The Effect of Nerolidol on Renal Dysfunction following Bilateral Ureteral Obstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Bilateral Ureteral Obstruction and Reversal of Obstruction
2.2. Nerolidol and Vehicle Administration
2.3. Experimental Groups
- G-sham (n = 12): rats that underwent sham manipulation of both ureters and received the vehicle only.
- G-BUO (n = 12): rats that underwent bilateral ureteral obstruction for 24 h and received only the vehicle.
- G-BUO/NR (n = 12): rats that underwent bilateral ureteral obstruction for 24 h and received nerolidol dissolved in the vehicle.
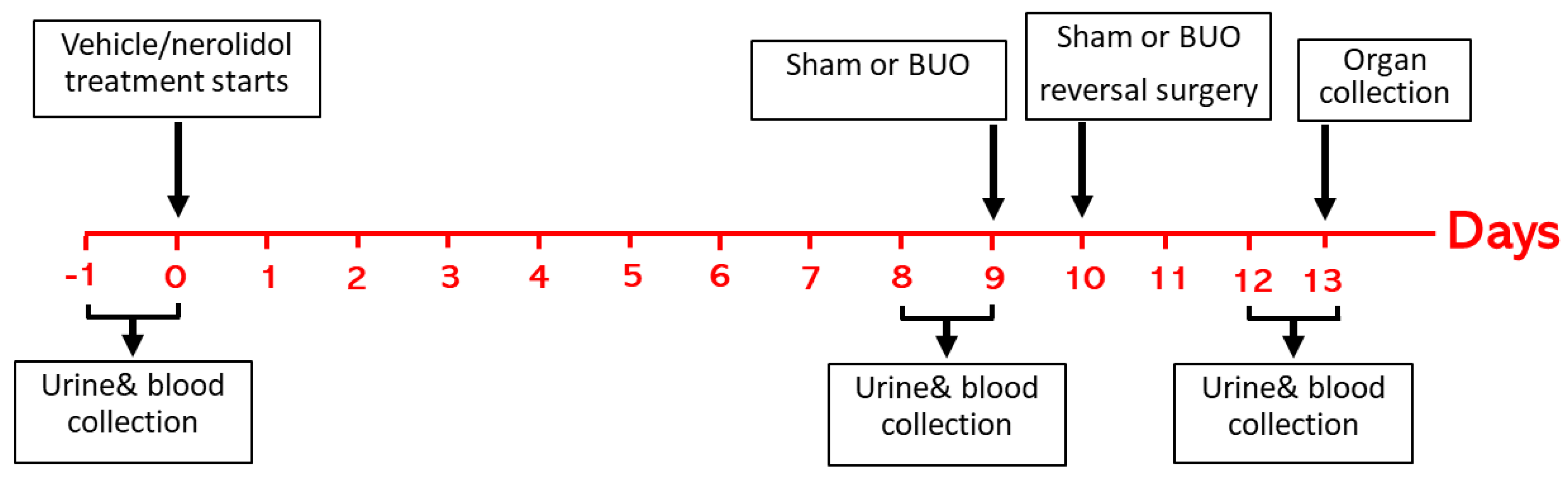
2.4. Experimental Protocol and Sample Collection
2.5. Gene Expression Analysis
- Acute kidney injury markers: kidney injury molecule-1 (KIM1) and neutrophil gelatinase-associated lipocalin (NGAL).
- Cytokines involved in inflammation and fibrosis: tumor necrosis factor-α (TNF-α), interlukin-1 beta (IL-1β), Interleukin-6 (IL-6), and plasminogen activator inhibitor-1 (PAI-1).
- Pro-apoptotic gene p53.
- Oxidative stress markers: glutathione peroxidase (GPX-1) and glutathione-disulfide reductase (GSR).
2.6. Histological Studies
2.7. Statistical Analysis
3. Results
3.1. Renal Functions
3.2. Gene Expression Analysis Results
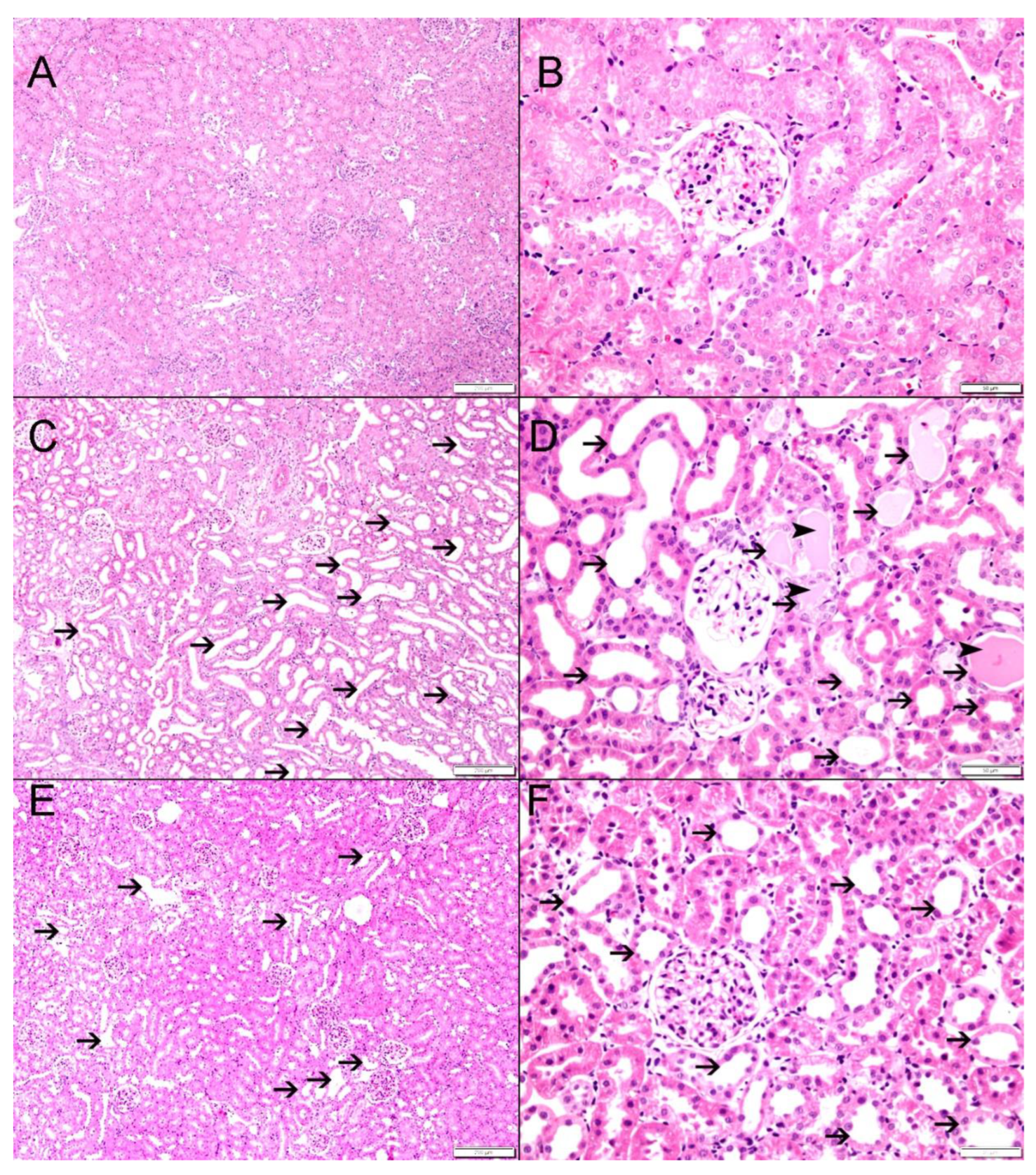
3.3. Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chávez-Iñiguez, J.S.; Navarro-Gallardo, G.J.; Medina-González, R.; Alcantar-Vallin, L.; García-García, G. Acute Kidney Injury Caused by Obstructive Nephropathy. Int. J. Nephrol. 2020, 2020, 8846622. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aizpurua, X.; Cabello Benavente, R.; Bueno Serrano, G.; Alcázar Peral, J.M.; Gómez-Jordana Mañas, B.; Tufet, I.J.J.; Ruiz de Castroviejo Blanco, J.; Osorio Ospina, F.; Gonzalez-Enguita, C. Obstructive uropathy: Overview of the pathogenesis, etiology and management of a prevalent cause of acute kidney injury. World J. Nephrol. 2024, 13, 93322. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Harris, K.; Purkerson, M.L. Effects of obstruction on renal functions. Pediatr. Nephrol. 1988, 2, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Modi, K.S.; Harris, K.P.; Klahr, S. Effects of unilateral or bilateral release of bilateral ureteral obstruction on renal function in rat. Nephron 1993, 64, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.G. Partial unilateral ureteral obstruction in rats. Neurourol. Urodyn. 2002, 21, 231–250. [Google Scholar] [CrossRef]
- Wen, J.G.; Pedersen, M.; Dissing, T.H.; Stodkilde-Jorgensen, H.; Jorgensen, T.M.; Djurhuus, J.C.; Frokiaer, J. Evaluation of complete and partially obstructed kidneys using Gd-DTPA enhanced dynamic MRI in adolescent swine. Eur. J. Pediatr. Surg. 2008, 18, 322–327. [Google Scholar] [CrossRef]
- Yaxley, J.; Yaxley, W. Obstructive uropathy—Acute and chronic medical management. World J. Nephrol. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Kim, S.W. Phytotherapy: Emerging therapeutic option in urologic disease. Transl. Androl. Urol. 2012, 1, 181–191. [Google Scholar]
- Ferreira, F.M.; Palmeira, C.M.; Oliveira, M.M.; Santos, D.; Simões, A.M.; Rocha, S.M.; Coimbra, M.A.; Peixoto, F. Nerolidol effects on mitochondrial and cellular energetics. Toxicol. Vitr. 2012, 26, 189–196. [Google Scholar] [CrossRef]
- Lapczynski, A.; Bhatia, S.P.; Letizia, C.S.; Api, A.M. Fragrance material review on nerolidol (isomer unspecified). Food Chem. Toxicol. 2008, 46 (Suppl. S11), S247–S250. [Google Scholar] [CrossRef]
- Chan, W.K.; Tan, L.T.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Nogueira Neto, J.D.; de Almeida, A.A.; da Silva Oliveira, J.; Dos Santos, P.S.; de Sousa, D.P.; de Freitas, R.M. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Asaikumar, L.; Vennila, L. Preventive effect of nerolidol on isoproterenol induced myocardial damage in Wistar rats: Evidences from biochemical and histopathological studies. Drug Dev. Res. 2019, 80, 814–823. [Google Scholar] [CrossRef]
- Krist, S.; Banovac, D.; Tabanca, N.; Wedge, D.E.; Gochev, V.K.; Wanner, J.; Schmidt, E.; Jirovetz, L. Antimicrobial activity of nerolidol and its derivatives against airborne microbes and further biological activities. Nat. Prod. Commun. 2015, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, D.; Bao, Y.; Shi, Y.; Cui, Y.; Guo, M. Nerolidol Protects Against LPS-induced Acute Kidney Injury via Inhibiting TLR4/NF-κB Signaling. Phytother. Res. 2017, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, A.; Najmi, A.K.; Md, S.; Alkreathy, H.M.; Ali, J.; Syed, M.A.; Haque, S.E. Oral delivery of nerolidol alleviates cyclophosphamide-induced renal inflammation, apoptosis, and fibrosis via modulation of NF-κB/cleaved caspase-3/TGF-β signaling molecules. Drug Deliv. 2023, 30, 2241661. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al-Salam, S.; Ahmad, R.; Yasin, J.; Hammad, A.F.; Rasheed, J.A.; Lubbad, L. The Effect of Nerolidol Renal Dysfunction following Ischemia-Reperfusion Injury in the Rat. Nutrients 2023, 15, 455. [Google Scholar] [CrossRef]
- Hammad, F.T.; Al-Salam, S.; Hammad, W.F.; Yasin, J.; Lubbad, L. Despite initial recovery of GFR, long-term renal functions deteriorate following short periods of unilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol. 2020, 319, F523–f533. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L. Does curcumin protect against renal dysfunction following reversible unilateral ureteric obstruction in the rat? Eur. Surg. Res. 2011, 46, 188–193. [Google Scholar] [CrossRef]
- Hammad, F.T.; Lubbad, L.; Al-Salam, S.; Hammad, W.F.; Yasin, J.; Meeran, M.F.N.; Ojha, S.; Arunachalam, S.; Hammad, A.F. Does Hypertension Affect the Recovery of Renal Functions after Reversal of Unilateral Ureteric Obstruction? Int. J. Mol. Sci. 2024, 25, 1540. [Google Scholar] [CrossRef]
- Hammad, F.T.; Wheatley, A.M.; Davis, G. Long-term renal effects of unilateral ureteral obstruction and the role of endothelin. Kidney Int. 2000, 58, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Capelouto, C.C.; Saltzman, B. The pathophysiology of ureteral obstruction. J. Endourol. 1993, 7, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Urinary tract obstruction. Semin. Nephrol. 2001, 21, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.R. Pathophysiology of obstructive nephropathy. Kidney Int. 1980, 18, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Leal, M.C.; Casabona, J.C.; Puntel, M.; Pitossi, F.J. Interleukin-1β and tumor necrosis factor-α: Reliable targets for protective therapies in Parkinson’s Disease? Front. Cell. Neurosci. 2013, 7, 53. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tanabe, K.; Takai, S.; Matsushima-Nishiwaki, R.; Adachi, S.; Iida, H.; Kozawa, O.; Dohi, S. Involvement of Rho-kinase in tumor necrosis factor-alpha-induced interleukin-6 release from C6 glioma cells. Neurochem. Int. 2009, 55, 438–445. [Google Scholar] [CrossRef]
- Lin, Y.M.; Badrealam, K.F.; Kuo, W.W.; Lai, P.F.; Shao-Tsu Chen, W.; Hsuan Day, C.; Ho, T.J.; Viswanadha, V.P.; Shibu, M.A.; Huang, C.Y. Nerolidol improves cardiac function in spontaneously hypertensive rats by inhibiting cardiac inflammation and remodelling associated TLR4/ NF-κB signalling cascade. Food Chem. Toxicol. 2021, 147, 111837. [Google Scholar] [CrossRef]
- Lin, X.; Kong, J.; Wu, Q.; Yang, Y.; Ji, P. Effect of TLR4/MyD88 signaling pathway on expression of IL-1β and TNF-α in synovial fibroblasts from temporomandibular joint exposed to lipopolysaccharide. Mediat. Inflamm. 2015, 2015, 329405. [Google Scholar] [CrossRef]
- Berkenbosch, F.; van Rooijen, N.; Tilders, F.H. Determining Role and Sources of Endogenous Interleukin 1 in Pituitary–Adrenal Activation in Response to Stressful and Inflammatory Stimuli. Methods Neurosci. 1993, 16, 211–231. [Google Scholar]
- Fonsêca, D.V.; Salgado, P.R.; de Carvalho, F.L.; Salvadori, M.G.; Penha, A.R.; Leite, F.C.; Borges, C.J.; Piuvezam, M.R.; Pordeus, L.C.; Sousa, D.P.; et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2016, 30, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Muse, K.E.; Oberley, T.D.; Sempf, J.M.; Oberley, L.W. Immunolocalization of antioxidant enzymes in adult hamster kidney. Histochem. J. 1994, 26, 734–753. [Google Scholar] [CrossRef]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef]
- Lüersen, K.; Stegehake, D.; Daniel, J.; Drescher, M.; Ajonina, I.; Ajonina, C.; Hertel, P.; Woltersdorf, C.; Liebau, E. The glutathione reductase GSR-1 determines stress tolerance and longevity in Caenorhabditis elegans. PLoS ONE 2013, 8, e60731. [Google Scholar] [CrossRef]
- Vinholes, J.; Gonçalves, P.; Martel, F.; Coimbra, M.A.; Rocha, S.M. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014, 156, 204–211. [Google Scholar] [CrossRef]
- Shan, Z.; Tan, D.; Satriano, J.; Silbiger, S.; Schlondorff, D. Intracellular glutathione influences collagen generation by mesangial cells. Kidney Int. 1994, 46, 388–395. [Google Scholar] [CrossRef][Green Version]
- Wen, J.; Shu, Y.; Zhang, W. ROS, P53, and ischemic acute kidney injury in diabetic models. Kidney Int. 2015, 88, 198–199. [Google Scholar] [CrossRef][Green Version]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Z. The Update of NGAL in Acute Kidney Injury. Curr. Protein Pept. Sci. 2017, 18, 1211–1217. [Google Scholar] [CrossRef] [PubMed]




| KIM-1 (NM_173149.2) | Forward | GCCTGGAATAATCACACTGTAAG |
| Reverse | GCAACGGACATGCCAACATAG | |
| Probe | d FAM-TCCCTTTGAGGAAGCCGCAGA-BHQ-1 | |
| NGAL (NM_130741.1) | Forward | CTGTTCCCACCGACCAATGC |
| Reverse | CCACTGCACATCCCAGTCA | |
| Probe | FAM-TGACAACTGAACAGACGGTGAGCG-BHQ-1 | |
| TNF-α (NM_012675.3) | Forward | CTCACACTCAGATCATCTTCTC |
| Reverse | CCGCTTGGTGGTTTGCTAC | |
| Probe | FAM-CTCGAGTGACAAGCCCGTAGCC-BHQ-1 | |
| PAI-1 (NM_012620.1) | Forward | GGCACAATCCAACAGAGACAA |
| Reverse | GGCTTCTCATCCCACTCTCAAG | |
| Probe | FAM-CCTCTTCATGGGCCAGCTGATGG-BHQ-1 | |
| IL-6 (NM_012589.2) | Forward | TCACAGAGGATACCACCCACAACA |
| Reverse | CACAAGTCCGGAGAGGAGAC | |
| Probe | FAM-TCAGAATTGCCATTGCACAACTCT-BHQ-1 | |
| IL-1β (NM_031512.2) | Forward | ATGCCTCGTGCTGTCTGACC |
| Reverse | GCTCATGGAGAATACCACTTGTTGG | |
| Probe | FAM-AGCTGAAAGCTCTCCACCTCAATGGA-BHQ-1 | |
| p53 (NM_030989.3) | Forward | CGAGATGTTCCGAGAGCTGAATG |
| Reverse | GTCTTCGGGTAGCTGGAGTG | |
| Probe | FAM-CCTTGGAATTAAAGGATGCCCGTGC-BHQ-1 | |
| GSR NM_053906.2 | Forward | CATCCCTACCGTGGTCTTCAG |
| Reverse | ATGGACGGCTTCATCTTCAGT | |
| Probe | FAM-CCACCCGCCTATCGGGACAGT-BHQ-1 | |
| GPx-1 NM_030826.4 | Forward | GTGCTGCTCATTGAGAATGTCG |
| Reverse | TCATTCTTGCCATTCTCCTGATG | |
| Probe | FAM-TCCCTCTGAGGCACCACGAC-BHQ-1 | |
| PPIA (NM_017101.1) | Forward | GCGTCTGCTTCGAGCTGT |
| Reverse | CACCCTGGCACATGAATCC | |
| Probe | Quasar 670-TGCAGACAAAGTTCCAAAGACAGCA-BHQ-2 |
| G-sham | G-BUO | G-BUO/NR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Basal | Pre-BUO | Post-BUO | Basal | Pre-BUO | Post-BUO | Basal | Pre-BUO | Post-BUO | |
| Serum Creatinine (mg/dL) | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.21 ± 0.02 | 0.19 ± 0.01 | 0.23 ± 0.01 | 0.32 ± 0.02 $ | 0.19 ± 0.01 | 0.21 ± 0.01 | 0.26 ± 0.02 * |
| Serum Urea (mg/dL) | 46.9 ± 1.3 | 44.7 ± 1.7 | 43.5 ± 2.8 | 48.0 ± 1.2 | 44.6 ± 1.2 | 56.5 ± 1.7 $ | 45.9 ± 2.9 | 45.5 ± 1.9 | 50.2 ± 1.1 * |
| Creatinine Clearance (mL/min/100 gm b.w.) | 1.01 ± 0.09 | 1.04 ± 0.08 | 1.08 ± 0.07 | 1.03 ± 0.06 | 1.06 ± 0.06 | 0.63 ± 0.07 $ | 0.99 ± 0.07 | 1.09 ± 0.09 | 0.95 ± 0.11 * |
| Albumin Creatinine Ratio | 13.8 ± 1.9 | 11.4 ± 2.7 | 14.1 ± 1.5 | 15.9 ± 2.6 | 12.0 ± 1.5 | 180.0 ± 34.0 $ | 15.0 ± 4.1 | 11.8 ± 2.7 | 73.5 ± 18.9 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toumi, H.R.; Sallabi, S.M.; Lubbad, L.; Al-Salam, S.; Hammad, F.T. The Effect of Nerolidol on Renal Dysfunction following Bilateral Ureteral Obstruction. Biomedicines 2024, 12, 2285. https://doi.org/10.3390/biomedicines12102285
Toumi HR, Sallabi SM, Lubbad L, Al-Salam S, Hammad FT. The Effect of Nerolidol on Renal Dysfunction following Bilateral Ureteral Obstruction. Biomedicines. 2024; 12(10):2285. https://doi.org/10.3390/biomedicines12102285
Chicago/Turabian StyleToumi, Harun R., Sundus M. Sallabi, Loay Lubbad, Suhail Al-Salam, and Fayez T. Hammad. 2024. "The Effect of Nerolidol on Renal Dysfunction following Bilateral Ureteral Obstruction" Biomedicines 12, no. 10: 2285. https://doi.org/10.3390/biomedicines12102285
APA StyleToumi, H. R., Sallabi, S. M., Lubbad, L., Al-Salam, S., & Hammad, F. T. (2024). The Effect of Nerolidol on Renal Dysfunction following Bilateral Ureteral Obstruction. Biomedicines, 12(10), 2285. https://doi.org/10.3390/biomedicines12102285





