en/documents/other/screening-adverse-reactions-eudravigilance_en.pdf (accessed on 7 September 2024).
- Morgovan, C.; Cosma, S.; Polinicencu, C.; Burta, C.; Ghibu, S. Comparative Study Regarding Commercial Policies for the Romanian Antidiabetics’ Market. Farmacia 2011, 59, 5. [Google Scholar]
- Saboo, B.; Chandalia, H.; Ghosh, S.; Kesavadev, J.; Kochar, I.; Prasannakumar, K.; Sarda, A.; Bantwal, G.; Mehrotra, R.; Rai, M. Insulin Glargine in Type 1 Diabetes Mellitus: A Review of Clinical Trials and Real-World Evidence Across Two Decades. Curr. Diabetes Rev. 2024, 20, E100323214554. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.; Roussel, R.; Giaccari, A.; Vora, J.; Brulle-Wohlhueter, C.; Yki-Järvinen, H. Better Glycaemic Control and Less Hypoglycaemia with Insulin Glargine 300 U/ML vs. Glargine 100 U/ML: 1-Year Patient-Level Meta-Analysis of the EDITION Clinical Studies in People with Type 2 Diabetes. Diabetes Obes. Metab. 2018, 20, 541–548. [Google Scholar] [CrossRef]
- Hong, E.G.; Min, K.W.; Lim, J.S.; Ahn, K.J.; Ahn, C.W.; Yu, J.M.; Kim, H.S.; Kim, H.J.; Kim, W.; Kim, D.H.; et al. Real-World Outcomes of Individualized Targeted Therapy with Insulin Glargine 300 Units/ML in Insulin-Naïve Korean People with Type 2 Diabetes: TOBE Study. Adv. Ther. 2024, 41, 1967–1982. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Vora, J. Insulin Aspart: A Review. Expert Opin. Drug Metab. Toxicol. 2006, 2, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Toledano, Y.; Hadar, E.; Hod, M. Safety of Insulin Analogues as Compared with Human Insulin in Pregnancy. Expert Opin. Drug Saf. 2016, 15, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Kapur, R.; Mittra, S.; Tonpe, G.; Adithi, P.; Raj, P.; Gudat, U.; Athalye, S.N. Comparison of the Efficacy and Safety of Rapid-Acting Insulin Analogs, Lispro versus Aspart, in the Treatment of Diabetes: A Systematic Review of Randomized Controlled Trials. Expert Opin. Biol. Ther. 2024, 24, 543–561. [Google Scholar] [CrossRef]
- Sullivan, M.M.; O’Brien, C.R.; Gitelman, S.E.; Shapiro, S.E.; Rushakoff, R.J. Impact of an Interactive Online Nursing Educational Module on Insulin Errors in Hospitalized Pediatric Patients. Diabetes Care 2010, 33, 1744–1746. [Google Scholar] [CrossRef]
- Datye, K.A.; Boyle, C.T.; Simmons, J.; Moore, D.J.; Jaser, S.S.; Sheanon, N.; Kittelsrud, J.M.; Woerner, S.E.; Miller, K.M. Timing of Meal Insulin and Its Relation to Adherence to Therapy in Type 1 Diabetes. J. Diabetes Sci. Technol. 2018, 12, 349–355. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Grey, M. Type 1 Diabetes Self-Management From Emerging Adulthood Through Older Adulthood. Diabetes Care 2018, 41, 1608–1614. [Google Scholar] [CrossRef]
- Robinson, S.; Newson, R.S.; Liao, B.; Kennedy-Martin, T.; Battelino, T. Missed and Mistimed Insulin Doses in People with Diabetes: A Systematic Literature Review. Diabetes Technol. Ther. 2021, 23, 844–856. [Google Scholar] [CrossRef]
- Langerman, C.; Forbes, A.; Robert, G. The Experiences of Insulin Use among Older People with Type 2 Diabetes Mellitus: A Thematic Synthesis. Prim. Care Diabetes 2022, 16, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.L.; Liu, X.M.; Ren, Y.F.; Sun, Y.X.; Luo, M.H.; Ye, L.; Ma, J.H.; Li, F.F. Sexual Differences in Response to Mid- or Low-Premixed Insulin Analogue in Patients with Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 8152640. [Google Scholar] [CrossRef]
- Aanstoot, H.J.; Rodriguez, H.; Weinzimer, S.; Vint, N.; Koeneman, L. Precision Dosing of Rapid-Acting Insulin Matters. Diabetes Technol. Ther. 2020, 22, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.I.; Shehab, N.; Lovegrove, M.C.; Kegler, S.R.; Weidenbach, K.N.; Ryan, G.J.; Budnitz, D.S. National Estimates of Insulin-Related Hypoglycemia and Errors Leading to Emergency Department Visits and Hospitalizations. JAMA Intern. Med. 2014, 174, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.R.; Hemmelgarn, B.R.; Lin, M.; McBrien, K.; Manns, B.J.; Tonelli, M. Hypoglycemia Associated with Hospitalization and Adverse Events in Older People: Population-Based Cohort Study. Diabetes Care 2013, 36, 3585–3590. [Google Scholar] [CrossRef]
- McCoy, R.G.; Lipska, K.J.; Van Houten, H.K.; Shah, N.D. Association of Cumulative Multimorbidity, Glycemic Control, and Medication Use with Hypoglycemia-Related Emergency Department Visits and Hospitalizations Among Adults with Diabetes. JAMA Netw. Open 2020, 3, e1919099. [Google Scholar] [CrossRef]
- Buchbinder, A.; Miodovnik, M.; McElvy, S.; Rosenn, B.; Kranias, G.; Khoury, J.; Siddiqi, T.A. Is Insulin Lispro Associated with the Development or Progression of Diabetic Retinopathy during Pregnancy? Am. J. Obstet. Gynecol. 2000, 183, 1162–1165. [Google Scholar] [CrossRef]
- Kaya, A.; Kar, T.; Aksoy, Y.; Özalper, V.; Başbuǧ, B. Insulin Analogues May Accelerate Progression of Diabetic Retinopathy after Impairment of Inner Blood-Retinal Barrier. Med. Hypotheses 2013, 81, 1012–1014. [Google Scholar] [CrossRef]
- Iliadis, F.; Kadoglou, N.; Didangelos, T. Insulin and the Heart. Diabetes Res. Clin. Pract. 2011, 93, S86–S91. [Google Scholar] [CrossRef]
- Kanamori, T.; Takeshita, Y.; Isobe, Y.; Kato, K.I.; Misu, H.; Kaneko, S.; Takamura, T. Mealtime Dosing of a Rapid-Acting Insulin Analog Reduces Glucose Variability and Suppresses Daytime Cardiac Sympathetic Activity: A Randomized Controlled Study in Hospitalized Patients with Type 2 Diabetes. BMJ Open Diabetes Res. Care 2018, 6, 588. [Google Scholar] [CrossRef]
- Rees, T.; Curtis, B.; Gaskins, K.; Sierra-Johnson, J.; Jiang, H.; Liu, R.; Holcombe, J. Efficacy and Safety of Insulin Lispro in Obese Patients with Type 2 Diabetes: A Retrospective Meta-Analysis of 7 Randomized Controlled Trials. Endocr. Pract. 2014, 20, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Taleb, N.; Messier, V.; Ott-Braschi, S.; Ardilouze, J.L.; Rabasa-Lhoret, R. Perceptions and Experiences of Adult Patients with Type 1 Diabetes Using Continuous Subcutaneous Insulin Infusion Therapy: Results of an Online Survey. Diabetes Res. Clin. Pract. 2018, 144, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Murao, S.; Murao, K.; Nagata, T.; Shimizu, M.; Miyai, Y. Repeated Insulin Injection without Site Rotation Affects Skin Thickness—Ultrasonographic and Histological Evaluation. J. Diabetes Investig. 2022, 13, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.-M.; Miftaraj, M.; Franzén, S.; Eliasson, B. Clinical Effects, Cardiovascular and Renal Outcomes Associated with Rapid-Acting Insulin Analogs among Individuals with Type 2 Diabetes: A Nation-Wide Observational Cohort Study. Clin. Diabetes Endocrinol. 2017, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Plank, J.; Siebenhofer, A.; Berghold, A.; Jeitler, K.; Horvath, K.; Mrak, P.; Pieber, T.R. Systematic Review and Meta-Analysis of Short-Acting Insulin Analogues in Patients With Diabetes Mellitus. Arch. Intern. Med. 2005, 165, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Bzowyckyj, A.S.; Stahnke, A.M. Hypersensitivity Reactions to Human Insulin Analogs in Insulin-Naïve Patients: A Systematic Review. Ther. Adv. Endocrinol. Metab. 2018, 9, 53–65. [Google Scholar] [CrossRef]
- Aberumand, B.; Jeimy, S. The Complexities of Insulin Allergy: A Case and Approach. Allergy Asthma Clin. Immunol. 2021, 17, 79. [Google Scholar] [CrossRef]
- Melo, K.F.S.; Bahia, L.R.; Pasinato, B.; Porfirio, G.J.M.; Martimbianco, A.L.; Riera, R.; Calliari, L.E.P.; Minicucci, W.J.; Turatti, L.A.A.; Pedrosa, H.C.; et al. Short-Acting Insulin Analogues versus Regular Human Insulin on Postprandial Glucose and Hypoglycemia in Type 1 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2019, 11, 2. [Google Scholar] [CrossRef]
- Nyiraty, S.; Pesei, F.; Orosz, A.; Coluzzi, S.; Vági, O.E.; Lengyel, C.; ábrahám, G.; Frontoni, S.; Kempler, P.; Várkonyi, T. Cardiovascular Autonomic Neuropathy and Glucose Variability in Patients with Type 1 Diabetes: Is There an Association? Front. Endocrinol. 2018, 9, 346973. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.H.; Song, K.H.; Ahn, Y.B.; Yoon, K.H.; Yoo, K.D.; Park, Y.M.; Ko, S.H. Cardiovascular Autonomic Dysfunction Predicts Severe Hypoglycemia in Patients With Type 2 Diabetes: A 10-Year Follow-up Study. Diabetes Care 2014, 37, 235–241. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Gosmanov, A.R. Safety of Rapid-Acting Insulin Analogs versus Regular Human Insulin. Am. J. Med. Sci. 2012, 344, 136–141. [Google Scholar] [CrossRef]
- Urata, H.; Mori, K.; Emoto, M.; Yamazaki, Y.; Motoyama, K.; Morioka, T.; Fukumoto, S.; Koyama, H.; Shoji, T.; Ishimura, E.; et al. Advantage of Insulin Glulisine Over Regular Insulin in Patients With Type 2 Diabetes and Severe Renal Insufficiency. J. Ren. Nutr. 2015, 25, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Alsahli, M.; Gerich, J.E. Hypoglycemia in Patients with Diabetes and Renal Disease. J. Clin. Med. 2015, 4, 948. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, A.A.; Wong, I.C.K.; Ma, T.; Lau, W.; Alhamed, M.; Alwafi, H.; Wei, L. The Association between Dementia and the Risk of Hypoglycaemia Events among Patients with Diabetes Mellitus: A Propensity-Score Matched Cohort Analysis. Front. Med. 2023, 10, 1177636. [Google Scholar] [CrossRef] [PubMed]
- Hemkens, L.G.; Grouven, U.; Bender, R.; Günster, C.; Gutschmidt, S.; Selke, G.W.; Sawicki, P.T. Risk of Malignancies in Patients with Diabetes Treated with Human Insulin or Insulin Analogues: A Cohort Study. Diabetologia 2009, 52, 1732–1744. [Google Scholar] [CrossRef]
- Bronsveld, H.K.; ter Braak, B.; Karlstad, Ø.; Vestergaard, P.; Starup-Linde, J.; Bazelier, M.T.; De Bruin, M.L.; de Boer, A.; Siezen, C.L.E.; van de Water, B.; et al. Treatment with Insulin (Analogues) and Breast Cancer Risk in Diabetics; a Systematic Review and Meta-Analysis of in Vitro, Animal and Human Evidence. Breast Cancer Res. 2015, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.B.; Neugebauer, R.; Reynolds, K.; Schmittdiel, J.A.; Loes, L.; Dyer, W.; Pimentel, N.; Desai, J.R.; Vazquez-Benitez, G.; Ho, P.M.; et al. Association of Cardiovascular Outcomes and Mortality With Sustained Long-Acting Insulin Only vs. Long-Acting Plus Short-Acting Insulin Treatment. JAMA Netw. Open 2021, 4, e2126605. [Google Scholar] [CrossRef]
- Burge, M.R.; Carey, J.D. Vitiligo Associated With Subcutaneous Insulin Lispro Infusion in Type 1 Diabetes. Diabetes Care 2004, 27, 275–276. [Google Scholar] [CrossRef]
- Van Hattem, S.; Bootsma, A.H.; Thio, H.B. Skin Manifestations of Diabetes. Cleve. Clin. J. Med. 2008, 75, 772–774. [Google Scholar] [CrossRef]
- Annuzzi, G.; Triggiani, R.; De Angelis, R.; Rainone, C.; Corrado, A.; Scidà, G.; Lupoli, R.; Bozzetto, L. Delayed Prandial Insulin Boluses Are an Important Determinant of Blood Glucose Control and Relate to Fear of Hypoglycemia in People with Type 1 Diabetes on Advanced Technologies. J. Diabetes Complicat. 2024, 38, 108689. [Google Scholar] [CrossRef]
- Johansen, N.J.; Christensen, M.B. A Systematic Review on Insulin Overdose Cases: Clinical Course, Complications and Treatment Options. Basic Clin. Pharmacol. Toxicol. 2018, 122, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, L.; van den Heuvel, C.; Humphries, M.; Byard, R.W. Characteristics of Fatal Insulin Overdoses. Forensic Sci. Med. Pathol. 2022, 18, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Rzepczyk, S.; Dolińska-Kaczmarek, K.; Uruska, A.; Żaba, C. The Other Face of Insulin—Overdose and Its Effects. Toxics 2022, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.K.; Rosano, T.G.; Chambers, E.E.; Pai, M.P.; Desemone, J. Insulin Glargine and Insulin Aspart Overdose With Pharmacokinetic Analysis. AACE Clin. Case Rep. 2016, 2, e122–e128. [Google Scholar] [CrossRef]
- Doǧan, F.S.; Onur, Ö.E.; Altinok, A.D.; Güneysel, Ö. Insulin Glargine Overdose. J. Pharmacol. Pharmacother. 2012, 3, 333. [Google Scholar] [CrossRef]
- Despras, J.; Guedj, A.M.; Soula-Dion, S.; Choukroun, C.; Leguelinel-Blache, G. Assessment of Insulin Adherence in Diabetic Outpatients: An Observational Study. Ann. Pharm. Françaises 2022, 80, 827–836. [Google Scholar] [CrossRef]
- Embaby, A.; Balai, M.; Franssen, E.J.F. Insulin Overdose with Fatal Outcome?: Two Forensic Cases. Toxicol. Rep. 2024, 12, 542–545. [Google Scholar] [CrossRef]
- Boonpattharatthiti, K.; Saensook, T.; Neelapaijit, N.; Sakunrag, I.; Krass, I.; Dhippayom, T. The Prevalence of Adherence to Insulin Therapy in Patients with Diabetes: A Systematic Review and Meta-Analysis. Res. Soc. Adm. Pharm. 2024, 20, 255–295. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Chatterjee, S.; Davies, M.J. Current Management of Diabetes Mellitus and Future Directions in Care. Postgrad. Med. J. 2015, 91, 612–621. [Google Scholar] [CrossRef]
- Tan, S.Y.; Mei Wong, J.L.; Sim, Y.J.; Wong, S.S.; Mohamed Elhassan, S.A.; Tan, S.H.; Ling Lim, G.P.; Rong Tay, N.W.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lingen, K.; Pikounis, T.; Bellini, N.; Isaacs, D. Advantages and Disadvantages of Connected Insulin Pens in Diabetes Management. Endocr. Connect. 2023, 12, e230108. [Google Scholar] [CrossRef]
- MacLeod, J.; Vigersky, R.A. A Review of Precision Insulin Management With Smart Insulin Pens: Opening Up the Digital Door to People on Insulin Injection Therapy. J. Diabetes Sci. Technol. 2023, 17, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Tanaka, J.H.; Beran, D.; Bernabe-Ortiz, A. Health System Responses for Type 1 Diabetes: A Scoping Review. Diabet. Med. 2022, 39, e14805. [Google Scholar] [CrossRef]
- EudraVigilance | European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 3 August 2024).
- Liu, F.; Demosthenes, P. Real-World Data: A Brief Review of the Methods, Applications, Challenges and Opportunities. BMC Med. Res. Methodol. 2022, 22, 287. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Ammendolia, I.; Cardia, L.; Currò, M.; Calapai, G.; Esposito, E.; Mannucci, C. Pharmacovigilance of Risankizumab in the Treatment of Psoriasis and Arthritic Psoriasis: Real-World Data from EudraVigilance Database. Pharmaceutics 2023, 15, 1933. [Google Scholar] [CrossRef]
- Caster, O.; Aoki, Y.; Gattepaille, L.M.; Grundmark, B. Disproportionality Analysis for Pharmacovigilance Signal Detection in Small Databases or Subsets: Recommendations for Limiting False-Positive Associations. Drug Saf. 2020, 43, 479. [Google Scholar] [CrossRef]
- Candore, G.; Monzon, S.; Slattery, J.; Piccolo, L.; Postigo, R.; Xurz, X.; Strauss, S.; Arlett, P. The Impact of Mandatory Reporting of Non-Serious Safety Reports to EudraVigilance on the Detection of Adverse Reactions. Drug Saf. 2022, 45, 83–95. [Google Scholar] [CrossRef]
- Perrone, S.; Imperatore, S.; Sucato, G.; Notarianni, E.; Corbingi, A.; Andriola, C.; Napolitano, M.; Pulsoni, A.; Molica, M. Gilteritinib and the Risk of Intracranial Hemorrhage: A Case Series of a Possible, under-Reported Side Effect. Ann. Hematol. 2023, 102, 3025–3030. [Google Scholar] [CrossRef]








| ADR | PT |
|---|---|
| Improper dose | Dose calculation error |
| Dose calculation error associated with device | |
| Drug dose titration not performed | |
| Drug titration error | |
| Incorrect dosage administered | |
| Incorrect dose administered | |
| Incorrect dose administered by device | |
| Incorrect dose administered by product | |
| Incorrect product dosage form administered | |
| Product dosage form confusion | |
| Wrong dosage formulation | |
| Wrong dose | |
| Overdose | Accidental overdose |
| Extra dose administered | |
| Overdose | |
| Prescribed overdose | |
| Underdose | Accidental underdose |
| Drug dose omission by device | |
| Incomplete dose administered | |
| Prescribed underdose | |
| Product dose omission | |
| Product dose omission in error | |
| Product dose omission issue | |
| Underdose |
| ASP | GLU | LIS | DEG | DET | GLA | HUM | ASP, DEG | GLA, LIX | |
|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | n | n | n | n | n | |
| % | % | % | % | % | % | % | % | % | |
| Total cases | 32,860 | 4396 | 26,589 | 6913 | 9198 | 44,613 | 26,887 | 677 | 674 |
| (100.00%) | (100.00%) | (100.00%) | (100.00%) | (100.00%) | (100.00%) | (100.00%) | (100.00%) | (100.00%) | |
| Age category | |||||||||
| Not Specified | 9511 | 1374 | 8228 | 2339 | 3131 | 14,408 | 7897 | 157 | 332 |
| (28.90%) | (31.30%) | (30.90%) | (33.80%) | (34.00%) | (32.30%) | (29.40%) | (23.20%) | (49.30%) | |
| 0–1 Month | 71 | 13 | 95 | 8 | 42 | 74 | 140 | 0 | 0 |
| (0.20%) | (0.30%) | (0.40%) | (0.10%) | (0.50%) | (0.20%) | (0.50%) | (0.00%) | (0.00%) | |
| 2 Months–2 Years | 100 | 6 | 59 | 17 | 16 | 51 | 92 | 0 | 0 |
| (0.30%) | (0.10%) | (0.20%) | (0.20%) | (0.20%) | (0.10%) | (0.30%) | (0.00%) | (0.00%) | |
| 3–11 Years | 1255 | 104 | 421 | 118 | 226 | 529 | 443 | 3 | 0 |
| (3.80%) | (2.40%) | (1.60%) | (1.70%) | (2.50%) | (1.20%) | (1.60%) | (0.40%) | (0.00%) | |
| 12–17 Years | 1439 | 119 | 433 | 149 | 205 | 608 | 463 | 11 | 0 |
| (4.40%) | (2.70%) | (1.60%) | (2.20%) | (2.20%) | (1.40%) | (1.70%) | (1.60%) | (0.00%) | |
| 18–64 Years | 12,801 | 1618 | 9601 | 2349 | 3153 | 14,097 | 9763 | 215 | 164 |
| (39.00%) | (36.80%) | (36.10%) | (34.00%) | (34.30%) | (31.60%) | (36.30%) | (31.80%) | (24.30%) | |
| 65–85 Years | 7026 | 1023 | 7011 | 1735 | 2194 | 13,312 | 7230 | 251 | 175 |
| (21.40%) | (23.30%) | (26.40%) | (25.10%) | (23.90%) | (29.80%) | (26.90%) | (37.10%) | (26.00%) | |
| More than 85 Years | 656 | 139 | 741 | 198 | 231 | 1534 | 859 | 40 | 3 |
| (2.00%) | (3.20%) | (2.80%) | (2.90%) | (2.50%) | (3.40%) | (3.20%) | (5.90%) | (0.40%) | |
| Sex | |||||||||
| Female | 16,449 | 2243 | 13,764 | 3724 | 5115 | 23,319 | 13,718 | 334 | 344 |
| (50.10%) | (51.00%) | (51.80%) | (53.90%) | (55.60%) | (52.30%) | (51.00%) | (49.30%) | (51.00%) | |
| Male | 14,501 | 1963 | 12,033 | 3025 | 3419 | 19,519 | 11,781 | 328 | 281 |
| (44.10%) | (44.70%) | (45.30%) | (43.80%) | (37.20%) | (43.80%) | (43.80%) | (48.40%) | (41.70%) | |
| NS | 1909 | 190 | 792 | 164 | 664 | 1775 | 1388 | 15 | 49 |
| (5.80%) | (4.30%) | (3.00%) | (2.40%) | (7.20%) | (4.00%) | (5.20%) | (2.20%) | (7.30%) | |
| Geographic origin | |||||||||
| EEA | 16,410 | 1822 | 7796 | 2845 | 3256 | 11,534 | 7251 | 66 | 259 |
| (49.90%) | (41.40%) | (29.30%) | (41.20%) | (35.40%) | (25.90%) | (27.00%) | (9.70%) | (38.40%) | |
| Non-EEA | 16,449 | 2574 | 18,793 | 4068 | 5942 | 33,079 | 19,636 | 611 | 415 |
| (50.10%) | (58.60%) | (70.70%) | (58.80%) | (64.60%) | (74.10%) | (73.00%) | (90.30%) | (61.60%) | |
| Reporter | |||||||||
| HP | 16,174 | 2429 | 12,073 | 3880 | 4664 | 18,712 | 15,324 | 444 | 488 |
| (49.20%) | (55.30%) | (45.40%) | (56.10%) | (50.70%) | (41.90%) | (57.00%) | (65.60%) | (72.40%) | |
| Non HP | 16,666 | 1956 | 14,494 | 3033 | 4532 | 25,670 | 11,409 | 233 | 186 |
| (50.70%) | (44.50%) | (54.50%) | (43.90%) | (49.30%) | (57.50%) | (42.40%) | (34.40%) | (27.60%) | |
| NS | 19 | 11 | 22 | 0 | 2 | 231 | 154 | 0 | 0 |
| (0.10%) | (0.30%) | (0.10%) | (0.00%) | (0.00%) | (0.50%) | (0.60%) | (0.00%) | (0.00%) | |
| ASP | GLU | LIS | |
|---|---|---|---|
| Blood and lymphatic system disorders | 0.24% | 0.18% | 0.37% |
| Cardiac disorders | 2.00% | 1.71% | 3.14% |
| Congenital, familial, and genetic disorders | 0.17% | 0.12% | 0.22% |
| Ear and labyrinth disorders | 0.25% | 0.33% | 0.64% |
| Endocrine disorders | 0.12% | 0.18% | 0.27% |
| Eye disorders | 2.03% | 2.67% | 5.18% |
| Gastrointestinal disorders | 2.51% | 2.83% | 3.19% |
| General disorders and administration site conditions | 10.92% | 12.56% | 12.04% |
| Hepatobiliary disorders | 0.44% | 0.42% | 0.59% |
| Immune system disorders | 1.18% | 1.28% | 1.18% |
| Infections and infestations | 2.39% | 2.13% | 3.47% |
| Injury, poisoning, and procedural complications | 8.95% | 11.85% | 11.74% |
| Investigations | 19.08% | 13.66% | 18.25% |
| Metabolism and nutrition disorders | 18.66% | 20.55% | 12.03% |
| Musculoskeletal and connective tissue disorders | 1.27% | 1.86% | 2.72% |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | 0.85% | 0.76% | 1.88% |
| Nervous system disorders | 7.61% | 8.20% | 9.69% |
| Pregnancy, puerperium, and perinatal conditions | 0.98% | 0.61% | 0.65% |
| Product issues | 10.40% | 4.59% | 0.32% |
| Psychiatric disorders | 1.54% | 2.73% | 2.67% |
| Renal and urinary disorders | 1.15% | 1.34% | 2.20% |
| Reproductive system and breast disorders | 0.14% | 0.23% | 0.23% |
| Respiratory, thoracic, and mediastinal disorders | 1.38% | 1.69% | 2.17% |
| Skin and subcutaneous tissue disorders | 2.88% | 4.31% | 2.47% |
| Social circumstances | 0.33% | 0.44% | 0.10% |
| Surgical and medical procedures | 1.54% | 1.46% | 0.81% |
| Vascular disorders | 0.97% | 1.30% | 1.76% |
| Fatal Outcome | Not Recovered/Not Resolved | |||||
|---|---|---|---|---|---|---|
| LIS | GLU | ASP | LIS | GLU | ASP | |
| improper doses | 0.1% | 0.0% | 0.3% | 2.7% | 2.3% | 1.8% |
| overdose | 4.5% | 0.0% | 3.2% | 3.6% | 2.2% | |
| underdose | 0.0% | 0.0% | 0.7% | 4.9% | 9.5% | 7.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa Ilie, I.R.; Vonica-Tincu, A.L.; Dobrea, C.M.; Butuca, A.; Frum, A.; Morgovan, C.; Gligor, F.G.; Ghibu, S. Safety Profiles Related to Dosing Errors of Rapid-Acting Insulin Analogs: A Comparative Analysis Using the EudraVigilance Database. Biomedicines 2024, 12, 2273. https://doi.org/10.3390/biomedicines12102273
Popa Ilie IR, Vonica-Tincu AL, Dobrea CM, Butuca A, Frum A, Morgovan C, Gligor FG, Ghibu S. Safety Profiles Related to Dosing Errors of Rapid-Acting Insulin Analogs: A Comparative Analysis Using the EudraVigilance Database. Biomedicines. 2024; 12(10):2273. https://doi.org/10.3390/biomedicines12102273
Chicago/Turabian StylePopa Ilie, Ioana Rada, Andreea Loredana Vonica-Tincu, Carmen Maximiliana Dobrea, Anca Butuca, Adina Frum, Claudiu Morgovan, Felicia Gabriela Gligor, and Steliana Ghibu. 2024. "Safety Profiles Related to Dosing Errors of Rapid-Acting Insulin Analogs: A Comparative Analysis Using the EudraVigilance Database" Biomedicines 12, no. 10: 2273. https://doi.org/10.3390/biomedicines12102273






