Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation
Abstract
1. Introduction
2. Drug-Induced Metabolic Effects in Patients from Clozapine Treatment
2.1. Inflammation
2.2. Hepatotoxicity
2.3. Hyperglycemia
2.4. Weight Gain
2.5. Iron Depletion
2.6. Sex Differences
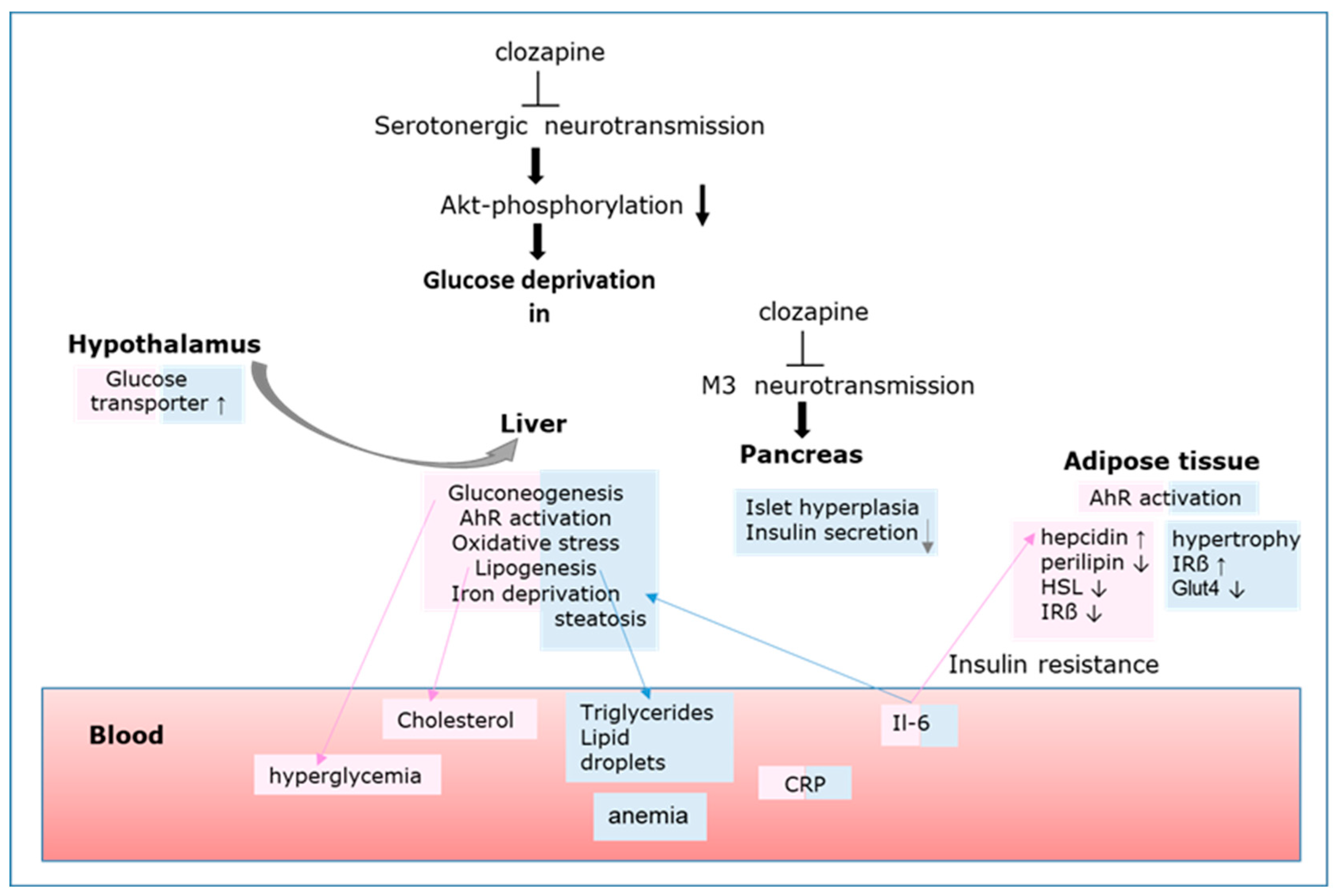
3. Mechanisms of Metabolic Changes Leading to MetS
3.1. Clozapine Inhibits Akt Activation and Cellular Glucose Uptake
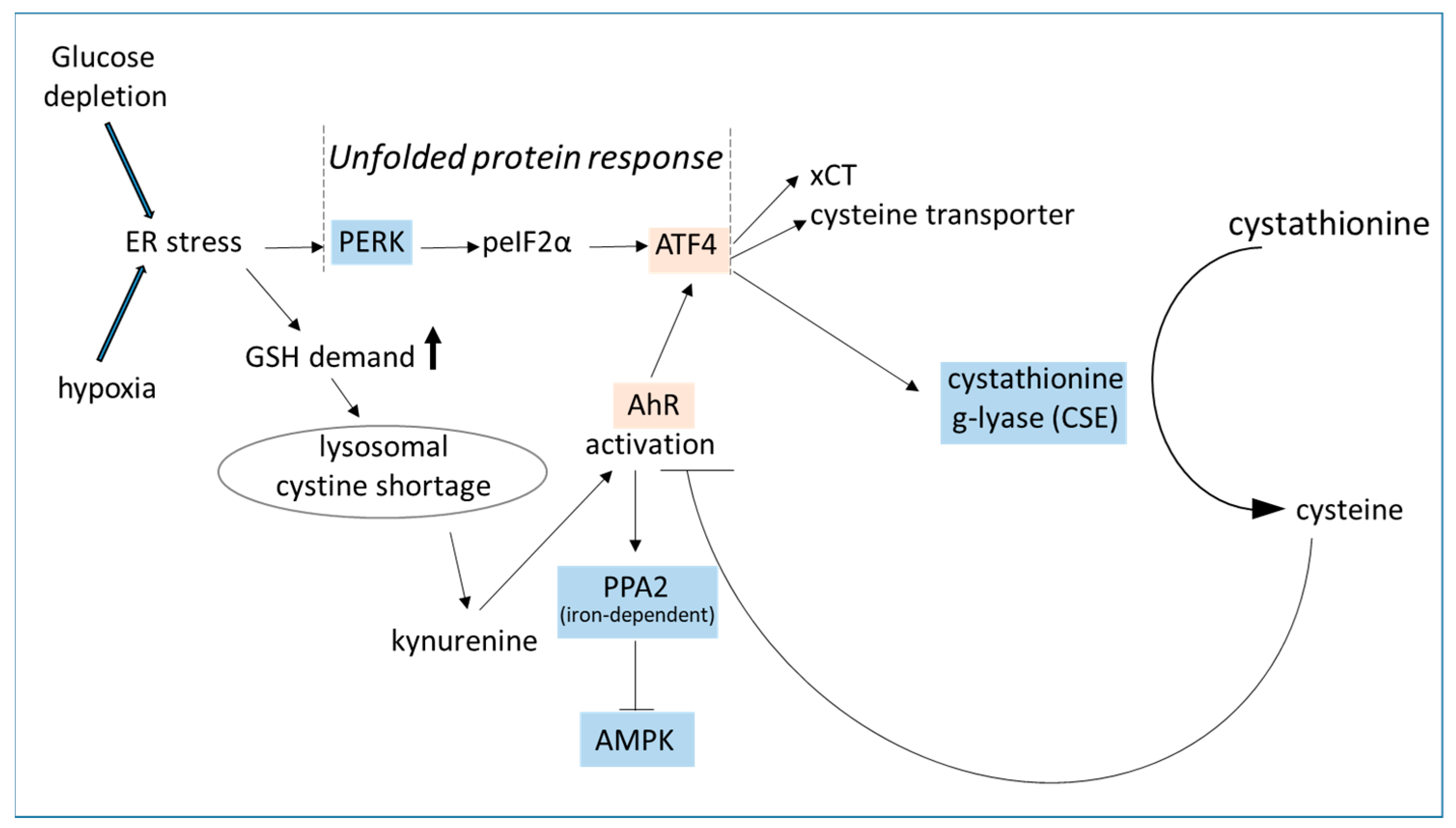
3.2. Glucose Deprivation Triggers AhR Activation
3.3. AhR Triggers ATF4 Activation
3.4. AhR Triggers HIF-1 Activation
3.5. Glucose Deprivation Triggers Proinflammatory Response
3.6. Viral Infection Reduces Glucose Availability
3.7. Clozapine Induces Metabolic Reprogramming in HL-60 Cells [66]
3.8. Akt Inhibition Entails All Facets of MetS
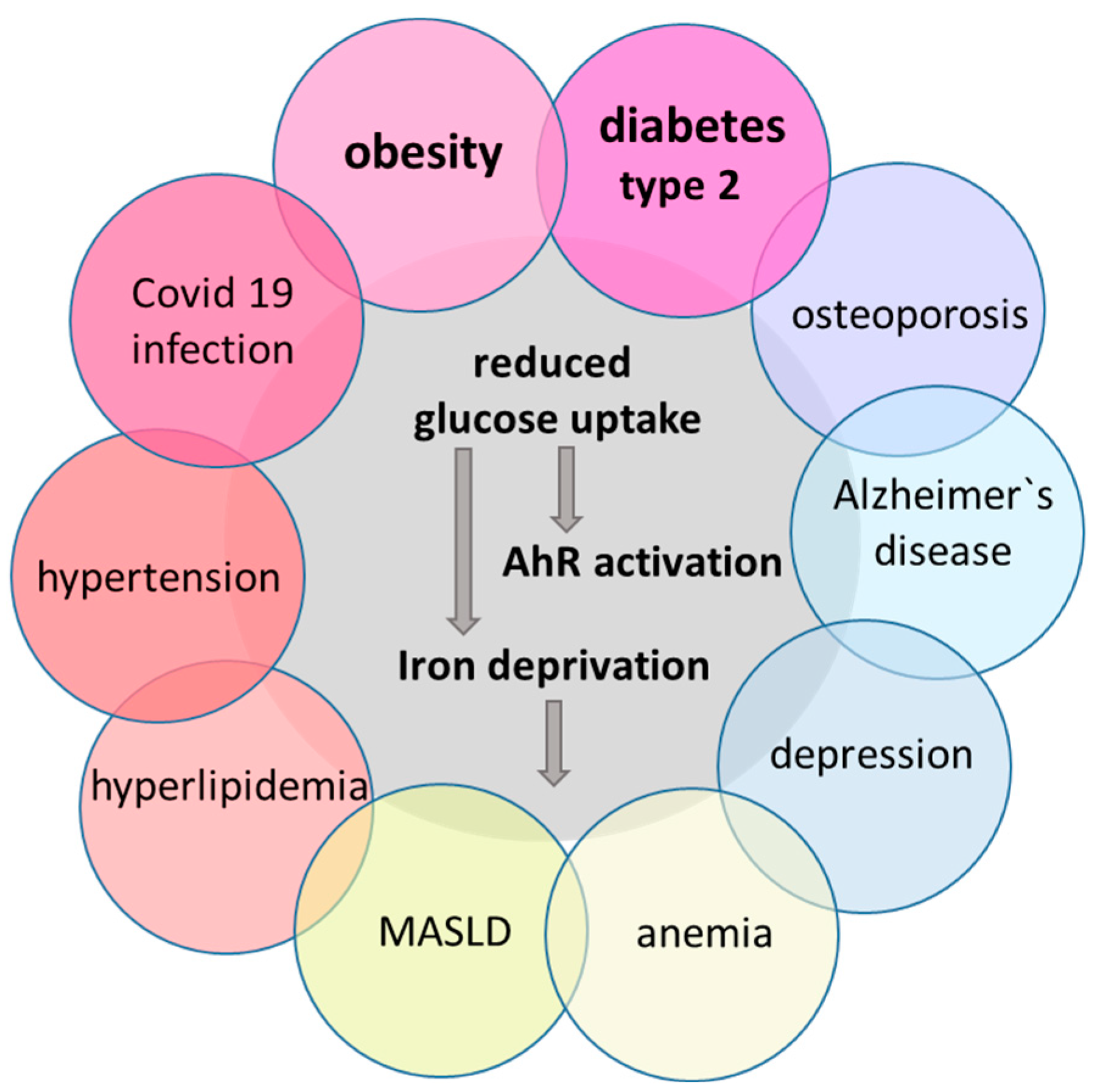
4. Animal Models of MetS with Known AhR Participation
5. Metabolic Changes in the Brain
5.1. The Brain–Liver Axis
5.2. The Brain–Fat Axis
5.3. The Gut–Brain Axis
5.4. Activation of the Noradrenergic System under MetS
6. Clinical Interventions
7. Conclusions
Funding
Conflicts of Interest
References
- Fehsel, K. Why is iron deficiency/anemia linked to Alzheimer's disease and its comorbidities, and how is it prevented? Biomedicines 2023, 11, 2421. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Jun, D.W.; Kim, J.T.; Jeong, J.H.; Park, H.; Chang, Y.S.; Park, K.S.; Lee, H.K.; Pak, Y.K. Novel cell-based assay reveals associations of circulating serum AhR-ligands with metabolic syndrome and mitochondrial dysfunction. Biofactors 2013, 39, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, Y.; Zhong, T.; Yu, X.; Wang, L.; Xiao, Y.; Peng, Y.; Sun, Q. A review of food contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin and its toxicity associated with metabolic disorders. Curr. Res. Food Sci. 2023, 7, 100617. [Google Scholar] [CrossRef]
- Kim, H.B.; Um, J.Y.; Chung, B.Y.; Kim, J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. Aryl Hydrocarbon Receptors: Evidence of Therapeutic Targets in Chronic Inflammatory Skin Diseases. Biomedicines 2022, 10, 1087. [Google Scholar] [CrossRef]
- Xiong, L.; Helm, E.Y.; Dean, J.W.; Sun, N.; Jimenez-Rondan, F.R.; Zhou, L. Nutrition impact on ILC3 maintenance and function centers on a cell-intrinsic CD71-iron axis. Nat. Immunol. 2023, 24, 1671–1684. [Google Scholar] [CrossRef]
- Hamano, H.; Niimura, T.; Horinouchi, Y.; Zamami, Y.; Takechi, K.; Goda, M.; Imanishi, M.; Chuma, M.; Izawa-Ishizawa, Y.; Miyamoto, L.; et al. Proton pump inhibitors block iron absorption through direct regulation of hepcidin via the aryl hydrocarbon receptor-mediated pathway. Toxicol. Lett. 2020, 318, 86–91. [Google Scholar] [CrossRef]
- Riaz, F.; Pan, F.; Wei, P. Aryl hydrocarbon receptor: The master regulator of immune responses in allergic diseases. Front. Immunol. 2022, 13, 1057555. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Yu, G.; Sun, L.; Guo, S.; Sai, L.; Bo, C.; Xing, C.; Shao, H.; Peng, C.; et al. Ligand-independent activation of AhR by hydroquinone mediates benzene-induced hematopoietic toxicity. Chem. Biol. Interact. 2022, 355, 109845. [Google Scholar] [CrossRef]
- da Silva, J.F.; Bolsoni, J.A.; da Costa, R.M.; Alves, J.V.; Bressan, A.F.M.; Silva, L.E.V.; Costa, T.J.; Oliveira, A.E.R.; Manzato, C.P.; Aguiar, C.A.; et al. Aryl hydrocarbon receptor (AhR) activation contributes to high-fat diet-induced vascular dysfunction. Br. J. Pharmacol. 2022, 179, 2938–2952. [Google Scholar] [CrossRef]
- Mo, Y.; Lu, Z.; Wang, L.; Ji, C.; Zou, C.; Liu, X. The Aryl Hydrocarbon Receptor in Chronic Kidney Disease: Friend or Foe? Front. Cell Dev. Biol. 2020, 8, 589752. [Google Scholar] [CrossRef] [PubMed]
- Bu, K.B.; Kim, M.; Shin, M.K.; Lee, S.H.; Sung, J.S. Regulation of Ben-zo[a]pyrene-Induced Hepatic Lipid Accumulation through CYP1B1-Induced mTOR-Mediated Lipophagy. Int. J. Mol. Sci. 2024, 25, 1324. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Liu, J.; Qu, F.; Chen, A.; He, W. Polychlorinated biphenyls exposure and type 2 diabetes: Molecular mechanism that causes insulin resistance and islet damage. Environ. Toxicol. 2024, 39, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Hoyeck, M.P.; Angela Ching, M.E.; Basu, L.; van Allen, K.; Palaniyandi, J.; Perera, I.; Poleo-Giordani, E.; Hanson, A.A.; Ghorbani, P.; Fullerton, M.D.; et al. The aryl hydrocarbon receptor in β-cells mediates the effects of TCDD on glucose homeostasis in mice. Mol. Metab. 2024, 81, 101893. [Google Scholar] [CrossRef]
- Haque, N.; Ojo, E.S.; Krager, S.L.; Tischkau, S.A. Deficiency of Adipose Aryl Hydrocarbon Receptor Protects against Diet-Induced Metabolic Dysfunction through Sexually Dimorphic Mechanisms. Cells 2023, 12, 1748. [Google Scholar] [CrossRef]
- Zhang, S.; An, X.; Huang, S.; Zeng, L.; Xu, Y.; Su, D.; Qu, Y.; Tang, X.; Ma, J.; Yang, J.; et al. AhR/miR-23a-3p/PKCα axis contributes to memory deficits in ovariectomized and normal aging female mice. Mol. Ther. Nucleic Acids 2021, 24, 79–91. [Google Scholar] [CrossRef]
- Henderson, D.C.; Cagliero, E.; Gray, C.; Nasrallah, R.A.; Hayden, D.L.; Schoenfeld, D.A.; Goff, D.C. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: A five-year naturalistic study. Am. J. Psychiatry 2000, 157, 975–981. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Chen, Y.; Ren, J.; Zhang, C. Association between IL-6 and metabolic syndrome in schizophrenia patients treated with second-generation antipsychotics. Neuropsychiatr. Dis. Treat. 2019, 15, 2161–2170. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Hong, C.J.; Tsai, S.J. Effects of subchronic clozapine administration on serum glucose, cholesterol and triglyceride levels, and body weight in male BALB/c mice. Life Sci. 2005, 76, 2269–2273. [Google Scholar] [CrossRef]
- Fehsel, K.; Bouvier, M.L. Sex-Specific Effects of Long-Term Antipsychotic Drug Treatment on Adipocyte Tissue and the Crosstalk to Liver and Brain in Rats. Int. J. Mol. Sci. 2024, 25, 2188. [Google Scholar] [CrossRef]
- Pisciotta, A.V. Drug-induced agranulocytosis. Peripheral destruction of polymorphonuclear leukocytes and their marrow precursors. Blood Rev. 1990, 4, 226–237. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, X.; Qin, Z.; Liu, J.; Song, E.; Song, Y. Polychlorinated biphenyl quinone metabolites cause neutrophil extracellular traps in mouse bone marrow neutrophils. Chem. Res. Toxicol. 2022, 35, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Rotella, F.; Cassioli, E.; Calderani, E.; Lazzeretti, L.; Ragghianti, B.; Ricca, V.; Mannucci, E. Long-term metabolic and cardiovascular effects of antipsychotic drugs. A meta-analysis of randomized controlled trials. Eur. Neuro-Psychopharmacol. 2020, 32, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. The impact of the aryl hydrocarbon receptor on antenatal chemical exposure-induced cardiovascular-kidney-metabolic programming. Int. J. Mol. Sci. 2024, 25, 4599. [Google Scholar] [CrossRef] [PubMed]
- Arsenescu, V.; Arsenescu, R.I.; King, V.; Swanson, H.; Cassis, L.A. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ. Health Perspect. 2008, 116, 761–768. [Google Scholar] [CrossRef]
- von Wilmsdorff, M.; Bouvier, M.L.; Henning, U.; Schmitt, A.; Schneider-Axmann, T.; Gaebel, W. The sex-dependent impact of chronic clozapine and haloperidol treatment on characteristics of the metabolic syndrome in a rat model. Pharmacopsychiatry 2013, 46, 1–9. [Google Scholar] [CrossRef]
- Vaddadi, K.S.; Soosai, E.; Vaddadi, G. Low blood selenium concentrations in schizophrenic patients on clozapine. Br. J. Clin. Pharmacol. 2003, 55, 307–309. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhang, T.; Yao, Y.; Shen, C.; Xue, Y. Association of Serum Trace Elements with Schizophrenia and Effects of Antipsychotic Treatment. Biol. Trace Elem. Res. 2018, 181, 22–30. [Google Scholar] [CrossRef]
- Lai, I.K.; Chai, Y.; Simmons, D.; Watson, W.H.; Tan, R.; Haschek, W.M.; Wang, K.; Wang, B.; Ludewig, G.; Rob-ertson, L.W. Dietary selenium as a modulator of PCB 126-induced hepatotoxicity in male Sprague-Dawley rats. Toxicol. Sci. 2011, 124, 202–214. [Google Scholar] [CrossRef]
- Fehsel, K.; Schwanke, K.; Kappel, B.A.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef]
- Löffler, S.; Klimke, A.; Kronenwett, R.; Kobbe, G.; Haas, R.; Fehsel, K. Clozapine mobilizes CD34+ hematopoietic stem and progenitor cells and increases plasma concentration of interleukin 6 in patients with schizophrenia. J. Clin. Psychopharmacol. 2010, 30, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Orlovska-Waast, S.; Köhler-Forsberg, O.; Brix, S.W.; Nordentoft, M.; Kondziella, D.; Krogh, J.; Benros, M.E. Cerebrospinal fluid markers of inflammation and infections in schizophrenia and affective disorders: A systematic review and meta-analysis. Mol. Psychiatry 2019, 24, 869–887. [Google Scholar] [CrossRef] [PubMed]
- Fabrazzo, M.; Prisco, V.; Sampogna, G.; Perris, F.; Catapano, F.; Monteleone, A.M.; Maj, M. Clozapine versus other antipsychotics during the first 18 weeks of treatment: A retrospective study on risk factor increase of blood dyscrasias. Psychiatry Res. 2017, 256, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Biffl, W.L.; Moore, E.E.; Moore, F.A.; Barnett, C.C., Jr.; Carl, V.S.; Peterson, V.N. Interleukin-6 delays neutrophil apoptosis. Arch Surg. 1996, 131, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Eftekharian, M.M.; Omrani, M.D.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. Serum cytokine profile in schizophrenic patients. Hum. Antibodies 2019, 27, 23–29. [Google Scholar] [CrossRef]
- Chen, S.M.; Hsiao, C.W.; Chen, Y.J.; Hong, C.J.; Lin, J.C.; Yang, C.P.; Chang, Y.H. Interleukin-4 inhibits the hypothalamic appetite control by modulating the insulin-AKT and JAK-STAT signaling in leptin mutant mice. Environ. Toxicol. 2024, 39, 3980–3990. [Google Scholar] [CrossRef]
- Zapata, R.C.; Zhang, D.; Libster, A.; Porcu, A.; Montilla-Perez, P.; Nur, A.; Xu, B.; Zhang, Z.; Correa, S.M.; Liu, C.; et al. Nuclear receptor 5A2 regulation of Agrp underlies olanzapine-induced hyperphagia. Mol. Psychiatry 2023, 28, 1857–1867. [Google Scholar] [CrossRef]
- Banks, L.B.; Sklarz, T.; Gohil, M.; O'Leary, C.; Behrens, E.M.; Sun, H.; Chen, Y.H.; Koretzky, G.A.; Jordan, M.S. Akt2 deficiency impairs Th17 differentiation, augments Th2 differentiation, and alters the peripheral response to immunization. bioRxiv 2024, 7, 598023. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O'Banion, M.K. Arginase 1+ microglia reduce Aβ plaque deposition during IL-1β-dependent neuroinflammation. J. Neuro-Inflamm. 2015, 12, 203. [Google Scholar] [CrossRef]
- Williams, D.; Hargrove-Wiley, E.; Bindeman, W.; Valent, D.; Miranda, A.X.; Beckstead, J.; Fingleton, B. Type II Interleukin-4 Receptor Activation in Basal Breast Cancer Cells Promotes Tumor Progression via Metabolic and Epigenetic Modulation. Int. J. Mol. Sci. 2024, 25, 4647. [Google Scholar] [CrossRef]
- Michurina, S.; Stafeev, I.; Beloglazova, I.; Zubkova, E.; Mamontova, E.; Kopylov, A.; Shevchenko, E.; Menshikov, M.; Parfyonova, Y. Regulation of Glucose Transport in Adipocytes by Interleukin-4. J. Interf. Cytokine Res. 2022, 42, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Alangari, A.A.; Ashoori, M.D.; Alwan, W.; Dawe, H.R.; Stockinger, B.; Barker, J.N.; Wincent, E.; Di Meglio, P. Manuka honey activates the aryl hydrocarbon receptor: Implications for skin inflammation. Pharmacol. Res. 2023, 194, 106848. [Google Scholar] [CrossRef]
- Choi, S.J.; Shin, I.J.; Je, K.H.; Min, E.K.; Kim, E.J.; Kim, H.S.; Choe, S.; Kim, D.E.; Lee, D.K. Hypoxia antagonizes glucose deprivation on interleukin 6 expression in an Akt dependent, but HIF-1/2α independent manner. PLoS ONE 2013, 8, e58662. [Google Scholar] [CrossRef][Green Version]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Hummer, M.; Kurz, M.; Kurzthaler, I.; Oberbauer, H.; Miller, C.; Fleischhacker, W.W. Hepatotoxicity of clozapine. J. Clin. Psychopharmacol. 1997, 17, 314–317. [Google Scholar] [CrossRef]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Fernández Gianotti, T.; Dopazo, H.; Rohr, C.; Gaj, G.; San Martino, J.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef]
- Lee, N.Y.; Roh, M.S.; Kim, S.H.; Jung, D.C.; Yu, H.Y.; Sung, K.H.; Chung, I.W.; Youn, T.; Kang, U.G.; Ahn, Y.M.; et al. The prevalence of metabolic syndrome and its association with alanine aminotransferase in clozapine-treated Korean patients with schizophrenia. Int. Clin. Psycho-Pharmacol. 2013, 28, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, T.; Ishikawa, S.; Okubo, R.; Hashimoto, N.; Sato, N.; Kusumi, I.; Ito, Y.M. Risk factors for abnormal glucose metabolism during antipsychotic treatment: A prospective cohort study. J. Psychiatr. Res. 2023, 168, 149–156. [Google Scholar] [CrossRef]
- Kowalchuk, C.; Kanagasundaram, P.; McIntyre, W.B.; Belsham, D.D.; Hahn, M.K. Direct effects of antipsychotic drugs on insulin, energy sensing and inflammatory pathways in hypothalamic mouse neurons. Psychoneuroendocrinology 2019, 109, 104400. [Google Scholar] [CrossRef]
- Kurita, H.; Yoshioka, W.; Nishimura, N.; Kubota, N.; Kadowaki, T.; Tohyama, C. Aryl hydrocarbon receptor-mediated effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on glucose-stimulated insulin secretion in mice. J. Appl. Toxicol. 2009, 29, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Grajales, D.; Ferreira, V.; Valverde, Á.M. Second-Generation Antipsychotics and Dysregulation of Glucose Metabolism: Beyond Weight Gain. Cells 2019, 8, 1336. [Google Scholar] [CrossRef] [PubMed]
- Møller, M.; Fredholm, S.; Jensen, M.E.; Wörtwein, G.; Larsen, J.R.; Vilsbøll, T.; Ødum, N.; Fink-Jensen, A. Proinflammatory biomarkers are associated with prediabetes in patients with schizophrenia. CNS Spectr. 2022, 27, 347–354. [Google Scholar] [CrossRef]
- Ferreira, V.; Grajales, D.; Valverde, Á.M. Adipose tissue as a target for second-generation (atypical) antipsychotics: A molecular view. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158534. [Google Scholar] [CrossRef]
- Kristóf, E.; Doan-Xuan, Q.M.; Sárvári, A.K.; Klusóczki, Á.; Fischer-Posovszky, P.; Wabitsch, M.; Bacso, Z.; Bai, P.; Balajthy, Z.; Fésüs, L. Clozapine modifies the differentiation program of human adipocytes inducing browning. Transl. Psychiatry 2016, 6, e963. [Google Scholar] [CrossRef] [PubMed]
- van Marum, R.J.; Wegewijs, M.A.; Loonen, A.J.; Beers, E. Hypothermia following antipsychotic drug use. Eur. J. Clin. Pharmacol. 2007, 63, 627–631. [Google Scholar] [CrossRef]
- Hemmrich, K.; Gummersbach, C.; Pallua, N.; Luckhaus, C.; Fehsel, K. Clozapine en-hances differentiation of adipocyte progenitor cells. Mol. Psychiatry 2006, 11, 980–981. [Google Scholar] [CrossRef]
- Lee, E.; Korf, H.; Vidal-Puig, A. An adipocentric perspective on the development and progression of non-alcoholic fatty liver disease. J. Hepatol. 2023, 78, 1048–1062. [Google Scholar] [CrossRef]
- Lee, J.; Bies, R.; Bhaloo, A.; Powell, V.; Remington, G. Clozapine and anemia: A 2-year follow-up study. J. Clin. Psychiatry 2015, 76, 1642–1647. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McLaren, G.D. Iron absorption and metabolism. Curr. Opin. Gastroenterol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- Bouvier, M.L.; Fehsel, K.; Schmitt, A.; Meisenzahl-Lechner, E.; Gaebel, W.; von Wilmsdorff, M. Sex-dependent effects of long-term clozapine or haloperidol medication on red blood cells and liver iron metabolism in Sprague Dawley rats as a model of metabolic syndrome. BMC Pharmacol. Toxicol. 2022, 23, 8. [Google Scholar] [CrossRef]
- Franc, M.A.; Moffat, I.D.; Boutros, P.C.; Tuomisto, J.T.; Tuomisto, J.; Pohjanvirta, R.; Okey, A.B. Patterns of dioxin-altered mRNA expression in livers of dioxin-sensitive versus dioxin-resistant rats. Arch. Toxicol. 2008, 82, 809–830. [Google Scholar] [CrossRef]
- Wang, C.M.; Chen, Y.H.; Lee, Y.C.; Chang, J.S. Endoplasmic reticulum stress contributes to ferritin molecules-mediated macrophage migration via P-selectin glycoprotein ligand-1. Mol. Nutr. Food Res. 2017, 61, 1600458. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.H.; Rouault, T.A. Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab. 2006, 3, 199–210. [Google Scholar] [CrossRef]
- Crooks, D.R.; Maio, N.; Lane, A.N.; Jarnik, M.; Higashi, R.M.; Haller, R.G.; Yang, Y.; Fan, T.W.; Linehan, W.M.; Rouault, T.A. Acute loss of iron-sulfur clusters results in metabolic re-programming and generation of lipid droplets in mammalian cells. J. Biol. Chem. 2018, 293, 8297–8311. [Google Scholar] [CrossRef]
- Fehsel, K.; Bouvier, M.L.; Capobianco, L.; Lunetti, P.; Klein, B.; Oldiges, M.; Majora, M.; Löffler, S. Neuroreceptor inhibition by clozapine triggers mitohormesis and metabolic reprogramming in human blood cells. Cells 2024, 13, 762. [Google Scholar] [CrossRef]
- Stallone, J.N.; Oloyo, A.K. Cardiovascular and metabolic actions of the androgens: Is testosterone a Janus-faced molecule? Biochem. Pharmacol. 2023, 208, 115347. [Google Scholar] [CrossRef]
- Duarte, A.I.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. Brain insulin signalling, glucose metabolism and females' reproductive aging: A dangerous triad in Alzheimer's disease. Neuro-pharmacology 2018, 136, 223–242. [Google Scholar] [CrossRef]
- Fehsel, K.; Christl, J. Comorbidity of osteoporosis and Alzheimer's disease: Is ‘AKT’-ing on cellular glucose up-take the missing link? Ageing Res. Rev. 2022, 76, 101592. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, G.; Sheikhvatan, M. Age and gender differences in the clustering of metabolic syndrome combinations: A prospective cohort research from the Kerman Coronary Artery Disease Risk Study (KERCADRS). Diabetes Metab. Syndr. 2015, 9, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Katare, D.P. Shared links between type 2 diabetes mellitus and Alzheimer's disease: A review. Diabetes Metab. Syndr. 2016, 10, S144–S149. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, M.Z.; Meijer, Y.; de Boer, N.; Guloksuz, S.; Hasan, A.; Siskind, D.; Wagner, E.; CLOZIN consortium; Okhuijsen-Pfeifer, C.; Luykx, J.J. Comprehensive dissection of prevalence rates, sex differences, and blood level-dependencies of clozapine-associated adverse drug reactions. Psychiatry Res. 2023, 330, 115539. [Google Scholar] [CrossRef]
- Shan, Y.; Cheung, L.; Zhou, Y.; Huang, Y.; Huang, R.S. A systematic review on sex differences in adverse drug reactions related to psychotropic, cardiovascular, and analgesic medications. Front. Pharmacol. 2023, 14, 1096366. [Google Scholar] [CrossRef] [PubMed]
- Martini, F.; Spangaro, M.; Buonocore, M.; Bechi, M.; Cocchi, F.; Guglielmino, C.; Bianchi, L.; Sapienza, J.; Agostoni, G.; Mastromatteo, A.; et al. Clozapine toler-ability in Treatment Resistant Schizophrenia: Exploring the role of sex. Psychiatry Res. 2021, 297, 113698. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, S.A.; Winckel, K.; Saiepour, N.; Wheeler, A.J.; Myles, N.; Siskind, D. Clozapine-related neutropenia, myocarditis and cardiomyopathy adverse event reports in Australia 1993–2014. Psychopharmacology 2018, 235, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.Y.; Pai, C.S.; Geng, J.H.; Wu, P.Y.; Huang, J.C.; Chen, S.C.; Chang, J.M. Sex difference in the associations among secondhand smoke with metabolic syndrome in non-smokers in a large Taiwanese population follow-up study. Int. J. Med. Sci. 2024, 21, 1518–1528. [Google Scholar] [CrossRef]
- Nikolić, T.; Petronijević, M.; Sopta, J.; Velimirović, M.; Stojković, T.; Dožudić, G.J.; Aksić, M.; Radonjić, N.V.; Petronijević, N. Haloperidol affects bones while clozapine alters metabolic parameter—Sex specific effects in rats perinatally treated with phencyclidine. BMC Pharmacol. Toxicol. 2017, 18, 65. [Google Scholar] [CrossRef]
- Allard, C.; Morford, J.J.; Xu, B.; Salwen, B.; Xu, W.; Desmoulins, L.; Zsombok, A.; Kim, J.K.; Levin, E.R.; Mauvais-Jarvis, F. Loss of Nuclear and Membrane Estrogen Receptor-alpha Dif-ferentially Impairs Insulin Secretion and Action in Male and Female Mice. Diabetes 2019, 68, 490–501. [Google Scholar] [CrossRef]
- Lee, H.J.; Min, L.; Gao, J.; Matta, S.; Drel, V.; Saliba, A.; Tamayo, I.; Montellano, R.; He-jazi, L.; Maity, S.; et al. Female Protection Against Diabetic Kidney Disease Is Regulated by Kidney-Specific AMPK Activity. Diabetes 2024, 73, 1167–1177. [Google Scholar] [CrossRef]
- Ray, A.; Wen, J.; Yammine, L.; Culver, J.; Garren, J.; Xue, L.; Hales, K.; Xiang, Q.; Birn-baum, M.J.; Zhang, B.B.; et al. GLUT4 dynamic subcellular localization is controlled by AMP kinase activation as revealed by proximal proteome mapping in human muscle cells. J. Cell Sci. 2023, 136, jcs261454. [Google Scholar] [CrossRef]
- Weston-Green, K.; Huang, X.F.; Deng, C. Second generation antipsychotic-induced type 2 diabetes: A role for the muscarinic M3 receptor. CNS Drugs 2013, 27, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, E.A. Histopathological change of the endocrine pancreas in male albino rat treated with the atypical antipsychotic clozapine. Rom. J. Morphol. Embryol. 2013, 54, 385–394. [Google Scholar] [PubMed]
- Muku, G.E.; Kusnadi, A.; Kuzu, G.; Tanos, R.; Murray, I.A.; Gowda, K.; Amin, S.; Perdew, G.H. Selective Ah receptor modulators attenuate NPC1L1-mediated cholesterol uptake through repression of SREBP-2 transcriptional activity. Lab. Investig. 2020, 100, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, G.; Yang, X.; Jia, X.; Li, J.; Bai, X.; Li, W.; Zhao, Y.; Li, Y.; Cheng, W.; et al. Low density lipoprotein mimics insulin action on autophagy and glucose uptake in endothelial cells. Sci. Rep. 2019, 9, 3020. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, C.; Yassin, K.; Wahlström, E.; Cheung, L.; Lindberg, J.; Brismar, K.; Ostenson, C.G.; Norstedt, G.; Tollet-Egnell, P. Sex-different hepatic glycogen content and glucose output in rats. BMC Biochem. 2010, 11, 38. [Google Scholar] [CrossRef]
- Pocai, A.; Obici, S.; Schwartz, G.J.; Rossetti, L. A brain-liver circuit regulates glucose homeostasis. Cell Metab. 2005, 1, 53–61. [Google Scholar] [CrossRef]
- Zhang, L.; Hatzakis, E.; Nichols, R.G.; Hao, R.; Correll, J.; Smith, P.B.; Chiaro, C.R.; Perdew, G.H.; Patterson, A.D. Metabolomics Reveals that Aryl Hydrocarbon Receptor Activation by Environmental Chemicals Induces Systemic Metabolic Dysfunction in Mice. Environ. Sci. Technol. 2015, 49, 8067–8077. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fas-ciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Burghardt, K.J.; Seyoum, B.; Dass, S.E.; Sanders, E.; Mallisho, A.; Yi, Z. Association of Protein Kinase B (AKT) DNA Hypermethylation with Maintenance Atypical Antipsychotic Treatment in Patients with Bipolar Disorder. Pharmacotherapy 2018, 38, 428–435. [Google Scholar] [CrossRef]
- Li, X.; Hu, S.; Cai, Y.; Liu, X.; Luo, J.; Wu, T. Revving the engine: PKB/AKT as a key regulator of cellular glucose metabolism. Front. Physiol. 2024, 14, 1320964. [Google Scholar] [CrossRef]
- de Oliveira, G.P.; Cortez, E.; Araujo, G.J.; de Carvalho Sabino, K.C.; Neves, F.A.; Bernardo, A.F.; de Carvalho, S.N.; Moura, A.S.; Carvalho, L.; Thole, A.A. Impaired mitochondrial function and reduced viability in bone marrow cells of obese mice. Cell Tissue Res. 2014, 357, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kipmen-Korgun, D.; Bilmen-Sarikcioglu, S.; Altunbas, H.; Demir, R.; Korgun, E.T. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand J. Clin. Lab. Investig. 2009, 69, 350–358. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, E.J.M.; de Jongh, R.T.; Brands, M.; Heijboer, A.C.; den Heijer, M.; Serlie, M.J.; van Raalte, D.H. The osteoblast: Linking glucocorticoid-induced osteoporosis and hyperglycaemia? A post-hoc analysis of a randomised clinical trial. Bone 2018, 112, 173–176. [Google Scholar] [CrossRef]
- Ni, Z.; Tang, J.; Cai, Z.; Yang, W.; Zhang, L.; Chen, Q.; Zhang, L.; Wang, X. A new pathway of glucocorticoid action for asthma treatment through the regulation of PTEN expres-sion. Respir. Res. 2011, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Picke, A.K.; Sylow, L.; Møller, L.L.V.; Kjøbsted, R.; Schmidt, F.N.; Steejn, M.W.; Sal-bach-Hirsch, J.; Hofbauer, C.; Blüher, M.; Saalbach, A.; et al. Differential effects of high-fat diet and exercise training on bone and energy metabolism. Bone 2018, 116, 120–134. [Google Scholar] [CrossRef]
- Umapathi, P.; Aggarwal, A.; Zahra, F.; Narayanan, B.; Zachara, N.E. The multifaceted role of intracellular glycosylation in cytoprotection and heart disease. J. Biol. Chem. 2024, 300, 107296. [Google Scholar] [CrossRef]
- Salguero, A.L.; Chen, M.; Balana, A.T.; Chu, N.; Jiang, H.; Palanski, B.A.; Bae, H.; Wright, K.M.; Nathan, S.; Zhu, H.; et al. Multifaceted regulation of Akt by diverse C-terminal post-translational modifications. ACS Chem. Biol. 2022, 17, 68–76. [Google Scholar] [CrossRef]
- Swanda, R.V.; Ji, Q.; Wu, X.; Yan, J.; Dong, L.; Mao, Y.; Uematsu, S.; Dong, Y.; Qian, S.B. Lysosomal cystine governs ferroptosis sensitivity in cancer via cysteine stress response. Mol. Cell 2023, 83, 3347–3359. [Google Scholar] [CrossRef]
- Terashima, J.; Tachikawa, C.; Kudo, K.; Habano, W.; Ozawa, S. An aryl hydrocarbon receptor induces VEGF expression through ATF4 under glucose deprivation in HepG2. BMC Mol Biol. 2013, 14, 27. [Google Scholar] [CrossRef]
- Statzer, C.; Meng, J.; Venz, R.; Bland, M.; Robida-Stubbs, S.; Patel, K.; Petrovic, D.; Emsley, R.; Liu, P.; Morantte, I.; et al. ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1. Nat. Commun. 2022, 13, 967. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogene-sis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129. [Google Scholar] [CrossRef]
- Ogata, T.; Ashimori, A.; Higashijima, F.; Sakuma, A.; Hamada, W.; Sunada, J.; Aoki, R.; Mikuni, M.; Hayashi, K.; Yoshimoto, T.; et al. HIF-1alpha-dependent regulation of angiogenic factor expression in Muller cells by mechanical stimulation. Exp. Eye Res. 2024, 247, 110051. [Google Scholar] [CrossRef]
- Basheeruddin, M.; Qausain, S. Hypoxia-Inducible Factor 1-Alpha (HIF-1alpha): An Essential Regulator in Cellular Metabolic Control. Cureus 2024, 16, e63852. [Google Scholar] [CrossRef]
- Yang, Y.; Chan, W.K. Glycogen Synthase Kinase 3 Beta Regulates the Human Aryl Hydrocarbon Receptor Cellular Content and Activity. Int. J. Mol. Sci. 2021, 22, 6097. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Yang, Z.; Wang, M.; Zhang, W.; Ren, Y.; Li, L.; Hu, J.; Sun, Z.; Nie, S. Ferulic acid positively modulates the inflammatory response to septic liver injury through the GSK-3β/NF-κB/CREB pathway. Life Sci. 2021, 277, 119584. [Google Scholar] [CrossRef]
- Jais, A.; Solas, M.; Backes, H.; Chaurasia, B.; Kleinridders, A.; Theurich, S.; Mauer, J.; Steculorum, S.M.; Hampel, B.; Goldau, J.; et al. Myeloid-Cell-Derived VEGF Maintains Brain Glucose Uptake and Limits Cognitive Impairment in Obesity. Cell 2016, 166, 1338–1340. [Google Scholar] [CrossRef]
- Du, W.; Jiang, S.; Yin, S.; Wang, R.; Zhang, C.; Yin, B.C.; Li, J.; Li, L.; Qi, N.; Zhou, Y.; et al. The microbiota-dependent tryptophan metabolite alleviates high-fat diet-induced insulin resistance through the hepatic AhR/TSC2/mTORC1 axis. Proc. Natl. Acad. Sci. USA 2024, 121, e2400385121. [Google Scholar] [CrossRef]
- Xie, T.; Lv, T.; Zhang, T.; Feng, D.; Zhu, F.; Xu, Y.; Zhang, L.; Gu, L.; Guo, Z.; Ding, C.; et al. Interleukin-6 promotes skeletal muscle catabolism by activating tryptophan-indoleamine 2,3-dioxygenase 1-kynurenine pathway during intraabdominal sepsis. J. Cachexia Sarcopenia Muscle 2023, 14, 1046–1059. [Google Scholar] [CrossRef]
- Rojas, I.Y.; Moyer, B.J.; Ringelberg, C.S.; Wilkins, O.M.; Pooler, D.B.; Ness, D.B.; Coker, S.; Tosteson, T.D.; Lewis, L.D.; Chamberlin, M.D.; et al. Kynurenine-Induced Aryl Hydrocarbon Receptor Signaling in Mice Causes Body Mass Gain, Liver Steatosis, and Hyperglycemia. Obesity (Silver Spring) 2021, 29, 337–349. [Google Scholar] [CrossRef]
- Kot, M.; Daujat-Chavanieu, M. The impact of serotonergic system dysfunction on the regulation of P4501A isoforms during liver insufficiency and consequences for thyroid hormone homeostasis. Food Chem. Toxicol. 2016, 97, 70–81. [Google Scholar] [CrossRef]
- Riabovol, O.O.; Tsymbal, D.O.; Minchenko, D.O.; Lebid-Biletska, K.M.; Sliusar, M.Y.; Rudnytska, O.V.; Minchenko, O.H. Effect of glucose deprivation on the expression of genes encoding glucocorticoid receptor and some related factors in ERN1-knockdown U87 glioma cells. Endocr. Regul. 2019, 53, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Tsou, T.C.; Chao, H.R.; Kuo, Y.T.; Tsai, F.Y.; Yeh, S.C. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3T3-L1 cells. J. Hazard. Mater. 2010, 182, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, R.; Xu, T.; Chen, Y.; Ding, Y.; Zuo, S.; Xu, L.; Xie, H.Q.; Zhao, B. Potential AhR-independent mechanisms of 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibition of human glioblastoma A172 cells migration. Ecotoxicol. Environ. Saf. 2024, 273, 116172. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Hu, K.L.; Li, X.X.; Ge, Y.M.; Yu, X.J.; Zhao, J. Bisphenol A downregulates GLUT4 expression by activating aryl hydrocarbon receptor to exacerbate polycystic ovary syndrome. Cell Commun. Signal 2024, 22, 28. [Google Scholar] [CrossRef]
- Quattrochi, L.C.; Tukey, R.H. Nuclear uptake of the Ah (dioxin) receptor in response to omeprazole: Transcriptional activation of the human CYP1A1 gene. Mol. Pharmacol. 1993, 43, 504–508. [Google Scholar]
- Ayed-Boussema, I.; Pascussi, J.M.; Maurel, P.; Bacha, H.; Hassen, W. Effect of aflatoxin B1 on nuclear receptors PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes. Int. J. Toxicol. 2012, 31, 86–93. [Google Scholar] [CrossRef]
- Volkova, M.; Palmeri, M.; Russell, K.S.; Russell, R.R. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc. Res. 2011, 90, 305–314. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Chang, Z.; Li, S.; Zhang, Z.; Liu, S.; Wang, S.; Wei, L.; Lv, Q.; Ding, K.; et al. SeMet alleviates AFB(1)-induced oxidative stress and apoptosis in rabbit kidney by regulating Nrf2//Keap1/NQO1 and PI3K/AKT signaling pathways. Ecotoxicol. Environ. Saf. 2024, 269, 115742. [Google Scholar] [CrossRef]
- Tulipano, G.; Rizzetti, C.; Bianchi, I.; Fanzani, A.; Spano, P.; Cocchi, D. Clozap-ine-induced alteration of glucose homeostasis in the rat: The contribution of hypothalam-ic-pituitary-adrenal axis activation. Neuroendocrinology 2007, 85, 61–70. [Google Scholar] [CrossRef]
- Dwyer, D.S.; Pinkofsky, H.B.; Liu, Y.; Bradley, R.J. Antipsychotic drugs affect glucose uptake and the expression of glucose transporters in PC12 cells. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 69–80. [Google Scholar] [CrossRef]
- Krijt, J.; Sokolová, J.; Šilhavý, J.; Mlejnek, P.; Kubovčiak, J.; Liška, F.; Malínská, H.; Hüttl, M.; Marková, I.; Křížková, M.; et al. High cysteine diet reduces insulin resistance in SHR-CRP rats. Physiol. Res. 2021, 70, 687–700. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Schmidt, E.E. Unresolved questions regarding cellular cysteine sources and their possible relationships to ferroptosis. Adv. Cancer Res. 2024, 162, 1–44. [Google Scholar] [CrossRef]
- Sapienza, J.; Agostoni, G.; Dall'Acqua, S.; Sut, S.; Nasini, S.; Martini, F.; Marchesi, A.; Bechi, M.; Buonocore, M.; Cocchi, F.; et al. The kynurenine pathway in treatment-resistant schizophrenia at the crossroads between pathophysiology and pharmacotherapy. Schizophr. Res. 2024, 264, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Domingues, D.S.; Crevelin, E.J.; de Moraes, L.A.; Cecilio Hallak, J.E.; de Souza Crippa, J.A.; Costa Queiroz, M.E. Simultaneous determination of amino acids and neurotransmitters in plasma samples from schizophrenic patients by hydrophilic interaction liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 780–787. [Google Scholar] [CrossRef]
- Calvo, B.; Fernandez, M.; Rincon, M.; Tranque, P. GSK3β Inhibition by Phosphorylation at Ser389 Controls Neuroinflammation. Int. J. Mol. Sci. 2022, 4, 337. [Google Scholar] [CrossRef]
- Ma, S.X.; Li, X.J.; Duan, T.T.; Pei, M.; Zou, L.; Yu, X.Y.; Zhao, Y.Y. Moshen granule ameliorates membranous nephropathy by regulating NF-kB/Nrf2 pathways via aryl hydrocarbon receptor signalling. Heliyon 2023, 9, e20019. [Google Scholar] [CrossRef]
- Tugnait, M.; Hawes, E.M.; McKay, G.; Eichelbaum, M.; Midha, K.K. Characterization of the human hepatic cytochromes P450 involved in the in vitro oxidation of clozapine. Chem. Biol. Interact. 1999, 118, 171–189. [Google Scholar] [CrossRef]
- Lane, H.Y.; Chang, Y.C.; Chang, W.H.; Lin, S.K.; Tseng, Y.T.; Jann, M.W. Effects of gender and age on plasma levels of clozapine and its metabolites: Analyzed by critical statistics. J. Clin. Psychiatry 1999, 60, 36–40. [Google Scholar] [CrossRef]
- Ardizzone, T.D.; Bradley, R.J.; Freeman, A.M., 3rd; Dwyer, D.S. Inhibition of glucose transport in PC12 cells by the atypical antipsychotic drugs risperidone and clozapine, and structural analogs of clozapine. Brain Res. 2001, 923, 82–90. [Google Scholar] [CrossRef]
- Fabrazzo, M.; Esposito, G.; Fusco, R.; Maj, M. Effect of treatment duration on plasma levels of clozapine and N-desmethylclozapine in men and women. Psychopharmacology 1996, 124, 197–200. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Y.H.; Song, W.; Ai, C.X.; Liu, Z.M.; Yu, M.H.; Zou, W.G. Different effects of continuous and pulsed Benzo[a]pyrene exposure on metabolism and antioxidant defense of large yellow croaker: Depend on exposure duration. Ecotoxicol. Environ. Saf. 2023, 263, 115370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, L.; He, Y.; Qin, L.; Tan, D.; Bai, Z.; Song, Y.; Wang, Y.H. The alteration of drug metabolism enzymes and pharmacokinetic parameters in nonalcoholic fatty liver disease: Current animal models and clinical practice. Drug Metab Rev. 2023, 55, 163–180. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, P.; Cheng, Y.; Wang, P.; Ma, X.; Liu, M.; Wang, X.; Xu, F. Diet-induced obese alters the expression and function of hepatic drug-metabolizing enzymes and transporters in rats. Biochem. Pharmacol. 2019, 164, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, J.; Guo, Y.; Zhang, Y.; Liu, J.; Huang, S.; Zhang, Y.; Gao, L.; Wang, X. Hypercholesterolemia reduces the expression and function of hepatic drug etabolizing enzymes and trans-porters in rats. Toxicol. Lett. 2022, 364, 1–11. [Google Scholar] [CrossRef]
- Das, D.N.; Naik, P.P.; Mukhopadhyay, S.; Panda, P.K.; Sinha, N.; Meher, B.R.; Bhutia, S.K. Elimination of dysfunctional mitochondria through mitophagy suppresses ben-zo[a]pyrene-induced apoptosis. Free Radic. Biol. Med. 2017, 112, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Mazzoccoli, G. Left Ventricular Hypertrophy: Roles of Mitochondria CYP1B1 and Melatonergic Pathways in Co-Ordinating Wider Pathophysiology. Int. J. Mol. Sci. 2019, 20, 4068. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cheung, G.; Zhang, Z.; Papadopoulos, V. Mitochondrial cytochrome P450 1B1 is involved in pregnenolone synthesis in human brain cells. J. Biol. Chem. 2023, 299, 105035. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Tian, S.; Na, S.; Wei, H.; Wu, Y.; Yang, Y.; Shen, Z.; Ding, J.; Bao, S.; et al. CYP1B1 affects the integrity of the blood-brain barrier and oxidative stress in the striatum: An investigation of manganese-induced neurotoxicity. CNS Neurosci. Ther. 2024, 30, e14633. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, H.; He, J.; Lai, J.; Lin, H.; Liu, X. Melatonin alleviates ischemic stroke by inhibiting ferroptosis through the CYP1B1/ACSL4 pathway. Environ. Toxicol. 2024, 39, 2623–2633. [Google Scholar] [CrossRef]
- Ron, D.; Harding, H.P. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb. Perspect. Biol. 2012, 4, a013177. [Google Scholar] [CrossRef]
- Kelly, B.; Carrizo, G.E.; Edwards-Hicks, J.; Sanin, D.E.; Stanczak, M.A.; Priesnitz, C.; Flachsmann, L.J.; Curtis, J.D.; Mittler, G.; Musa, Y.; et al. Sulfur sequestration promotes multicellularity during nutrient limitation. Nature 2021, 591, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, K.; Janik, K.; Rydz, L.; Wróbel, M.; Jurkowska, H. Bridging the Gap in Cancer Research: Sulfur Metabolism of Leukemic Cells with a Focus on L-Cysteine Metabolism and Hydrogen Sulfide-Producing Enzymes. Biomolecules 2024, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.V.; Shin, E.J.; Jeong, J.H.; Lee, J.W.; Lee, Y.; Jang, C.G.; Nah, S.Y.; Lei, X.G.; Toriumi, K.; Yamada, K.; et al. Protective Potential of the Glutathione Peroxidase-1 Gene in Abnormal Behaviors Induced by Phencyclidine in Mice. Mol. Neurobiol. 2017, 54, 7042–7062. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef]
- Blevins, L.K.; Zhou, J.; Crawford, R.; Kaminski, N.E. TCDD-mediated suppression of naive hu-man B cell IgM secretion involves aryl hydrocarbon receptor-mediated reduction in STAT3 serine 727 phosphorylation and is restored by interferon-gamma. Cell Signal. 2020, 65, 109447. [Google Scholar] [CrossRef]
- Yu, J.S. Activation of protein phosphatase 2A by the Fe2+/ascorbate system. J. Biochem. 1998, 124, 225–230. [Google Scholar] [CrossRef]
- Maguire, M.; Larsen, M.C.; Foong, Y.H.; Tanumihardjo, S.; Jefcoate, C.R. Cyp1b1 deletion and retinol deficiency coordinately suppress mouse liver lipogenic genes and hepcidin expression during postnatal development. Mol. Cell Endocrinol. 2017, 454, 50–68. [Google Scholar] [CrossRef]
- Alva, R.; Wiebe, J.E.; Stuart, J.A. Revisiting reactive oxygen species production in hypoxia. Pflug. Arch. 2024, 476, 1423–1444. [Google Scholar] [CrossRef]
- Yang, X.; Zhong, M.; Chen, J.; Li, T.; Cheng, Q.; Dai, Y. HIF-1 repression of PTEN transcription mediates protective effects of BMSCs on neurons during hypoxia. Neuroscience 2018, 392, 57–65. [Google Scholar] [CrossRef]
- Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Schwarz, F.; Sadlon, A.; Abderhalden, L.A.; de Godoi Rezende Costa Molino, C.; Spahn, D.R.; Schaer, D.J.; Orav, E.J.; Egli, A.; Bischoff-Ferrari, H.A. DO-HEALTH Research group. Iron deficiency and biomarkers of inflammation: A 3-year prospective analysis of the DO-HEALTH trial. Aging Clin. Exp. Res. 2022, 34, 515–525. [Google Scholar] [CrossRef]
- Löffler, S.; Löffler-Ensgraber, M.; Fehsel, K.; Klimke, A. Clozapine therapy raises serum concentrations of high sensitive C-reactive protein in schizophrenic patients. Int. Clin. Psychopharmacol. 2010, 25, 101–106. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Hu, C.; Cui, J.; Li, H.; Luo, X.; Hao, Y. IL-6 Enhances the Activation of PI3K-AKT/mTOR-GSK-3β by Upregulating GRPR in Hippocampal Neurons of Autistic Mice. J. Neuroimmune Pharmacol. 2024, 19, 12. [Google Scholar] [CrossRef]
- Smieszek, S.P.; Przychodzen, B.P.; Polymeropoulos, V.M.; Polymeropoulos, C.M.; Polymeropoulos, M.H. Assessing the potential correlation of polymorphisms in the IL6R with relative IL6 elevation in severely ill COVID-19 patients'. Cytokine 2021, 148, 155662. [Google Scholar] [CrossRef]
- Li, X.; Qiu, W.; Li, N.; Da, X.; Ma, Q.; Hou, Y.; Wang, T.; Song, M.; Chen, J. Susceptibility to hyperglycemia in rats with stress-induced depressive-like behavior: Involvement of IL-6 mediated glucose homeostasis signaling. Front. Psychiatry 2020, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, Y.; Ju, P.; Gao, J.; Zhang, L.; Li, J.; Wang, K.; Zhang, J.; Li, C.; Xia, Q.; et al. Network association of biochemical and inflammatory abnormalities with psychiatric symptoms in first-episode schizophrenia Patients. Front. Psychiatry 2022, 13, 834539. [Google Scholar] [CrossRef]
- Costantini, E.; Carrarini, C.; Calisi, D.; De Rosa, M.; Simone, M.; Di Crosta, A.; Pa-lumbo, R.; Cipollone, A.; Aielli, L.; De Laurentis, M.; et al. Search in the periphery for potential inflammatory biomarkers of dementia with Lewy Bodies and Alzheimer's disease. J. Alzheimers Dis. 2024, 99, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.S.; Daneshpour, M.S.; Akbarzadeh, M.; Hedayati, M.; Azizi, F.; Zarkesh, M. Association of baseline and changes in adiponectin, homocysteine, high-sensitivity C-reactive protein, interleukin-6, and interleukin-10 levels and metabolic syndrome incidence: Tehran lipid and glucose study. Heliyon 2023, 9, e19911. [Google Scholar] [CrossRef]
- Zhu, M.; Peng, L.; Huo, S.; Peng, D.; Gou, J.; Shi, W.; Tao, J.; Jiang, T.; Jiang, Y.; Wang, Q.; et al. STAT3 signaling promotes cardiac injury by upregulating NCOA4-mediated ferritinophagy and ferroptosis in high-fat-diet fed mice. Free Radic. Biol. Med. 2023, 201, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhang, Y.; Ye, T.; Kong, Y.; Cui, X.; Yuan, S.; Liu, J.; Zhang, Y. A high-tryptophan diet alleviated cognitive impairment and neuroinflammation in APP/PS1 mice through activating aryl hydrocarbon receptor via the regulation of gut microbiota. Mol. Nutr. Food Res. 2024, 68, e2300601. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; Lee, J.R.; Lee, J.Y.; Lee, H. Metabolic health is more strongly associated with the severity and mortality of coronavirus disease 2019 than obesity. Arch. Public Health 2024, 82, 131. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, T. Heterogeneity of the effect of the COVID-19 pandemic on the incidence of Metabolic Syndrome onset at a Japanese campus. PeerJ 2024, 12, e17013. [Google Scholar] [CrossRef]
- Rochowski, M.T.; Jayathilake, K.; Balcerak, J.M.; Tamil Selvan, M.; Gunasekara, S.; Rudd, J.; Miller, C.; Lacombe, V.A. Alterations of whole body glucose metabolism in a feline SARS-CoV-2 infection model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2024, 326, R499–R506. [Google Scholar] [CrossRef]
- Gugo, K.; Tandara, L.; Juricic, G.; Pavicic Ivelja, M.; Rumora, L. Effects of Hypoxia and Inflammation on Hepcidin Concentration in Non-Anaemic COVID-19 Patients. J. Clin. Med. 2024, 13, 3201. [Google Scholar] [CrossRef]
- Mostaghim, A.; Sathe, N.A.; Mabrey, F.L.; Sahi, S.; O'Connor, N.; Morrell, E.D.; Fitzpatrick, M.; Smith, C.H.; Wurfel, M.M.; Liles, W.C.; et al. Normalization of IL-6 levels is associated with survival in critically ill patients with COVID-19. J. Crit. Care 2024, 84, 154896. [Google Scholar] [CrossRef]
- AbdelMassih, A.; Yacoub, E.; Husseiny, R.J.; Kamel, A.; Hozaien, R.; El Shershaby, M.; Rajab, M.; Yacoub, S.; Eid, M.A.; Elahmady, M.; et al. Hypoxia-inducible factor (HIF): The link between obesity and COVID-19. Obes. Med. 2021, 22, 100317. [Google Scholar] [CrossRef]
- Icard, P.; Lincet, H.; Wu, Z.; Coquerel, A.; Forgez, P.; Alifano, M.; Fournel, L. The key role of Warburg effect in SARS-CoV-2 replication and associated inflammatory response. Biochimie 2021, 180, 169–177. [Google Scholar] [CrossRef]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Onodera, T.; Fukuda, S.; Kita, S.; Fukuhara, A.; Shimomura, I. SARS-CoV-2 infection impairs the insulin/IGF signaling pathway in the lung, liver, adipose tissue, and pancreatic cells via IRF1. Metabolism 2022, 133, 155236. [Google Scholar] [CrossRef] [PubMed]
- Rochowski, M.T.; Jayathilake, K.; Balcerak, J.M.; Selvan, M.T.; Gunasekara, S.; Miller, C.; Rudd, J.M.; Lacombe, V.A. Impact of Delta SARS-CoV-2 infection on glucose metabolism: Insights on host metabolism and virus crosstalk in a feline model. Viruses 2024, 16, 295. [Google Scholar] [CrossRef]
- Giovannoni, F.; Li, Z.; Remes-Lenicov, F.; Dávola, M.E.; Elizalde, M.; Paletta, A.; Ash-kar, A.A.; Mossman, K.L.; Dugour, A.V.; Figueroa, J.M.; et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 2021, 12, 5148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.W.; Chen, W.; Culpepper, J.; Jiang, H.; Ball, L.E.; Mehrotra, S.; Blumental-Perry, A.; Tew, K.D.; Townsend, D.M. Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radic. Biol. Med. 2020, 160, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, K.R.H.; Katshu, M.Z.U.H.; Chakrabarti, L. Second-generation antipsychotics and metabolic syndrome: A role for mitochondria. Front. Psychiatry 2023, 14, 1257460. [Google Scholar] [CrossRef]
- Da, W.; Chen, Q.; Shen, B. The current insights of mitochondrial hormesis in the occurrence and treatment of bone and cartilage degeneration. Biol. Res. 2024, 57, 37. [Google Scholar] [CrossRef]
- Fehsel, K.; Loeffler, S.; Krieger, K.; Henning, U.; Agelink, M.; Kolb-Bachofen, V.; Klimke, A. Clozapine induces oxidative stress and proapoptotic gene expression in neutrophils of schizo-phrenic patients. J. Clin. Psychopharmacol. 2005, 25, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.M.; Skaria, D.S.; James, T.; Kanthlal, S.K. Clozapine Disrupts Endothelial Nitric Oxide Signaling and Antioxidant System for its Cardiovascular Complications. Drug Res. 2019, 69, 695–698. [Google Scholar] [CrossRef]
- Zaulet, M.; Kevorkian, S.E.M.; Dinescu, S.; Cotoraci, C.; Suciu, M.; Herman, H.; Buburuzan, L.; Badulescu, L.; Ardelean, A.; Hermenean, A. Protective effects of silymarin against bisphenol A-induced hepatotoxicity in mouse liver. Exp. Ther. Med. 2017, 13, 821–828. [Google Scholar] [CrossRef]
- Cahuzac, K.M.; Lubin, A.; Bosch, K.; Stokes, N.; Shoenfeld, S.M.; Zhou, R.; Lemon, H.; Asara, J.; Parsons, R.E. AKT activation because of PTEN loss upregulates xCT via GSK3β/NRF2, leading to inhibition of ferroptosis in PTEN-mutant tumor cells. Cell Rep. 2023, 42, 112536. [Google Scholar] [CrossRef]
- Mayén-Lobo, Y.G.; Alcaraz-Zubeldia, M.; Dávila-Ortiz de Montellano, D.J.; Motilla-Frías, B.A.; García-Manteca, M.Y.; Ortega-Vázquez, A.; Aviña-Cervantes, C.L.; Crail-Meléndez, E.D.; Ríos, C.; López-López, M.; et al. Influence of glutathione-related genetic variants on the oxidative stress profile of Mexican patients with psychotic disorders. Braz. J. Psychiatry 2023, 45, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Rivas, J.C.; Galindo-A, J.; Zambrano, L.F.; Miranda-B, C.A.; Ramírez, S.M.; Rivas-Grajales, A.M.; Hernández-Carrillo, M.; Rincón, E.A.; Perafán, P.E.; Gómez-Mesa, J.E. Risk of corrected QT interval prolongation in patients receiving antipsychotics. Int. Clin. Psychopharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, N.; Madhukar, M. Assessment of Corrected QT Interval and QT Dispersion in Patients with Uncomplicated Metabolic Syndrome. J. Pharm. Bioallied. Sci. 2023, 15 (Suppl. S2), S1097–S1100. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Srole, D.N.; Palaskas, N.J.; Meriwether, D.; Reddy, S.T.; Ganz, T.; Nemeth, E. Iron loading induces cholesterol synthesis and sensitizes endothelial cells to TNFα-mediated apoptosis. J. Biol. Chem. 2021, 297, 101156. [Google Scholar] [CrossRef]
- Lee, E.H.; Lee, J.H.; Kim, D.Y.; Lee, Y.S.; Jo, Y.; Dao, T.; Kim, K.E.; Song, D.K.; Seo, J.H.; Seo, Y.K.; et al. Loss of SREBP-1c ameliorates iron-induced liver fibrosis by decreasing lipocalin-2. Exp. Mol. Med. 2024, 56, 1001–1012. [Google Scholar] [CrossRef]
- Eti, N.A.; Flor, S.; Iqbal, K.; Scott, R.L.; Klenov, V.E.; Gibson-Corley, K.N.; Soares, M.J.; Ludewig, G.; Robertson, L.W. PCB126 induced toxic actions on liver energy metabolism is mediated by AhR in rats. Toxicology 2022, 466, 153054. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.; Han, J.; Lei, Y.; Cao, Z.; Pan, J.; Pan, Z.; Zhang, Z.; Qu, N.; Luo, H.; et al. Tiaogan Jiejiu Tongluo Formula attenuated alcohol-induced chronic liver injury by regulating lipid metabolism in rats. J. Ethnopharmacol. 2023, 317, 116838. [Google Scholar] [CrossRef]
- Abdul Muneer, P.M.; Alikunju, S.; Szlachetka, A.M.; Haorah, J. Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: Preventive role of acetyl-L: -carnitine. Psychopharmacology 2011, 214, 707–718. [Google Scholar] [CrossRef]
- Lefebvre, P.; Chinetti, G.; Fruchart, J.C.; Staels, B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J. Clin. Investig. 2006, 116, 571–580. [Google Scholar] [CrossRef]
- Löffler, D.; Landgraf, K.; Körner, A.; Kratzsch, J.; Kirkby, K.C.; Himmerich, H. Modulation of triglyceride accumulation in adipocytes by psychopharmacological agents in vitro. J. Psychiatr. Res. 2016, 72, 37–42. [Google Scholar] [CrossRef]
- Brandl, E.J.; Tiwari, A.K.; Zai, C.C.; Chowdhury, N.I.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L.; Müller, D.J. No evidence for a role of the peroxisome proliferator-activated receptor gamma (PPARG) and adiponectin (ADIPOQ) genes in antipsychotic-induced weight gain. Psychiatry Res. 2014, 219, 255–260. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, J.Y.; Gong, Z.C.; Huang, Q.; Chen, H.; Zhang, W.; Zhou, H.H.; Liu, Z.Q. Evidence for an effect of clozapine on the regulation of fat-cell derived factors. Clin. Chim. Acta 2009, 408, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gadupudi, G.; Gourronc, F.A.; Ludewig, G.; Robertson, L.W.; Klingelhutz, A.J. PCB126 inhibits adipogenesis of human preadipocytes. Toxicol. Vitr. 2015, 29, 132–141. [Google Scholar] [CrossRef]
- Tong, M.; Ziplow, J.L.; Mark, P.; de la Monte, S.M. Dietary Soy Prevents Alcohol-Mediated Neurocognitive Dysfunction and Associated Impairments in Brain Insulin Pathway Signaling in an Adolescent Rat Model. Biomolecules 2022, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Fernø, J.; Vik-Mo, A.O.; Jassim, G.; Håvik, B.; Berge, K.; Skrede, S.; Gudbrandsen, O.A.; Waage, J.; Lunder, N.; Mørk, S.; et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology 2009, 203, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Fan, S.; Gao, Y.; Cai, C.; Li, H.; Li, X.; Yang, X.; Xing, Y.; Huang, M.; et al. The reversal of PXR or PPARα activation-induced hepatomegaly. Toxicol. Lett. 2024, 397, 79–88. [Google Scholar] [CrossRef]
- Wickramasinghe, P.B.; Qian, S.; Langley, L.E.; Liu, C.; Jia, L. Hepatocyte toll-Like receptor 4 mediates alcohol-induced insulin resistance in mice. Biomolecules 2023, 13, 454. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ko, B.; Kim, D.J.; Tak, J.; Han, C.Y.; Cho, J.Y.; Kim, W.; Kim, S.G. Induction of the hepatic aryl hydrocarbon receptor by alcohol dysregulates autophagy and phospholipid metabolism via PPP2R2D. Nat. Commun. 2022, 13, 6080. [Google Scholar] [CrossRef]
- Harrison-Findik, D.D.; Klein, E.; Crist, C.; Evans, J.; Timchenko, N.; Gollan, J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology 2007, 46, 1979–1985. [Google Scholar] [CrossRef]
- Gallage, S.; Ali, A.; Barragan, A.J.E.; Seymen, N.; Ramadori, P.; Joerke, V.; Zizmare, L.; Aicher, D.; Gopalsamy, I.K.; Fong, W.; et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. 2024, 36, 1371–1393.e7. [Google Scholar] [CrossRef]
- Pang, M.; de la Monte, S.M.; Longato, L.; Tong, M.; He, J.; Chaudhry, R.; Duan, K.; Ouh, J.; Wands, J.R. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J. Hepatol. 2009, 50, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Ndakotsu, A.; Vivekanandan, G. The Role of Thiazolidinediones in the Amelioration of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e25380. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, D.K.; Dwyer, D.S.; Bodhankar, S.L. Antipsychotic induced metabolic abnormalities: An interaction study with various PPAR modulators in mice. Life Sci. 2006, 79, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Barroso, E.; Peña, L.; Rada, P.; Valverde, Á.M.; Wahli, W.; Palomer, X.; Vázquez-Carrera, M. PPARβ/δ attenuates hepatic fibrosis by reducing SMAD3 phosphorylation and p300 levels via AMPK in hepatic stellate cells. Biomed. Pharmacother. 2024, 179, 117303. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Zhang, Y.; Zhang, T.; Qi, X.; Hou, L.; Ma, Z.; Xu, F. Hepatic polypeptide nutrient solution improves high-cholesterol diet-induced rats with nonalcoholic fatty liver disease by activating AMP-activated protein kinase signaling pathway. Food Sci. Nutr. 2024, 12, 3225–3236. [Google Scholar] [CrossRef]
- Bao, J.; Zhao, Y.; Xu, X.; Ling, S. Advance of Metformin in Liver Disease. Curr. Med. Chem. 2024. [Google Scholar] [CrossRef]
- Plowman, T.J.; Christensen, H.; Aiges, M.; Fernandez, E.; Shah, M.H.; Ramana, K.V. Anti-inflammatory potential of the anti-diabetic drug metformin in the prevention of inflammatory complications and infectious diseases including COVID-19: A narrative review. Int. J. Mol. Sci. 2024, 25, 5190. [Google Scholar] [CrossRef]
- Ladeiras-Lopes, R.; Sampaio, F.; Leite, S.; Santos-Ferreira, D.; Vilela, E.; Leite-Moreira, A.; Bettencourt, N.; Gama, V.; Braga, P.; Fontes-Carvalho, R. Metformin in non-diabetic patients with metabolic syndrome and diastolic dysfunction: The MET-DIME randomized trial. Endocrine 2021, 72, 699–710. [Google Scholar] [CrossRef]
- Battini, V.; Cirnigliaro, G.; Leuzzi, R.; Rissotto, E.; Mosini, G.; Benatti, B.; Pozzi, M.; Nobile, M.; Radice, S.; Carnovale, C.; et al. The potential effect of metformin on cognitive and other symptom dimensions in patients with schizophrenia and antipsychotic-induced weight gain: A systematic review, meta-analysis, and meta-regression. Front. Psychiatry 2023, 14, 1215807. [Google Scholar] [CrossRef]
- Possik, E.; Klein, L.L.; Sanjab, P.; Zhu, R.; Côté, L.; Bai, Y.; Zhang, D.; Sun, H.; Al-Mass, A.; Oppong, A.; et al. Glycerol 3-phosphate phosphatase/PGPH-2 counters metabolic stress and promotes healthy aging via a glycogen sensing-AMPK-HLH-30-autophagy axis in C. elegans. Nat. Commun. 2023, 14, 5214. [Google Scholar] [CrossRef]
- Ren, Q.; Sun, Q.; Fu, J. Dysfunction of autophagy in high-fat diet-induced non-alcoholic fatty liver disease. Autophagy 2024, 20, 221–241. [Google Scholar] [CrossRef]
- Camacho-Castillo, L.; Phillips-Farfán, B.V.; Rosas-Mendoza, G.; Baires-López, A.; Toral-Ríos, D.; Campos-Peña, V.; Carvajal, K. Increased oxidative stress contributes to enhance brain amyloidogenesis and blunts energy metabolism in sucrose-fed rat: Effect of AMPK activation. Sci. Rep. 2021, 11, 19547. [Google Scholar] [CrossRef] [PubMed]
- Lindtner, C.; Scherer, T.; Zielinski, E.; Filatova, N.; Fasshauer, M.; Tonks, N.K.; Puchowicz, M.; Buettner, C. Binge drinking induces whole-body insulin resistance by impairing hypothalamic insulin action. Sci. Transl. Med. 2013, 5, 170ra14. [Google Scholar] [CrossRef]
- Hirato, Y.; Seiriki, K.; Kojima, L.; Yamada, S.; Rokujo, H.; Takemoto, T.; Nakazawa, T.; Kasai, A.; Hashimoto, H. Clozapine induces neuronal activation in the medial prefrontal cortex in a projection target-biased manner. Biol. Pharm. Bull. 2024, 47, 478–485. [Google Scholar] [CrossRef]
- Stanley, S.; Devarakonda, K.; O'Connor, R.; Jimenez-Gonzalez, M.; Alvarsson, A.; Hampton, R.; Espinoza, D.; Li, R.; Shtekler, A.; Conner, K.; et al. Amygdala-liver signaling orchestrates rapid glycemic responses to stress and drives stress-induced metabolic dysfunction. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Roth, C.L.; Zenno, A. Treatment of hypothalamic obesity in people with hypothalamic injury: New drugs are on the horizon. Front. Endocrinol. 2023, 14, 1256514. [Google Scholar] [CrossRef]
- Refaat, H.; Dowidar, M.F.; Ahmed, A.I.; Khamis, T.; Abdelhaleem, S.E.; Abdo, S.A. The corrective role of super-paramagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity-associated secondary hypogonadism. Open Vet. J. 2024, 14, 428–437. [Google Scholar] [CrossRef] [PubMed]
- von Wilmsdorff, M.; Bouvier, M.L.; Henning, U.; Schmitt, A.; Gaebel, W. The impact of antipsychotic drugs on food intake and body weight and on leptin levels in blood and hypotha-lamic ob-r leptin receptor expression in Wistar rats. Clinics 2010, 65, 885–894. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, L.K.; Ciaraldi, T.P.; Henry, R.R.; Wittgrove, A.C.; Phillips, S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte 2013, 2, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Meier, U.; Gressner, A.M. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin. Chem. 2004, 50, 1511–1525. [Google Scholar] [CrossRef]
- Tsubai, T.; Yoshimi, A.; Hamada, Y.; Nakao, M.; Arima, H.; Oiso, Y.; Noda, Y. Effects of clozapine on adipokine secretions/productions and lipid droplets in 3T3-L1 adipocytes. J. Pharmacol. Sci. 2017, 133, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, P.; Martinez, M.; Ahima, R.S.; William Wong, G.; Inestrosa, N.C. Modulation of glucose metabolism in hippocampal neurons by adiponectin and resistin. Mol. Neurobiol. 2019, 56, 3024–3037. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, P.; Gherardelli, C.; Gutierrez, J.; Salazar, P.; Mendez-Orellana, C.; Wong, G.W.; Inestrosa, N.C. Adiponectin and resistin modulate the progression of Alzheimers disease in a metabolic syndrome model. Front. Endocrinol. 2023, 14, 1237796. [Google Scholar] [CrossRef]
- Iwaniak, P.; Owe-Larsson, M.; Urbańska, E.M. Microbiota, tryptophan and aryl hydrocarbon receptors as the target triad in Parkinson's disease-a narrative review. Int. J. Mol. Sci. 2024, 25, 2915. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O'Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef]
- Cuartero, M.I.; Ballesteros, I.; de la Parra, J.; Harkin, A.L.; Abautret-Daly, A.; Sherwin, E.; Fernández-Salguero, P.; Corbí, A.L.; Lizasoain, I.; Moro, M.A. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation 2014, 130, 2040–2051. [Google Scholar] [CrossRef]
- Rajkumar, R.; Suri, S.; Deng, H.M.; Dawe, G.S. Nicotine and clozapine cross-prime the locus coeruleus noradrenergic system to induce long-lasting potentiation in the rat hippocampus. Hippocampus 2013, 23, 616–624. [Google Scholar] [CrossRef]
- He, C.; An, Y.; Shi, L.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Du, Y.; Xie, J.; et al. Xiasangju alleviate metabolic syndrome by enhancing noradrenaline biosynthesis and activating brown adipose tissue. Front. Pharmacol. 2024, 15, 1371929. [Google Scholar] [CrossRef]
- Gourronc, F.A.; Perdew, G.H.; Robertson, L.W.; Klingelhutz, A.J. PCB126 blocks the thermogenic beiging response of adipocytes. Environ. Sci. Pollut. Res. Int. 2020, 27, 8897–8904. [Google Scholar] [CrossRef]
- Lelou, E.; Corlu, A.; Nesseler, N.; Rauch, C.; Mallédant, Y.; Seguin, P.; Aninat, C. The Role of Catecholamines in Pathophysiological Liver Processes. Cells 2022, 11, 1021. [Google Scholar] [CrossRef]
- Nagaoka, S.; Kato, M.; Aoyama, Y.; Yoshida, A. Comparative studies on the hypercholesterolaemia induced by excess dietary tyrosine or polychlorinated biphenyls in rats. Br. J. Nutr. 1986, 56, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.S.; Zaher, Z.F.; Ashour, O.M.; Abdel-Naim, A.B. 2-Methoxyestradiol ameliorates metabolic syndrome-induced hypertension and catechol-O-methyltransferase inhibited expression and activity in rats. Eur. J. Pharmacol. 2020, 882, 173278. [Google Scholar] [CrossRef] [PubMed]
- Desaulniers, D.; Xiao, G.H.; Leingartner, K.; Chu, I.; Musicki, B.; Tsang, B.K. Comparisons of brain, uterus, and liver mRNA expression for cytochrome p450s, DNA methyltransferase-1, and catechol-o-methyltransferase in prepubertal female Sprague-Dawley rats exposed to a mixture of aryl hydrocarbon receptor agonists. Toxicol. Sci. 2005, 86, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Dima, L.; Correll, C.U.; Manu, P. Pharmacological Management of Glucose Dysregulation in Patients Treated with Second-Generation Antipsychotics. Drugs 2020, 80, 1763–1781. [Google Scholar] [CrossRef]
- Zimbron, J.; Khandaker, G.M.; Toschi, C.; Jones, P.B.; Fernandez-Egea, E. A systematic review and meta-analysis of randomised controlled trials of treatments for clozapine-induced obesity and metabolic syndrome. Eur. Neuropsychopharmacol. 2016, 26, 1353–1365. [Google Scholar] [CrossRef]
- Zhuo, C.; Xu, Y.; Wang, H.; Zhou, C.; Liu, J.; Yu, X.; Shao, H.; Tian, H.; Fang, T.; Li, Q.; et al. Clozapine induces metformin-resistant prediabetes/diabetes that is associated with poor clinical efficacy in patients with early treatment-resistant schizophrenia. J. Affect Disord. 2021, 295, 163–172. [Google Scholar] [CrossRef]
- Vasiliu, O. Impact of SGLT2 inhibitors on metabolic status in patients with psychiatric disorders undergoing treatment with second-generation antipsychotics. Exp. Ther. Med. 2023, 25, 125. [Google Scholar] [CrossRef]
- Möller, M.; Du Preez, J.L.; Viljoen, F.P.; Berk, M.; Emsley, R.; Harvey, B.H. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain Behav. Immun. 2013, 30, 156–167. [Google Scholar] [CrossRef]
- Liu, J.; Su, H.; Jin, X.; Wang, L.; Huang, J. The effects of N-acetylcysteine supplement on metabolic parameters in women with polycystic ovary syndrome: A systematic review and me-ta-analysis. Front. Nutr. 2023, 10, 1209614. [Google Scholar] [CrossRef]
- Abdelhaffez, A.S.; Abd El-Aziz, E.A.; Tohamy, M.B.; Ahmed, A.M. N-acetyl cysteine can blunt metabolic and cardiovascular effects via down-regulation of cardiotrophin-1 in rat model of fructose-induced metabolic syndrome. Arch. Physiol. Biochem. 2023, 129, 854–869. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.; Chen, W.J.; Bunck, M.C.; van Raalte, D.H.; van der Zijl, N.J.; van Genugten, R.E.; van Bloemendaal, L.; Baggen, J.M.; Serné, E.H.; Diamant, M.; et al. Metabolic changes in type 2 diabetes are reflected in peripheral blood cells, revealing aberrant cytotoxicity, a viral signature, and hypoxia inducible factor activity. BMC Med. Genom. 2015, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.Y.; Friedman, J.E.; Joshi, A.D. Role of Hepatic Aryl Hydrocarbon Receptor in Non-Alcoholic Fatty Liver Disease. Receptors 2023, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Liao, C.H.; Wu, W.B.; Zheng, C.M.; Lu, K.C.; Ma, M.C. Uremic Toxin Indoxyl Sulfate Impairs Hydrogen Sulfide Formation in Renal Tubular Cells. Antioxidants 2022, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Jin, S.; Wu, D.D.; Wang, M.J.; Zhu, Y.C. Hydrogen sulfide improves glucose metabolism and prevents hypertrophy in cardiomyocytes. Nitric Oxide 2015, 46, 114–122. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, W.; Li, B.; Qiao, X.; Wang, X.; Yang, G.; Li, S. The potential role of hydrogen sulfide in regulating macrophage phenotypic changes via PINK1/parkin-mediated mitophagy in sepsis-related cardiorenal syndrome. Immunopharmacol. Immunotoxicol. 2024, 46, 139–151. [Google Scholar] [CrossRef]
- Smimmo, M.; Casale, V.; Casillo, G.M.; Mitidieri, E.; d'Emmanuele di Villa, B.R.; Bello, I.; Schettino, A.; Montanaro, R.; Brancaleone, V.; Indolfi, C.; et al. Hydrogen sulfide dysfunction in metabolic syndrome-associated vascular complications involves cGMP regulation through soluble guanylyl cyclase persulfidation. Biomed Pharmacother. 2024, 174, 116466. [Google Scholar] [CrossRef]
- Sun, H.J.; Xiong, S.P.; Wang, Z.C.; Nie, X.W.; Bian, J.S. Hydrogen sulfide in diabetic complications revisited: The state of the art, challenges, and future directions. Antioxid. Redox Signal 2023, 38, 18–44. [Google Scholar] [CrossRef]
- Aslami, H.; Heinen, A.; Roelofs, J.J.; Zuurbier, C.J.; Schultz, M.J.; Juffermans, N.P. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med. 2010, 36, 1946–1952. [Google Scholar] [CrossRef]


| Glucose Uptake | Mediators | Trigger | Pathways to Reconstitute Glucose Uptake | References | |
|---|---|---|---|---|---|
 | Akt | GFs, hormones, neurotransmitter | translocation of Gluts into the membrane | glucose uptake↑ | [69] |
| AhR | Cystine depletion→kynurenine (?) | HIF-1 and ATF4 | glucose uptake↑ | [98] | |
| ATF4 | ER/mitochondrial stress | H2S production → AMPK-P, Glut5↑ | glucose uptake↑ | [99,100] | |
| VEGF expression ↑ → Akt-P | glucose uptake↑ | [101,102] | |||
| H1F1 | iron deprivation | expression of Gluts | [103] | ||
| AMPK | High AMP, iron deprivation, H2S | Glut4 translocation ↑, Glut4 recycling ↓ | glucose uptake↑ | [80] | |
| Akt inhibition | GSK3β | AhR activation | [104] | ||
| NFkB → IL6 | help of immune cells → VEGF ↑ | glucose uptake↑ | [105,106] |
| AhR wt Female | AhR wt Male | AhR wt Female | AhR wt Male | AhR wt Male | ||
|---|---|---|---|---|---|---|
| AhR Induction by | PCB126 | Clozapine | EtOH | |||
| blood | weight |  |  |  |  | |
| blood glucose |  |  |  | ? | ||
| Cholesterol |  |  | 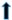 | |||
| TGs | ? | ? |  | 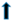 | ||
| liver | weight |  | 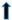 | 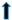 | ? | |
| vacuolation |  |  |  | 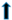 | ||
| hypertrophy | 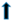 | 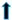 |  |  |  | |
| glycogen |  |  |  | 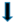 | ||
| gluconeogenesis | 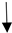 | 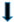 |  | |||
| fatty acid oxidation | 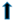 |  |  | |||
| pAMPK | ? | ? |  | |||
| SREBP-1C | ? | ? | 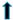 |  | ||
| MDA | ? | ? |  |  | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehsel, K. Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation. Biomedicines 2024, 12, 2294. https://doi.org/10.3390/biomedicines12102294
Fehsel K. Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation. Biomedicines. 2024; 12(10):2294. https://doi.org/10.3390/biomedicines12102294
Chicago/Turabian StyleFehsel, Karin. 2024. "Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation" Biomedicines 12, no. 10: 2294. https://doi.org/10.3390/biomedicines12102294
APA StyleFehsel, K. (2024). Metabolic Side Effects from Antipsychotic Treatment with Clozapine Linked to Aryl Hydrocarbon Receptor (AhR) Activation. Biomedicines, 12(10), 2294. https://doi.org/10.3390/biomedicines12102294




