Weight Loss Therapies and Hypertension Benefits
Abstract
:1. Introduction
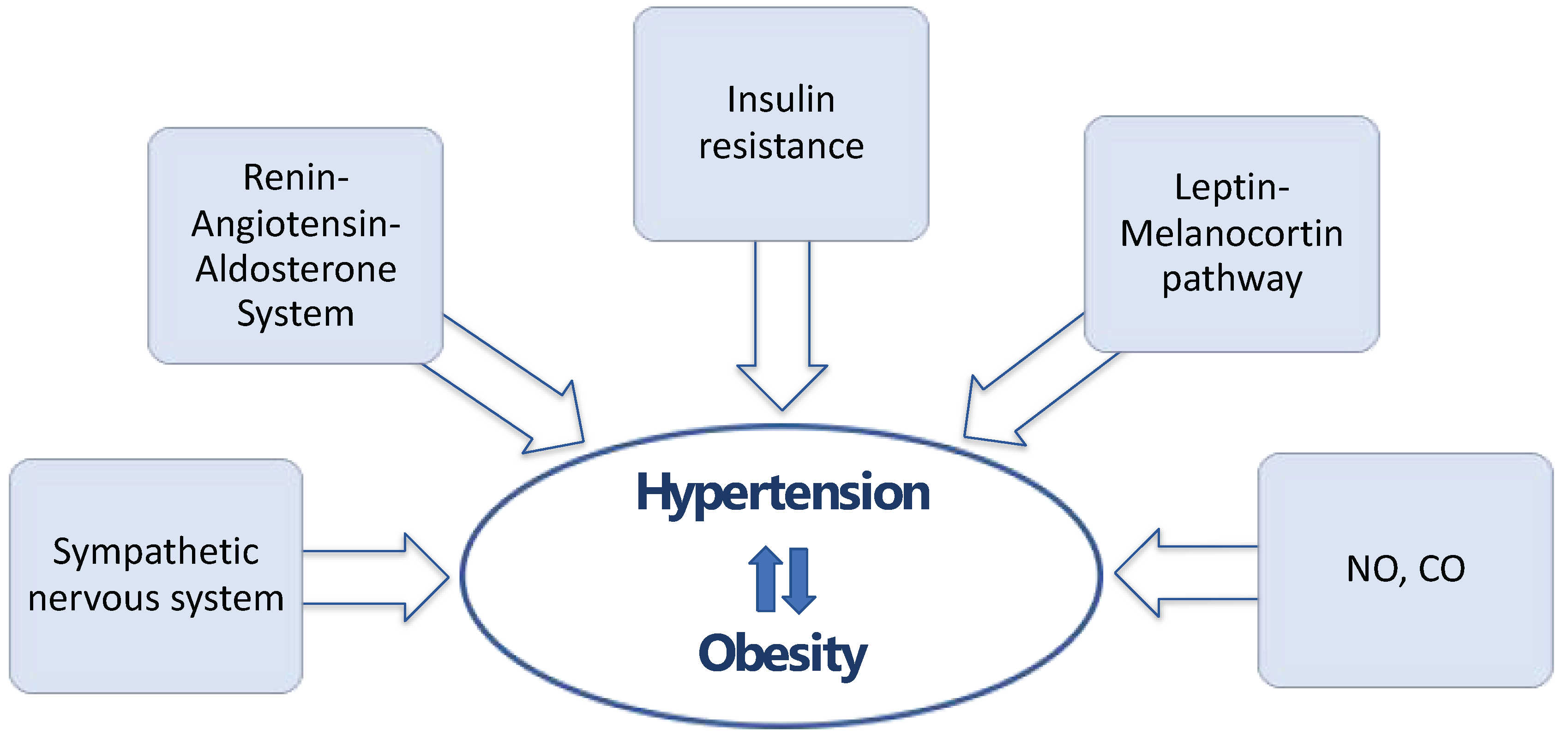
2. The Link between Obesity and Hypertension
2.1. Sympathetic Nervous System
2.2. Renin–Angiotensin–Aldosterone System
2.3. Insulin Resistance
2.4. Leptin–Melanocortin Pathway
2.5. New Mediators in Hypertension
3. Blood Pressure and Weight Loss
4. Lifestyle Interventions
5. Pharmacological Therapy for Weight Reduction
5.1. Phentermine/Topiramate
5.1.1. Mechanism of Action
5.1.2. Clinical Evidence on Blood Pressure
5.1.3. Mechanisms Affecting Blood Pressure
5.2. Orlistat
5.2.1. Mechanism of Action
5.2.2. Clinical Evidence on Blood Pressure
5.2.3. Mechanisms Affecting Blood Pressure
5.3. Naltrexone/Bupropion
5.3.1. Mechanism of Action
5.3.2. Clinical Evidence on Blood Pressure
5.3.3. Mechanisms Affecting Blood Pressure
5.4. Glucagon-like Peptide-1 Receptor Agonists
5.4.1. Mechanism of Action
5.4.2. Clinical Evidence on Blood Pressure
Liraglutide
Semaglutide
5.4.3. Mechanisms Affecting Blood Pressure
5.5. Tirzepatide
5.5.1. Mechanism of Action
5.5.2. Clinical Evidence on Blood Pressure
SURPASS Trials in Diabetic Patients
SURMOUNT Trials in Overweight/Obese Individuals
Data from Meta-Analyses
5.5.3. Mechanisms Affecting Blood Pressure
6. Bariatric Surgery
6.1. Mechanism of Action
6.2. Clinical Evidence on Blood Pressure
6.3. Mechanisms Affecting Blood Pressure
7. Discussion
8. Limitations
9. Future Directions
10. Conclusions
Funding
Conflicts of Interest
References
- Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 28 August 2024).
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the Management of Arterial Hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309. [Google Scholar] [CrossRef]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D.; et al. A Call to Action and a Lifecourse Strategy to Address the Global Burden of Raised Blood Pressure on Current and Future Generations: The Lancet Commission on Hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 28 August 2024).
- Seravalle, G.; Grassi, G. Obesity and Hypertension. Pharmacol. Res. 2017, 122, 65–79. [Google Scholar] [CrossRef]
- Fantin, F.; Giani, A.; Zoico, E.; Rossi, A.P.; Mazzali, G.; Zamboni, M. Weight Loss and Hypertension in Obese Subjects. Nutrients 2019, 11, 1667. [Google Scholar] [CrossRef]
- Grassi, G. Assessment of Sympathetic Cardiovascular Drive in Human Hypertension: Achievements and Perspectives. Hypertension 2009, 54, 690–697. [Google Scholar] [CrossRef]
- Shariq, O.A.; McKenzie, T.J. Obesity-Related Hypertension: A Review of Pathophysiology, Management, and the Role of Metabolic Surgery. Gland. Surg. 2020, 9, 80–93. [Google Scholar] [CrossRef]
- Reid, I.A. Interactions between ANG II, Sympathetic Nervous System, and Baroreceptor Reflexes in Regulation of Blood Pressure. Am. J. Physiol. 1992, 262, E763–E778. [Google Scholar] [CrossRef]
- Fisher, J.P.; Paton, J.F.R. The Sympathetic Nervous System and Blood Pressure in Humans: Implications for Hypertension. J. Hum. Hypertens. 2012, 26, 463–475. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. The Autonomic Nervous System and Hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef]
- Mancia, G.; Grassi, G. The Central Sympathetic Nervous System in Hypertension. Handb. Clin. Neurol. 2013, 117, 329–335. [Google Scholar] [CrossRef]
- El Meouchy, P.; Wahoud, M.; Allam, S.; Chedid, R.; Karam, W.; Karam, S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. Int. J. Mol. Sci. 2022, 23, 12305. [Google Scholar] [CrossRef]
- Da Silva, G.; Da Silva, M.; Nascimento, D.; Lima Silva, E.; Gouvêa, F.; De França Lopes, L.; Araújo, A.; Ferraz Pereira, K.; De Queiroz, T. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef]
- Stec, D.E.; Drummond, H.A.; Vera, T. Role of Carbon Monoxide in Blood Pressure Regulation. Hypertension 2008, 51, 597–604. [Google Scholar] [CrossRef]
- Johnson, F.K.; Johnson, R.A.; Durante, W.; Jackson, K.E.; Stevenson, B.K.; Peyton, K.J. Metabolic Syndrome Increases Endogenous Carbon Monoxide Production to Promote Hypertension and Endothelial Dysfunction in Obese Zucker Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R601–R608. [Google Scholar] [CrossRef]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of Weight Reduction on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef]
- Aucott, L.; Rothnie, H.; McIntyre, L.; Thapa, M.; Waweru, C.; Gray, D. Long-Term Weight Loss from Lifestyle Intervention Benefits Blood Pressure?: A Systematic Review. Hypertension 2009, 54, 756–762. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and Cardiovascular Disease: An ESC Clinical Consensus Statement. Eur. Heart J. 2024, 45, 4063–4098. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- Viera, A.J.; Kshirsagar, A.V.; Hinderliter, A.L. Lifestyle Modifications to Lower or Control High Blood Pressure: Is Advice Associated with Action? The Behavioral Risk Factor Surveillance Survey. J. Clin. Hypertens. 2008, 10, 105–111. [Google Scholar] [CrossRef]
- Engeli, S.; Jordan, J. Novel Metabolic Drugs and Blood Pressure: Implications for the Treatment of Obese Hypertensive Patients? Curr. Hypertens. Rep. 2013, 15, 470–474. [Google Scholar] [CrossRef]
- Allison, D.B.; Gadde, K.M.; Garvey, W.T.; Peterson, C.A.; Schwiers, M.L.; Najarian, T.; Tam, P.Y.; Troupin, B.; Day, W.W. Controlled-Release Phentermine/Topiramate in Severely Obese Adults: A Randomized Controlled Trial (EQUIP). Obesity 2012, 20, 330–342. [Google Scholar] [CrossRef]
- Davidson, M.H.; Tonstad, S.; Oparil, S.; Schwiers, M.; Day, W.W.; Bowden, C.H. Changes in Cardiovascular Risk Associated with Phentermine and Topiramate Extended-Release in Participants With Comorbidities and a Body Mass Index ≥27 kg/m2. Am. J. Cardiol. 2013, 111, 1131–1138. [Google Scholar] [CrossRef]
- Aronne, L.J.; Wadden, T.A.; Peterson, C.; Winslow, D.; Odeh, S.; Gadde, K.M. Evaluation of Phentermine and Topiramate versus Phentermine/Topiramate Extended-release in Obese Adults. Obesity 2013, 21, 2163–2171. [Google Scholar] [CrossRef]
- Garvey, W.T.; Ryan, D.H.; Look, M.; Gadde, K.M.; Allison, D.B.; Peterson, C.A.; Schwiers, M.; Day, W.W.; Bowden, C.H. Two-Year Sustained Weight Loss and Metabolic Benefits with Controlled-Release Phentermine/Topiramate in Obese and Overweight Adults (SEQUEL): A Randomized, Placebo-Controlled, Phase 3 Extension Study. Am. J. Clin. Nutr. 2012, 95, 297–308. [Google Scholar] [CrossRef]
- Blüher, M.; Aras, M.; Aronne, L.J.; Batterham, R.L.; Giorgino, F.; Ji, L.; Pietiläinen, K.H.; Schnell, O.; Tonchevska, E.; Wilding, J.P.H. New Insights into the Treatment of Obesity. Diabetes Obes. Metab. 2023, 25, 2058–2072. [Google Scholar] [CrossRef]
- Cohen, J.B.; Gadde, K.M. Weight Loss Medications in the Treatment of Obesity and Hypertension. Curr. Hypertens. Rep. 2019, 21, 16. [Google Scholar] [CrossRef]
- Sahebkar, A.; Simental-Mendía, L.E.; Kovanen, P.T.; Pedone, C.; Simental-Mendía, M.; Cicero, A.F.G. Effects of Orlistat on Blood Pressure: A Systematic Review and Meta-Analysis of 27 Randomized Controlled Clinical Trials. J. Am. Soc. Hypertens. 2018, 12, 80–96. [Google Scholar] [CrossRef]
- Sharma, A.M.; Golay, A. Effect of Orlistat-Induced Weight Loss on Blood Pressure and Heart Rate in Obese Patients with Hypertension. J. Hypertens. 2002, 20, 1873–1878. [Google Scholar] [CrossRef]
- Al-Tahami, B.A.M.; Ismail, A.A.A.-S.; Bee, Y.T.G.; Awang, S.A.; Salha Wan Abdul Rani, W.R.; Sanip, Z.; Rasool, A.H.G. The Effects of Anti-Obesity Intervention with Orlistat and Sibutramine on Microvascular Endothelial Function. Clin. Hemorheol. Microcirc. 2015, 59, 323–334. [Google Scholar] [CrossRef]
- Billes, S.K.; Sinnayah, P.; Cowley, M.A. Naltrexone/Bupropion for Obesity: An Investigational Combination Pharmacotherapy for Weight Loss. Pharmacol. Res. 2014, 84, 1–11. [Google Scholar] [CrossRef]
- Greenway, F.L.; Fujioka, K.; Plodkowski, R.A.; Mudaliar, S.; Guttadauria, M.; Erickson, J.; Kim, D.D.; Dunayevich, E. Effect of Naltrexone plus Bupropion on Weight Loss in Overweight and Obese Adults (COR-I): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2010, 376, 595–605. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.; Rubino, D.; Still, C.; Wyatt, H.; Burns, C.; Kim, D.; Dunayevich, E.; for the COR-II Study Group. A Randomized, Phase 3 Trial of Naltrexone SR/Bupropion SR on Weight and Obesity-related Risk Factors (COR-II). Obesity 2013, 21, 935–943. [Google Scholar] [CrossRef]
- Wadden, T.A.; Foreyt, J.P.; Foster, G.D.; Hill, J.O.; Klein, S.; O’Neil, P.M.; Perri, M.G.; Pi-Sunyer, F.X.; Rock, C.L.; Erickson, J.S.; et al. Weight Loss With Naltrexone SR/Bupropion SR Combination Therapy as an Adjunct to Behavior Modification: The COR-BMOD Trial. Obesity 2011, 19, 110–120. [Google Scholar] [CrossRef]
- Hollander, P.; Gupta, A.K.; Plodkowski, R.; Greenway, F.; Bays, H.; Burns, C.; Klassen, P.; Fujioka, K.; for the COR-Diabetes Study Group. Effects of Naltrexone Sustained- Release/Bupropion Sustained-Release Combination Therapy on Body Weight and Glycemic Parameters in Overweight and Obese Patients with Type 2 Diabetes. Diabetes Care 2013, 36, 4022–4029. [Google Scholar] [CrossRef]
- Jiang, Q.; Velu, P.; Sohouli, M.H.; Ziamanesh, F.; Shojaie, S.; Fatahi, S.; Li, Q. The Effects of Bupropion Alone and Combined with Naltrexone on Blood Pressure and CRP Concentration: A Systematic Review and Meta-regression Analysis of Randomized Controlled Trials. Eur. J. Clin. Investig. 2023, 54, e14118. [Google Scholar] [CrossRef]
- Cataldi, M.; Cignarelli, A.; Giallauria, F.; Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A.; on behalf of Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Cardiovascular Effects of Antiobesity Drugs: Are the New Medicines All the Same? Int. J. Obes. Suppl. 2020, 10, 14–26. [Google Scholar] [CrossRef]
- Arias, H.R. Is the Inhibition of Nicotinic Acetylcholine Receptors by Bupropion Involved in Its Clinical Actions? Int. J. Biochem. Cell Biol. 2009, 41, 2098–2108. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. The Dual Glucose-Dependent Insulinotropic Polypeptide (GIP) and Glucagon-like Peptide-1 (GLP-1) Receptor Agonist Tirzepatide: A Novel Cardiometabolic Therapeutic Prospect. Cardiovasc. Diabetol. 2021, 20, 225. [Google Scholar] [CrossRef]
- Hu, E.-H.; Tsai, M.-L.; Lin, Y.; Chou, T.-S.; Chen, T.-H. A Review and Meta-Analysis of the Safety and Efficacy of Using Glucagon-like Peptide-1 Receptor Agonists. Medicina 2024, 60, 357. [Google Scholar] [CrossRef]
- Puglisi, S.; Rossini, A.; Poli, R.; Dughera, F.; Pia, A.; Terzolo, M.; Reimondo, G. Effects of SGLT2 Inhibitors and GLP-1 Receptor Agonists on Renin-Angiotensin-Aldosterone System. Front. Endocrinol. 2021, 12, 738848. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Astrup, A.; Fujioka, K.; Greenway, F.; Halpern, A.; Krempf, M.; Lau, D.C.W.; Le Roux, C.W.; Violante Ortiz, R.; Jensen, C.B.; et al. A Randomized, Controlled Trial of 3.0 Mg of Liraglutide in Weight Management. N. Engl. J. Med. 2015, 373, 11–22. [Google Scholar] [CrossRef]
- Davies, M.J.; Bergenstal, R.; Bode, B.; Kushner, R.F.; Lewin, A.; Skjøth, T.V.; Andreasen, A.H.; Jensen, C.B.; DeFronzo, R.A.; for the NN8022-1922 Study Group. Efficacy of Liraglutide for Weight Loss Among Patients with Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA 2015, 314, 687. [Google Scholar] [CrossRef]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L.; on behalf of the NN8022-1923 Investigators. Weight Maintenance and Additional Weight Loss with Liraglutide after Low-Calorie-Diet-Induced Weight Loss: The SCALE Maintenance Randomized Study. Int. J. Obes. 2013, 37, 1443–1451. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 Mg Once a Week in Adults with Overweight or Obesity, and Type 2 Diabetes (STEP 2): A Randomised, Double-Blind, Double-Dummy, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]
- Wadden, T.A.; Bailey, T.S.; Billings, L.K.; Davies, M.; Frias, J.P.; Koroleva, A.; Lingvay, I.; O’Neil, P.M.; Rubino, D.M.; Skovgaard, D.; et al. Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults with Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA 2021, 325, 1403. [Google Scholar] [CrossRef]
- Rubino, D.; Abrahamsson, N.; Davies, M.; Hesse, D.; Greenway, F.L.; Jensen, C.; Lingvay, I.; Mosenzon, O.; Rosenstock, J.; Rubio, M.A.; et al. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults with Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021, 325, 1414. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 Receptor Activation and Epac2 Link Atrial Natriuretic Peptide Secretion to Control of Blood Pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]
- Goud, A.; Zhong, J.; Peters, M.; Brook, R.D.; Rajagopalan, S. GLP-1 Agonists and Blood Pressure: A Review of the Evidence. Curr. Hypertens. Rep. 2016, 18, 16. [Google Scholar] [CrossRef]
- Rosenstock, J.; Wysham, C.; Frías, J.P.; Kaneko, S.; Lee, C.J.; Fernández Landó, L.; Mao, H.; Cui, X.; Karanikas, C.A.; Thieu, V.T. Efficacy and Safety of a Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide in Patients with Type 2 Diabetes (SURPASS-1): A Double-Blind, Randomised, Phase 3 Trial. Lancet 2021, 398, 143–155. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Ludvik, B.; Giorgino, F.; Jódar, E.; Frias, J.P.; Fernández Landó, L.; Brown, K.; Bray, R.; Rodríguez, Á. Once-Weekly Tirzepatide versus Once-Daily Insulin Degludec as Add-on to Metformin with or without SGLT2 Inhibitors in Patients with Type 2 Diabetes (SURPASS-3): A Randomised, Open-Label, Parallel-Group, Phase 3 Trial. Lancet 2021, 398, 583–598. [Google Scholar] [CrossRef]
- Del Prato, S.; Kahn, S.E.; Pavo, I.; Weerakkody, G.J.; Yang, Z.; Doupis, J.; Aizenberg, D.; Wynne, A.G.; Riesmeyer, J.S.; Heine, R.J.; et al. Tirzepatide versus Insulin Glargine in Type 2 Diabetes and Increased Cardiovascular Risk (SURPASS-4): A Randomised, Open-Label, Parallel-Group, Multicentre, Phase 3 Trial. Lancet 2021, 398, 1811–1824. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- De Lemos, J.A.; Linetzky, B.; Le Roux, C.W.; Laffin, L.J.; Vongpatanasin, W.; Fan, L.; Hemmingway, A.; Ahmad, N.N.; Bunck, M.C.; Stefanski, A. Tirzepatide Reduces 24-Hour Ambulatory Blood Pressure in Adults with Body Mass Index ≥27 Kg/m2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 2024, 81, e41–e43. [Google Scholar] [CrossRef]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; Le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity in People with Type 2 Diabetes (SURMOUNT-2): A Double-Blind, Randomised, Multicentre, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef]
- Wadden, T.A.; Chao, A.M.; Machineni, S.; Kushner, R.; Ard, J.; Srivastava, G.; Halpern, B.; Zhang, S.; Chen, J.; Bunck, M.C.; et al. Tirzepatide after Intensive Lifestyle Intervention in Adults with Overweight or Obesity: The SURMOUNT-3 Phase 3 Trial. Nat. Med. 2023, 29, 2909–2918. [Google Scholar] [CrossRef]
- Aronne, L.J.; Sattar, N.; Horn, D.B.; Bays, H.E.; Wharton, S.; Lin, W.-Y.; Ahmad, N.N.; Zhang, S.; Liao, R.; Bunck, M.C.; et al. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults with Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Siriopol, D.; Yildiz, A.B.; Gaipov, A.; Van Raalte, D.H.; Tuttle, K.R. Effect of Tirzepatide on Blood Pressure and Lipids: A Meta-analysis of Randomized Controlled Trials. Diabetes Obes. Metab. 2023, 25, 3766–3778. [Google Scholar] [CrossRef]
- Lingvay, I.; Mosenzon, O.; Brown, K.; Cui, X.; O’Neill, C.; Fernández Landó, L.; Patel, H. Systolic Blood Pressure Reduction with Tirzepatide in Patients with Type 2 Diabetes: Insights from SURPASS Clinical Program. Cardiovasc. Diabetol. 2023, 22, 66. [Google Scholar] [CrossRef]
- Lv, X.; Wang, H.; Chen, C.; Zhao, Y.; Li, K.; Wang, Y.; Wang, L.; Fu, S.; Liu, J. The Effect of Tirzepatide on Weight, Lipid Metabolism and Blood Pressure in Overweight/Obese Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. 2024, 17, 701–714. [Google Scholar] [CrossRef]
- Taktaz, F.; Fontanella, R.A.; Scisciola, L.; Pesapane, A.; Basilicata, M.G.; Ghosh, P.; Franzese, M.; Tortorella, G.; Puocci, A.; Vietri, M.T.; et al. Bridging the Gap between GLP1-Receptor Agonists and Cardiovascular Outcomes: Evidence for the Role of Tirzepatide. Cardiovasc. Diabetol. 2024, 23, 242. [Google Scholar] [CrossRef]
- Cho, Y.K.; La Lee, Y.; Jung, C.H. The Cardiovascular Effect of Tirzepatide: A Glucagon-Like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide Dual Agonist. J. Lipid Atheroscler. 2023, 12, 213. [Google Scholar] [CrossRef]
- Pirro, V.; Roth, K.D.; Lin, Y.; Willency, J.A.; Milligan, P.L.; Wilson, J.M.; Ruotolo, G.; Haupt, A.; Newgard, C.B.; Duffin, K.L. Effects of Tirzepatide, a Dual GIP and GLP-1 RA, on Lipid and Metabolite Profiles in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 363–378. [Google Scholar] [CrossRef]
- Woodman, R.J.; Chew, G.T.; Watts, G.F. Mechanisms, Significance and Treatment of Vascular Dysfunction in Type 2 Diabetes Mellitus: Focus on Lipid-Regulating Therapy. Drugs 2005, 65, 31–74. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Bottino, R.; Carbone, A.; Formisano, T.; D’Elia, S.; Orlandi, M.; Sperlongano, S.; Molinari, D.; Castaldo, P.; Palladino, A.; Barbareschi, C.; et al. Cardiovascular Effects of Weight Loss in Obese Patients with Diabetes: Is Bariatric Surgery the Additional Arrow in the Quiver? Life 2023, 13, 1552. [Google Scholar] [CrossRef]
- Wang, L.; Lin, M.; Yu, J.; Fan, Z.; Zhang, S.; Lin, Y.; Chen, X.; Peng, F. The Impact of Bariatric Surgery Versus Non-Surgical Treatment on Blood Pressure: Systematic Review and Meta-Analysis. Obes. Surg. 2021, 31, 4970–4984. [Google Scholar] [CrossRef]
- Wiggins, T.; Guidozzi, N.; Welbourn, R.; Ahmed, A.R.; Markar, S.R. Association of Bariatric Surgery with All-Cause Mortality and Incidence of Obesity-Related Disease at a Population Level: A Systematic Review and Meta-Analysis. PLoS Med. 2020, 17, e1003206. [Google Scholar] [CrossRef]
- Hallersund, P.; Sjöström, L.; Olbers, T.; Lönroth, H.; Jacobson, P.; Wallenius, V.; Näslund, I.; Carlsson, L.M.; Fändriks, L. Gastric Bypass Surgery Is Followed by Lowered Blood Pressure and Increased Diuresis—Long Term Results from the Swedish Obese Subjects (SOS) Study. PLoS ONE 2012, 7, e49696. [Google Scholar] [CrossRef]
- Samson, R.; Ayinapudi, K.; Le Jemtel, T.H.; Oparil, S. Obesity, Hypertension, and Bariatric Surgery. Curr. Hypertens. Rep. 2020, 22, 46. [Google Scholar] [CrossRef]
- Antza, C.; Grassi, G.; Weber, T.; Persu, A.; Jordan, J.; Nilsson, P.M.; Redon, J.; Stabouli, S.; Kreutz, R.; Kotsis, V. Assessment and Management of Patients with Obesity and Hypertension in European Society of Hypertension Excellence Centres. A Survey from the ESH Working Group on Diabetes and Metabolic Risk Factors. Blood Press. 2024, 33, 2317256. [Google Scholar] [CrossRef]
- Hui, C.Y.; Creamer, E.; Pinnock, H.; McKinstry, B. Apps to Support Self-Management for People with Hypertension: Content Analysis. JMIR mHealth uHealth 2019, 7, e13257. [Google Scholar] [CrossRef]



| Trial | Total Population, n | Hypertension | Anti-Hypertension Therapy | Comparison | Difference in SBP, mmHg | Difference in DBP, mmHg |
|---|---|---|---|---|---|---|
| SCALE Obesity and Prediabetes [46] | 3731 | mixed | mixed | Liraglutide 3 mg vs. placebo | −4.2 mmHg vs. −1.5 mmHg p < 0.001 | −2.6 mmHg vs. −1.9 mmHg p < 0.001 |
| SCALE Diabetes [47] | 846 | mixed | mixed | Liraglutide 3 mg vs. Liraglutide 1.8 mg vs. placebo | ETD −2.59 mmHg for liraglutide 3 mg vs. placebo p = 0.01 | ETD −0.36 mmHg for liraglutide 3 mg vs. placebo p = 0.59 |
| SCALE Maintenance [48] | 422 | mixed | mixed | Liraglutide 3 mg vs. placebo | ETD −2.7 mmHg for liraglutide 3 mg vs. placebo p = 0.007 | ETD −0.3 mmHg for liraglutide 3 mg vs. placebo p = 0.64 |
| Trial | Total Population, n | Hypertension | Anti-Hypertension Therapy | Comparison | Difference in SBP, mmHg | Difference in DBP, mmHg |
|---|---|---|---|---|---|---|
| STEP 1 [49] | 1961 | mixed | mixed | Semaglutide 2.4 mg vs placebo | −6.16 mmHg vs. −1.06 mmHg p < 0.001 | −2.83 mmHg vs. −0.42 mmHg p NR |
| STEP 2 [50] | 1210 | mixed | mixed | Semaglutide 2.4 mg vs. Semaglutide 1 mg vs. placebo | ETD −3.4 mmHg for semaglutide 2.4 mg vs. placebo p = 0.0016 | ETD −0.7 mmHg for semaglutide 2.4 mg vs. placebo p NR |
| STEP 3 [51] | 611 | mixed | mixed | Semaglutide 2.4 mg vs. placebo | −5.6 mmHg vs. −1.6 mmHg p = 0.001 | −3.0 mmHg vs. −0.8 mmHg p = 0.008 |
| STEP 4 [52] | 803 | mixed | mixed | Semaglutide 2.4 mg vs. placebo | ETD −3.9 mmHg for semaglutide 2.4 mg vs. placebo p < 0.001 | ETD −0.6 mmHg for semaglutide 2.4 mg vs. placebo p = 0.46 |
| Trial | Total Population, n | Hypertension | Anti-Hypertension Therapy | Comparison | Difference in SBP, mmHg | Difference in DBP, mmHg |
|---|---|---|---|---|---|---|
| SURPASS-1 [55] | 478 | mixed | mixed | Tirzepatide 5 mg vs placebo | −4.7 mmHg vs. −2.0 mmHg p NR | −2.9 mmHg vs. −1.4 mmHg p NR |
| Tirzepatide 10 mg vs. placebo | −5.2 mmHg vs. −2.0 mmHg p < 0.05 | −3.1 mmHg vs. −1.4 mmHg p NR | ||||
| Tirzepatide 15 mg vs. placebo | −4.7 mmHg vs. −2.0 mmHg p NR | −3.4 mmHg vs. −1.4 mmHg p NR | ||||
| SURPASS-2 [56] | 1878 | mixed | mixed | Tirzepatide 5 mg vs. Semaglutide 1 mg | −4.8 mmHg vs. −3.6 mmHg p NR | −1.9 mmHg vs. −1.0 mmHg p NR |
| Tirzepatide 10 mg vs. Semaglutide 1 mg | −5.3 mmHg vs. −3.6 mmHg p < 0.05 | −2.5 mmHg vs. −1.0 mmHg p NR | ||||
| Tirzepatide 15 mg vs. Semaglutide 1 mg | −6.5 mmHg vs. −3.6 mmHg p < 0.001 | −2.9 mmHg vs. −1.0 mmHg p NR | ||||
| SURPASS-3 [57] | 1444 | mixed | mixed | Tirzepatide 5 mg vs. Insulin Degludec | −4.9 mmHg vs. +0.5 mmHg | −2.0 mmHg vs. +0.4 mmHg |
| Tirzepatide 10 mg vs. Insulin Degludec | −6.6 mmHg vs. +0.5 mmHg | −2.5 mmHg vs. +0.4 mmHg | ||||
| Tirzepatide 15 mg vs. Insulin Degludec | −5.5 mmHg vs. +0.5 mmHg | −1.9 mmHg vs. +0.4 mmHg | ||||
| SURPASS-4 [58] | 2002 | mixed | mixed | Tirzepatide 5 mg vs. Insulin Glargine | −2.8 mmHg vs. +1.3 mmHg | −1.0 mmHg vs. + 0.7 mmHg |
| Tirzepatide 10 mg vs. Insulin Glargine | −3.7 mmHg vs. +1.3 mmHg | −0.8 mmHg vs. +0.7 mmHg | ||||
| Tirzepatide 15 mg vs. Insulin Glargine | −4.8 mmHg vs. +1.3 mmHg | −1.0 mmHg vs. +0.7 mmHg | ||||
| SURPASS-5 [59] | 475 | mixed | mixed | Tirzepatide 5 mg + Insulin Glargine vs. Placebo + Insulin Glargine | −6.1 mmHg vs. −1.7 mmHg p = 0.012 | −2.0 mmHg vs. −2.1 mmHg p = 0.958 |
| Tirzepatide 10 mg + Insulin Glargine vs. Placebo + Insulin Glargine | −8.3 mmHg vs. −1.7 mmHg p < 0.001 | −3.3 mmHg vs. −2.1 mmHg p = 0.218 | ||||
| Tirzepatide 15 mg + Insulin Glargine vs. Placebo + Insulin Glargine | −12.6 mmHg vs. −1.7 mmHg p < 0.001 | −4.5 mmHg vs. −2.1 mmHg p = 0.017 |
| Trial | Total Population, n | Hypertension | Anti-Hypertension Therapy | Comparison | Difference in SBP, mmHg | Difference in DBP, mmHg |
|---|---|---|---|---|---|---|
| SURMOUNT-1 [60] | 2539 | mixed | mixed | Tirzepatide 5 mg or 10 mg or 15 mg vs. placebo | Pooled Tirzepatide groups −7.2 mmHg vs. −1.0 mmHg p < 0.001 | Pooled Tirzepatide groups −4.8 mmHg vs. −0.8 mmHg p < 0.001 |
| SURMOUNT-2 [62] | 938 | mixed | mixed | Tirzepatide 10 or 15 mg vs. placebo | Pooled Tirzepatide groups −6.3 mmHg vs. −1.2 mmHg p < 0.0001 | Pooled Tirzepatide groups −2.5 mmHg vs. −0.3 mmHg p = 0.0012 |
| SURMOUNT-3 [63] | 579 | mixed | mixed | Tirzepatide 10 or 15 mg vs. placebo | −5.1 mmHg vs. +4.1 mmHg | −3.2 mmHg vs. +2.3 mmHg |
| SURMOUNT-4 [64] | 783 | mixed | mixed | Tirzepatide 10 or 15 mg vs. placebo | −9.7 mmHg vs. −2.0 mmHg p < 0.001 | −5.5 mmHg vs. −1.7 mmHg p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsi, V.; Manta, E.; Fragoulis, C.; Tsioufis, K. Weight Loss Therapies and Hypertension Benefits. Biomedicines 2024, 12, 2293. https://doi.org/10.3390/biomedicines12102293
Katsi V, Manta E, Fragoulis C, Tsioufis K. Weight Loss Therapies and Hypertension Benefits. Biomedicines. 2024; 12(10):2293. https://doi.org/10.3390/biomedicines12102293
Chicago/Turabian StyleKatsi, Vasiliki, Eleni Manta, Christos Fragoulis, and Konstantinos Tsioufis. 2024. "Weight Loss Therapies and Hypertension Benefits" Biomedicines 12, no. 10: 2293. https://doi.org/10.3390/biomedicines12102293






